
Case Report
J Pediatr & Child Health Care. 2017; 2(1): 1013.
Question of Prophylactic Anticoagulation in Steroid Sensitive Nephrotic Syndrome – Rare Complication in Pediatrics
Orosz P¹, Durányik M¹, Bíró E¹, Körhegyi I¹, Balogh I² and Szabó T¹*
¹Department of Pediatrics, University and Clinical Center of Debrecen, Hungary
²Department of Laboratory Medicine, University and Clinical Center of Debrecen, Hungary
*Corresponding author: Szabó T, Department of Pediatrics, University and Clinical Center of Debrecen, 4032 Debrecen, Nagyerdei krt. 98, Hungary
Received: February 02, 2017; Accepted: April 12, 2017; Published: April 21, 2017
Abstract
Nephrotic syndrome occasionally may be associated with complications of acquired hypercoagulable state. An eight-year-old boy with steroid responsive nephrotic syndrome developed his fourth relaps after nearly 15 months of remission. Acute neurological symptoms developed on the third day of his hospital stay. Cranial MRI demonstrated severe superior sagital bilateral transverse and rectal sinus thrombosis. Unfractionated heparin was switched to LMWH on the 6th day of treatment as progression-hemiparesis and seizures in the neurologic state were observed. Repeated MRI revealed new hyperacute vascular lesions. Besides the well known acquired prothrombotic state, we decided to search for inherited factors of procoagulant activity. We detected the activated Protein C rate below the lowest normal value. Furthermore, mutational analysis revealed heterozygous FV Leiden mutation in combination with prothrombin 20210A polymorphism (heterozygous mutation). LMWH followed by warfarin therapy led to successful complete recanalisation and neurological recovery. Warfarin was discontinued after 18 months follow up period. Lack of evidence based therapeutic protocols highlights the importance of sharing case reports where multiple aquired and/or genetical causes are detected.
Keywords: Nephrosis; Thrombosis; Pediatric; Leiden; Prothrombin; LMWH
Case Presentation
An eight-year-old boy with Steroid Sensitive Nephrotic Syndrome (SSNS) developed his fourth relaps after nearly 15 months of remission phase. He was diagnosed with Nephrotic Syndrome (NS) at the age of 4 and had experienced three steroid responsive relapses Family history is unremarkable.
On admission the patient was hospitalized with relapse of nephrotic syndrome and was treated with oral steroid at a dose of 60 mg/m2/day.
On the third day of his hospital stay he acutely developed severe headache, somnolent state followed by vomiting and seizures.
Coagulation studies showed the following parameters: Prothrombin Time (PT) of 8.2 sec (controll: 8.8 sec), an Activated Partial Thromboplastin Time (APTT) of 25, 2 sec (controll: 29, 2 sec), a Thrombin Time (TT) of 24.3 sec (controll: 18.2 sec), an INR of 0.97, a D-dimer of 33.85 mg/L FEU, a positive fibrin monomer test, an FDP of higher than 20 mg/L, and a platelet count of 224 G/L.
Cranial MRI demonstrated superior sagittal, bilateral transverseand rectal sinus thrombosis (Figure 2).
A loading dose of Un-Fractionated-Heparin (UFH) of 75 U/kg was given IV, followed by a continous infusion of 15-25 U/kg/hour. We attempted to maintain the APTT between 70 to 90 sec, with a control of 32 sec.
On the 6th day of his treatment the boy developed new symptoms: left sided hemiparesis with faciobrachial dominance, transient visual disturbance, repeatedly occuring seizures. Repeated MRI revealed new hyperacute vascular lesions on territories supplied by the right anterior and medial cerebral artery. The previously detected sinus thrombosis showed unchanged spread.
Anticoagulant therapy was immediately changed to LMWH administration (2mg/kg/day). 11 days after the admission, on reaching the desired level of anticoagulation warfarin was added with successful control of PT while LMWH was withdrawn Warfarin administration was discontinued after 18 months of problem free period.
The child gradually made a complete clinical recovery, proteinuria ceased by the end of the second week of steroid treatment, with no relapse occuring after withdrawal of steroid therapy. Cranial MRI performed 11 months later showed complete recanalisation with regularly filling sinuses (Figure 3).
Besides the well known acquired prothrombotic state, we decided to search for inherited causes of increased procoagulant activity. With functional tests we detected Protein S and ATIII activity within the reference range, however Protein C activity and activated Protein C rate remained below the lowest normal values. Further molecular genetic investigation, using nucleic acid hybridization assays, confirmed the diagnosis of heterozygous FV Leiden mutation that was suspected by previous APC resistance testing method. Prothrombin 20210A polymorphism analysis verified heterozygous mutation (Figure 1), but the presence of lupus “anticoagulant” was excluded.
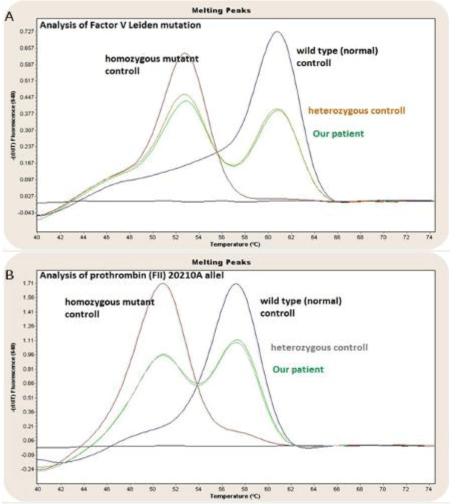
Figure 1: Factor V Leiden heterozygous mutation (A) Prothrombin 20210A
allel heterozyguos mutation (B) In the case of our patient, compared with wild
type, homozygous and heterozygous controlls-hybridisation assay.
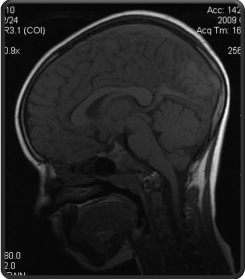
Figure 2: Acute sinus thrombosis in the sinus sagittal superior, rectus and
bilateral transversus.
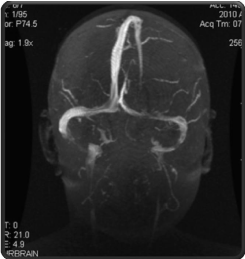
Figure 3: Regularly filled sinuses. Complete recanalisation of the superior,
rectus and both transverse sinuses.
Conclusion
NS is known to be associated with a hypercoagulable state and thromboembolic complications, however such episodes are reportedly less frequent in children than adults [1,2]. Thrombotic Episodes (TE) have been reported in patients with established glomerular disease based on activation of the hemostatic system with a complex interplay between increased hepatic synthesis and intravascular consumption of coagulation factors and increased urinary loss of proteins.
Higher incidence of TE was reported in adults with membranous nephropathy. Children with secondary form of NS have a higher incidence of TE than those with minimal change disease [3]. Studies, like the Mid-West Pediatric Nephrology Consortium (MWPNC) Study [3], reported 9.2% prevalence of venous thromboembolic event in the examined group. Similar data was shown in a recent report by Bryce A Kerlin [4].
Higher risk of TE in NS, especially within the first 3-6 months, has been reported by Mahmoodi BK [5]. Intravascular hypovolemia due to hypoalbuminemia and the use of steroid are important risk factors of thrombosis development. Therefore, thromboprophylaxis may be indicated in selected cases especially with high grade proteinuria in the early weeks after diagnosis. Most clinicians do not give prophylactic anticoagulation initially. The incidence of TE tends to increase in case of serum albumin concentration <20g/L, fibrinogen level >6g/L, antithrombin III level <70% of the normal, in neonates and adolescents with systemic vasculitis associated “secondary” NS [3,6]. Among the prophilactic strategies, some authors suggested using aspirin [7], but in vitro observations shows it less beneficial, and there are no controlled trials confirming its efficacy [8]. Another choice for oral profilactic therapy can be warfarin.
Our patient had consolidated laboratory alterations and quick response to previous steroid treatments by the time promised a rather “low risk” SSNS patient with modest nephrotic range proteinuria.
The initial treatment was UFH. Available trials in pediatric patients that evaluate the efficacy of UFH compared to LMWH found no significant difference in the outcome [9]. However, LMWH seems preferable because of the lower side effect rate. In our case the child developed new intracranial vascular lesions six days after the initation of UFH. However, after switching anticoagulation method to LMWH a convincing clinical improvement and complete recovery was observed. LMWH therapy was followed by prolonged oral anticoagulation with warfarin, when nephrotic-range proteinuria disappeared and the albumine level stabilized above 25g/l. Total recanalisation of the affected sinuses were reached successfully (Figure 3). Thromboembolic phenomenon in NS has been attributed mainly to low plasma AT III levels [10], although such events has been reported with normal AT III levels as well [11]. Higher fibrinogen levels has been detected in serum of NS patients in relapse, such alterations were attributed to hyperthrombotic state observed in these patients. Variable levels of protein C and S has been measured in NS patients as well, and there have been conflicting reports published concerning alterations in functional activity of protein C [12]. We detected the APC rate below the lowest normal value in relapse, however upon stable remission such alteration in PC value was not observed.
Loss of AT III in the nephrotic urine may be an important cause of the failure of anticoagulation with heparin. Large amounts of administered heparin may also be lost in the nephrotic urine. LMWH has a lot of difference from UFH in its anticoagulant mechanism, let alone the different antifactor Xa to antifactor IIa ratios.
Finally, about our interesting findings of genetic factors. Beyond obvious acquired prothrombotic state in NS, screening for genetical background may be indicated in selected cases. It is no doubt to search for genetic background if a previously healthy child experiences a TE. Similarly, predisposing thrombophylic factors are to be searched for when a child with nephrotic syndrome has thrombotic complication when no obvious explanation (high grade proteinuria, severe hypovolemia etc.) for such an event exist. Some authors found a synergic interaction between increased prothrombin levels caused by 20210A mutation and delayed inactivation of FV Leiden substrate [13]. Others believe that clinicians must decide about testing thrombophilia in every case of TE in affected children, or only with positive familial history of thrombosis [14]. Usually small children with thrombosis have both hereditary and acquired factors. According to the American College guideline about antithrombotic therapy and prevention of thrombosis in children, knowlegde of background factors does not really influence the therapeutic recommendations [15]. Lack of evidence based therapeutic protocols highlights the importance of sharing case reports where multiple aquired and/or genetical causes are detected. Long term anticoagulation is indicated in adult patients with serious TE associated with NS especially when genetical predisposing factor(s) are detected. However, there are no clear therapeutic recommendations for similar cases in children with NS. It might be justified to perform basic genetic testing among pediatric patients with NS, who once faced with thromboembolism, even if aquired predisposing factors are already at hand.
References
- Citak A, Emre S, Sâirin A, Bilge I, Nayir A. Hemostatic problems and thromboembolic complications in nephrotic children. Pediatr Nephrol. 2000; 14: 138-142.
- Louis CU, Morgenstern BZ, Butani L. Thrombotic complications in childhood-onset idiopathic membranous nephropathy. Pediatr Nephrol. 2003; 18: 1298-1300.
- Kerlin BA, Blatt NB, Fuh B, Zhao S, Lehman A, Blanchang C, et al. Epidemiology and risk factors for thromboembolic complications of childhood nephrotic syndrome: a Mid-West Pediatric Nephrology Consortium (MWPNC) study. J Pediatr. 2009; 155: 105-110.
- Kerlin BA, Ayoog R, Smoyer WE. Epidemiology and pathophysiology of nephrotic syndrome-associated thromboembolic disease. Clin J Am Soc Nephrol. 2012; 7: 513-520.
- Mahmoodi BK, ten Kate MK, Waanders F, Veeger NJ, Brouwer JL, Vogt L, et al. High absolute risks and predictors of venous and arterial thromboembolic events in patients with nephrotic syndrome: results from a large retrospective cohort study. Circulation. 2008; 117: 224-230.
- Hamed RM, Shomaf M. Congenital nephrotic syndrome: a clinico-pathologic study of thirty children. J Nephrol. 2001; 14; 104-109.
- Hodson E. The management of idiopathic nephrotic syndrome in children. Pediatr Drugs. 2003; 5: 335-349.
- Kerlin BA, Haworth K, Smoyer WE. Venous thromboembolism in pediatric nephrotic syndrome. Pediatr Nephrol. 2014; 29: 989-997.
- Coutinho JM, Ferro JM, Canhao P, Barinagarrementeria F, Bousser MG, Stam J. Unfractionated or low-molecular weight heparin for the treatment of cerebral venous thrombosis. Stroke. 2010; 41: 2575-2580.
- Lau SO, Tkachuck JY, Hasegawa DK, Edson JR. Plasminogen and antithrombin III deficiences in the childhood nephrotic syndrome associated with plasminogenuria and antithrombinuria. J Pediatr. 1980; 3: 390-392.
- Llach F. Hypercoagulability, renal vein thrombosis, and other thrombotic complications of nephrotic syndrome. Kidney Int. 1985; 28: 429-439.
- Anand NK, Chand G, Talib VH, Chellani H, Pande J. Hemostatic profile in nephrotic syndrome. Indian Pediatr. 1996; 33: 1005-1012.
- Kalafatis M, Haley PE, Lu D, Bertina RM, Long GL, Mann KG. Proteolytic events that regulate factor V activity in whole plasma from normal and Activated Protein C (APC)-resistant individuals during clotting: an insight into the APC-resistance assay. Blood. 1996; 87: 4695-4707.
- Raffini L, Thornburg C. Testing children for inherited thrombophilia: more questions than answers. Br J Hematol. 2009; 147: 277-288.
- Monagle P, Chan AK, Goldenberg NA, Ichord RN, Journeycake JM, Nowak-Gottl U, et al. Antithrombotic Therapy in Neonates and Children. Chest. 2012.