
Special Issue - Pediatric Cardiology
J Pediatr & Child Health Care. 2017; 2(1): 1015.
Infective Endarteritis and Massive Pulmonary Artery Aneurysm Formation in a Child with Untreated Large Patent Ductus Arteriosus: Case Report from Tanzania
Zuechner A1,2*, Kabyemera R1,2, Mhada T1,2, Majani N³, Warrier G4 and Freund MW5
¹Department of Pediatrics, Bugando Medical Centre, Tanzania
²Department of Pediatrics, Catholic University of Health and Allied Sciences, Tanzania
³JakayaKikwete Cardiac Institute, Tanzania
4Department of Cardiothoracic Surgery, Apollo Hospital Hyderabad, India
5Department of Paediatric Cardiology, Germany
*Corresponding author: Zuechner A, Department of Pediatrics, BugandoMedical Centre, Wurzburg Road, Mwanza, Tanzania
Received: May 04, 2017; Accepted: July 05, 2017; Published: July 11, 2017
Abstract
Indication for Patent Ductus Arteriosus (PDA) closure is widely accepted for hemodynamic reasons only. However, patients with PDA are at risk for Infective Endarteritis (IE), which may cause serious complications and even lead to death. We report a case of infective endarteritis with the rare complication of a massive pulmonary artery aneurysm formation in a child with an undiagnosed large PDA.
A seven-year-old girl was referred to a tertiary referral hospital in Tanzania with two weeks of fever, difficult breathing and general body weakness. Furthermore, she complained of long-standing fatigability. Prior to referral, an oral antibiotic treatment was started elsewhere.
On examination the girl was ill-looking with left sided chest bulging, precordial hyperactivity and a grade IV continuous systolic-diastolic murmur at the left upper sternal border with palpable thrill.
Two-dimensional echocardiography revealed a large PDA with turbulent left to right shunt. Furthermore, a large vegetation in the distal main pulmonary artery and a small aneurysm were detected. Follow-up echocardiography over the course of six weeks revealed an increasing aneurysm of the Pulmonary Artery (PA), finally covering a large part of the left chest. Antibiotic treatment was started and five months after the onset of the IE a successful surgical resection of the PA-aneurysm formation and PDA-closure was performed with full clinical recovery.
In conclusion, patients with relevant PDA are at risk developing IE and other serious complications. We therefore recommend that a significant PDA should not only be closed for hemodynamic reasons, but also to prevent life-threatening endarteritis.
Keywords: Patent Ductus Arteriosus (PDA); Infective endarteritis; Vegetation; Pulmonary artery aneurysm
Introduction
Patent Ductus Arteriosus (PDA), among other congenital and acquired heart diseases, increases the risk of infective endarteritis [1]. Before the introduction of antibiotic therapy and surgical closure, infective end arteritis was a possible cause of death in patients with PDA [2]. However, currently, indication for PDA closure is accepted mainly for hemodynamic reasons [3,4].
Case Report
A seven-year-old girl was presented to Bugando Medical Centre (BMC) in Mwanza, North Western Tanzania, with fever, difficult breathing and general body weakness for two weeks. Furthermore, for three years she complained about fatigability. Before admission to BMC the girl was treated in a district hospital for the diagnosis of rheumatic heart disease and received penicillin.
On examination the girl was weak and ill-looking with a temperature of 38.50C, respiratory rate of 52 breaths/minute and pulse rate of 138 beats/min. The oxygen saturation was 96% in room air. Her weight was 22 kg and height was 125 cm, equivalent to the body mass index of 14.1. Left sided chest bulging, precordial hyperactivity and raised jugular venous pressure were observed. The apex beat was palpated at seventh inter-costal space and a grade IV continuous systolic-diastolic murmur with palpable thrill was auscultated at the left upper sternal border.
Two-dimensional echocardiography revealed dilated left atrium and left ventricle (LVID 58/36 mm, Z-score LVIDd+4.0 and LVIDs+3.2 [5], hyperdynamic contractility (SF 39%), a large PDA with a floating vegetation in the distal main and proximal left pulmonary artery and a wall defect of the pulmonary artery resulting in an aneurysm formation (Figure1 and 2).The chest X-ray showed severe cardiomegaly with enlarged pulmonary arteries (Figure 4). The diagnosis of large PDA with infective endarteritis and formation of a pulmonary artery aneurysm was made. Intravenous antibiotic therapy (X-penicillin and gentamycin) was initiated after blood cultures were taken. Furthermore, anti-congestive therapy with furosemide (1mg/ kg 8 hourly), captopril (0.5 mg/kg 8 hourly) and digoxin was started accompanied by high caloric feeding. Serial echocardiography studies, in the following six weeks, showed a continuous increase in the size of the aneurysm formation. The defect in the pulmonary artery wall, being the entrance to the aneurysm, additionally increased in size, measuring two cm (Figure 2 and 3). Finally, the aneurysm nearly occupied the entire left hemi-thorax (Figure 4a).
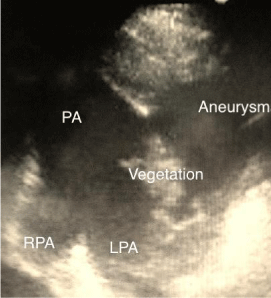
Figure 1: Echocardiography. Short axis of the pulmonary artery showing the
vegetation and the aneurysm.
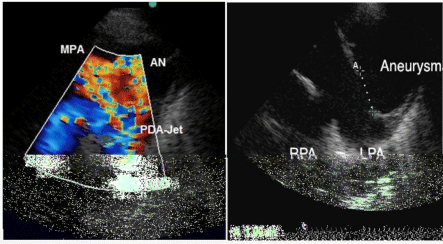
Figure 2: Echocardiography. Short axis of the pulmonary artery.
Left (2A) with colour Doppler illustrating the PDA-jetarising from the
Descending Aorta (DAO) and right (2B) showing the defect of the pulmonary
wall measuring 1, 99 cm and the large aneurysm.
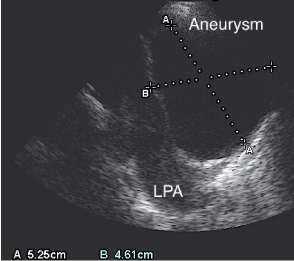
Figure 3: Echocardiography. Showing the increasing size (5, 25x4, 6cm) of
aneurysm within 3 weeks compared with Figure 1.
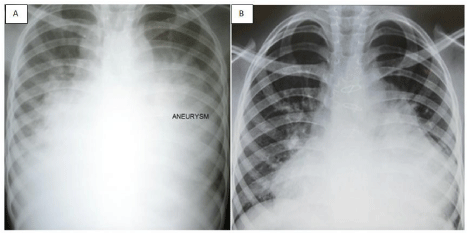
Figure 4: Chest X-ray (left, 4A) before surgery showing severe
cardiomegaly and aneurysm of the pulmonary artery and (right, 4B) three
months after surgery.
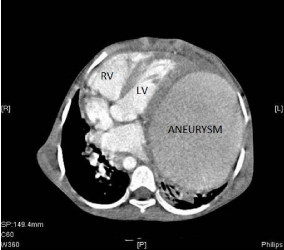
Figure 5: Angiographic computer tomography before surgery with
presentation of the huge aneurysm and displacement of the heart to the right
(LV: Left Ventricle; RV: Right Ventricle).
With the initial antibiotic therapy, the girl remained febrile and worsened clinically. Despite negative blood culture results, Staphylococcus aureus was suspected and intravenous cloxacillin was added without improvement. Therefore, the antibiotic treatment was changed to Vancomycin and very slowly the girl recovered clinically.
As complex paediatric cardiothoracic surgery is not yet available in Tanzania, a referral for heart surgery in India was prepared and five months after the onset of the IE with the formation of the PAaneurysm, the girl underwent successful surgical correction with closure of the PDA and resection of the aneurysm with patchplasty of the defect in the pulmonary artery. Prior to the surgery, a contrast computer tomography was performed and demonstrated the large aneurysm of the left pulmonary artery in the lower left chest, shifting the heart into the right thorax (Figure5). The left lung and the left bronchus were compressed and shifted posteriorly. The intraoperative findings were dense intra-pericardial adhesions, severe dextro-positioning of the heart due to the large pulsating aneurysm occupying the entire left chest, compressing the left lung. The entrance to the massive aneurysm was the defect in the wall of the left pulmonary artery, located opposite to the origin of the large PDA.
Four and 18 months post surgery, echocardiography revealed excellent results; no residual PDA, no residual aneurysm, a good ventricular contractility and normal LV size (LVID 43/30 mm, Z-score LVIDd+1.1, SF 32%). Clinically the girl developed well; she had gained weight, her physical capacity was normal and she did not report any complaints.
Discussion
Infective endarteritis is thought to be a rare complication of PDA [6-9]. However, the incidence of IE in resource-limited countries is reported to be significantly higher than in developed countries [10,11]. Before introduction of antibiotic therapy and surgical closure, IE was a possible cause of death in patients with PDA [2]. However, indication for PDA closure is widely accepted for hemodynamic reasons only [3,4].
Our patient presented with a vegetation on 2-D-echocardiography. The echocardiographic detection of vegetations, in spite of negative blood culture results, is frequently reported [10-12] and may be an almost typical pattern of IE in developing countries. One of the reasons of negative blood cultures is the use of inadequate antibiotic therapy prior to the sampling of blood cultures [1], as in the reported case.
The complication of IE with an aneurysm formation of the pulmonary artery is life threatening and extremely rare, only single cases with successful outcomes have been reported [13]. Other causes of PA-aneurysm formation in PDA are long-lasting pulmonary artery hypertension in adults [14]. Possible complications of PA-aneurysms are pulmonary emboli, dissection, compression of coronary arteries or bronchial tubes [15,16]. In our case no emboli were detected and the coronary arteries were not affected, but the left bronchus was compressed by the huge aneurysm as demonstrated in the contrast computer tomography prior to surgery.
Conclusion
In a young patient with hyperactive precordium and a continuous systolic-diastolic murmur with palpable thrill at the left upper sternal border the main diagnosis to be considered by the pediatrician or family doctor is a PDA. Rheumatic heart disease, which was the working diagnosis in the district hospital, mainly presents with signs of mitral valve regurgitation, such as systolic murmur over the apex with radiation into the axilla.
Patients with hemodynamic relevant PDA are not only at risk of developing heart failure and pulmonary hypertension. Yet, these patients are also at risk of developing IE, and even the formation of a massive aneurysm due to an endarteritis of the pulmonary artery wall. Timely antibiotic treatment prior to surgery can be an appropriate strategy in this rare complication of an endarteritis.
Nonetheless, the indication for PDA closure in developing countries has to be revisited, and a recommendation for the closure of all PDAs has to be determined. Even in countries with limited resources, surgical and percutaneous closure of PDA is a simple procedure, which has the potential to prevent life-threatening complications.
References
- Choi KN, Yang TH, Park BS, Jun HJ, Lim SJ, Seol SH, et al. A Case with Patent Ductus Arteriosus Complicated by Pulmonary Artery Endarteritis. J Cardiovasc Ultrasound. 2008; 16: 90-92.
- Campbell M. Natural History of persistent ductus arteriosus. Br Heart J. 1968; 30: 4-13.
- Doyle T, Kavanaugh-McHugh A, Soslow J, Hill K. Management of patent ductus arteriosus. UpToDate. 2017.
- Schneider DJ, Moore JW. Patent ductus arteriosus. Circulation. 2006; 114: 1873-1882.
- Pettersen MD, Du W, Skeens ME, Humes RA. Regression equations for calculation of z scores of cardiac structures in a large cohort of healthy infants, children, and adolescents: an echocardiographic study. J Am Soc Echocardiogr. 2008; 21: 922-934.
- Satoh T, Nishida N. Patent ductus arteriosus with infective endocarditis at age 92. Intern Med. 2008; 47: 263-268.
- Huggon IC, Quereshi SA. Is the prevention of infective endarteritis a valid reason for closure of the patent arterial duct? Eur Heart J. 1997; 18: 364-366.
- Knirsch W, Nadal D. Infective endocarditis in congenital heart disease. Eur J Pediatr. 2011: 170: 1111-1127.
- Di Filippo S. Prophylaxis of infective endocarditis in patients with congenital heart disease in the context of recent modified guidelines. Arch Cardiovasc Dis. 2012; 105: 454-460.
- Sadiq M, Nazir M, Sheikh SA. Infective endocarditis in children--Incidence, pattern, diagnosis and management in a developing country. Int J Cardiol. 2001; 78: 175-182.
- Siddiqui BK, Tariq M, Jadoon A, Murtaza G, Syed A, Abid MB, et al. Infective endocarditis in patients with congenitally malformed hearts: characterization of the syndrome in a developing country. Cardiol Young. 2007; 17: 623-630.
- Garg N, Kandpal B, Garg N, Tewari S, Kapoor A, Goel P, et al. Characteristics of infective endocarditis in a developing country-clinical profile and outcome in 192 Indian patients, 1992-2001. Int J Cardiol. 2005; 98: 253-260.
- Kiani A, Radmehr H, Shabanian R. Pulmonary Artery Aneurysm in a Child Secondary to Infective Endarteritis. Pediatr Cardiol. 2008; 29: 471-472.
- Bezgin T, Demircan HC, Kaymaz C. Giant pulmonary artery aneurysm secondary to patent ductus arteriosus: a case report. Curr Cardiol Rev. 2015; 11: 163-166.
- Tiwari N, Ganguly G, Garg A, Nagi GS, Hasnain S, Dikshit V. Pulmonary artery aneurysm with dissection and hemopericardium. Asian CardiovascThorac Ann. 2013; 21: 71-73.
- Kreibich M, Siepe M, Kroll J, Hoehn R, Grohmann J, Beyersdorf F. Aneurysms of the Pulmonary Artery. Circulation. 2015; 131: 310-316.