
Special Article - Pediatric Endocrinology
J Pediatr & Child Health Care. 2017; 2(2): 1016.
Low-Dose Pamidronate Therapy for Pediatric Osteoporosis: Influence of Diagnosis on Changes in Fracture Rate and Bone Mineral Density
Crossen SS¹, Pederson J², Kumar RB³ and Bachrach LK³*
¹Department of Pediatrics, University of California at Davis, USA
²Department of Pediatrics, Feinberg School of Medicine, Northwestern University, USA
³Stanford University School of Medicine, USA
*Corresponding author: Laura K. Bachrach, Room H 314, Stanford Medical Center, 300 Pasteur Drive, Stanford, USA
Received: June 20, 2017; Accepted: September 12, 2017; Published: September 19, 2017
Abstract
Controversy surrounds the optimal agent, dose and duration of bisphosphonate therapy for pediatric osteoporosis. We conducted a prospective, observational study of low-dose (4 mg/kg/year) intravenous pamidronate in 31 children with Osteogenesis Imperfecta (OI) or non-OI osteoporosis treated for a median of 39 months (range 6.5-164). Subjects in both diagnostic groups showed significant gains in spine areal Bone Mineral Density (aBMD) during the first year of therapy (29% median gain in children with OI and 15% in children with non-OI osteoporosis). Fracture frequency also declined significantly in both patient groups during the first year of treatment, including for two patients who had <10% improvement in spine aBMD over this time frame. The correlation between % change in aBMD and % change in fracture rate for our study population was weak, as demonstrated by a Spearman’s rank correlation coefficient (rho) of 0.13 (p-value 0.32, 95% confidence interval -0.32 to 1.00). Minor side effects of bisphosphonate therapy were self-limited, and no osteopetrosis, jaw osteonecrosis, or atypical femur fractures occurred during treatment for up to 13.6 years. These data suggest that low dose pamidronate is safe and effective for long-term use in pediatric osteoporosis, and that change in aBMD is an imperfect predictor of reduction in fracture risk.
Keywords: Bisphosphonate; Bone mineral density; Fracture; Pediatric osteoporosis; Osteogenesis imperfecta; Pamidronate
Introduction
Bone fragility and osteoporosis (OP) are common complications of several genetic and acquired disorders of childhood. Pediatric patients with Osteogenesis Imperfecta (OI), inflammatory bowel disease, rheumatologic disorders, cerebral palsy, muscular dystrophy, cystic fibrosis, or a history of transplantation may develop low bone mass and fragility fractures [1]. Treatment of pediatric osteoporosis begins with optimizing nutrition, vitamin D stores, endocrine function, and weight-bearing physical activity [2]. When these measures are insufficient to prevent bone loss and fracture, use of pharmacologic therapies is considered [3].
Pharmacologic options for treating OP in adults include bisphosphonates to reduce bone resorption and anabolic agents to stimulate bone formation. The safety and efficacy of these medications in older patients have been established in large randomized controlled trials (RCTs), but data are limited in pediatrics [3-5]. The best studied anabolic agent, synthetic parathyroid hormone, should not be used in children due to a black box warning about the risk of osteosarcoma [6]. The anti-resorptive bisphosphonates have been used to treat primary and secondary osteoporosis in children, but the optimal agent, dose and duration of therapy remain controversial due to a lack of RCTs comparing different drugs and dosing regimens [3,7]. The response to bisphosphonates in children has been studied most extensively in patients with OI with less information on the response in children with secondary osteoporosis from chronic disease [3,4,7,8]. The most common outcome measure in these studies has been the change in areal bone mineral density (aBMD) by dual energy x-ray absorptiometry (DXA) rather than clinically relevant endpoints such as improvements in bone pain, mobility or fragility fractures [3].
Pamidronate is the bisphosphonate that has been used most extensively in pediatric patients, administered intravenously either in a high-dose protocol of 9mg/kg/year divided every 4 months [9] or low-dose at 4mg/kg/year divided every 2-3 months [10- 12]. Data comparing the relative efficacy and safety of the two regimens are limited. One retrospective study of 15 patients with non-OI osteoporosis treated for a year with low-dose or high-dose pamidronate found no dose-related differences in the changes observed for spine areal bone mineral density (aBMD) and fracture frequency [13].
Whether adverse effects vary by dose, particularly with long-term use, has also not been determined. In older adults, a “drug holiday” is often recommended after five years of bisphosphonate therapy due to concerns for over-suppression of bone turnover, osteonecrosis of the jaw, and atypical femur fractures [14]. In children, the maximal benefit from bisphosphonate therapy is achieved after 2-3 years [15], but there are risks to discontinuing drug therapy in growing patients. Fractures may occur at the junction of older “treated” bone and the more distal “untreated” bone added during growth [16]. Therefore, children with OI or ongoing risk factors for secondary osteoporosis are often maintained on a lower dose of bisphosphonates after their initial treatment period until final height is reached [3,16].
Prescribing the lowest effective dose is a priority, given concerns for over-suppression of bone turnover during many years of bisphosphonate therapy. One study of patients with OI found bone turnover markers to be suppressed below the expected range as long as two years after high dose pamidronate was discontinued [17]. Reassuringly, the same investigators found no clinical signs of oversuppression in patients treated for 10 years or more with pamidronate or zoledronic acid. No studies to date have reported long-term followup data for patients treated with low dose pamidronate.
We have previously shown that 4mg/kg/year pamidronate given for up to 30 months in 11 osteoporotic children resulted in reduced fracture rates and improved spinal aBMD [12]. This low dose was derived by extrapolation from pamidronate protocols for adults with secondary OP due to glucocorticoid therapy, transplantation, and other chronic diseases. This report summarizes the changes in spine aBMD and fracture rates and the adverse effects in 31 children treated with low dose pamidronate for up to 13.6 years for bone fragility related to OI or non-OI disorders.
Methods
Pamidronate therapy was offered on a compassionate use basis to pediatric patients with a history of low-impact long bone or vertebral fracture. Two patients without a prior fracture history were also treated due to a perceived high risk for fracture. One was a patient with Duchenne Muscular Dystrophy, chronic high dose glucocorticoid therapy, evidence of decreasing bone mineral density, and anticipated loss of ambulation. The other was a patient on chronic high-dose glucocorticoids for management of Crohn’s disease who had declining bone density; pamidronate was provided for the duration of glucocorticoid therapy. Both of these patients had baseline spine aBMD z-scores less than -2 prior to treatment. All subjects were bisphosphonate-naïve except one OI patient who had been treated at another center; this patient’s continued bone fragility at the time of entry in our study qualified her for ongoing therapy.
All patients underwent screening tests prior to the initiation of treatment, which included a complete blood count (CBC), serum calcium, phosphorus, magnesium, alkaline phosphatase, albumin, creatinine, intact parathyroid hormone, 25-hydroxy and 1,25-dihydroxy vitamin D levels, and urine calcium, phosphorus, and creatinine. The purpose of these tests was to identify any underlying bone disorder or vitamin D deficiency that would need to be addressed prior to bisphosphonate administration. Pamidronate was not administered to any child with a 25-hydroxy vitamin D concentration <50 nmol/L (20ng/ml).
Pretreatment densitometry of the lumbar spine, whole body and total hip by dual energy x-ray absorptiometry (DXA) was performed in children overage three if the examinations could be conducted without sedation. Sites with metal implants in the region of interest were excluded from analysis. Baseline lateral thoraco-lumbar spine x-rays were performed in younger children in whom a DXA scan was not feasible and in any patient suspected of having a vertebral fracture. Prior fracture history was recorded based on patient and parent reports. Clinically significant fractures were defined as any long bone or vertebral compression fracture, excluding fractures of the fingers, toes, hands, feet, ribs, and clavicles.
For children three years or older, pamidronate disodium (mixed with 0.9% normal saline) was administered intravenously every three months at a dose of 1mg/kg, up to a maximum of 30mg per dose. Children under age three received a dose of 0.75mg/kg every eight weeks due to more rapid bone turnover. Each dose was infused over four hours in an inpatient or day hospital setting at Lucile Packard Children’s Hospital. After the initial treatment period of three years, maintenance therapy was continue data dose of 1mg/kg (maximum 30mg) every six months until growth plates closed or the underlying risk factors resolved.
To monitor for acute adverse effects following the first infusion, serum calcium, magnesium, phosphorus and creatinine were measured within 3-7 days post treatment. Serum calcium, phosphorus, magnesium, creatinine and CBC were also measured prior to each subsequent infusion; 25-hydroxyvitamin D was measured annually. All follow-up infusion visits included a systematic review of interval events including fractures, symptoms or side effects and any laboratory or imaging studies. Bone densitometry by DXA was repeated at 6, 12, 24, and 36 months where feasible without sedation; spine x-rays were performed annually if a DXA could not be obtained or there was suspicion of new vertebral fracture.
Statistical analyses
Study data were managed using Research Electronic Data Capture (REDCap) [18], a secure web-based research application hosted at the Stanford Center for Clinical Informatics. Data were analyzed for OI and non-OI cohorts separately. Fracture rates were assessed as total fractures divided by total years at risk for each patient during the time frame in question. Years at risk prior to study entry was defined as 1) years lived for OI patients, 2) years since onset of highdose steroids for patients with glucocorticoid induced osteoporosis (GIO), 3) date of diagnosis for a patient with osteosarcoma, and 4) date of first fracture for other diagnoses including idiopathic juvenile osteoporosis (IJO), congenital hypomyelination syndrome, and cerebral palsy, where onset of risk was difficult to determine. Change in spine aBMD was calculated for each patient as a percent change in numeric score from baseline value, and these calculations were performed only when serial data were obtained using the same DXA equipment.
Fracture and densitometry data are reported as median and range (Table 2) or median, quartiles, and range (Figure 1) because the data were not normally distributed. P-values were calculated using Fisher’s Exact Test for categorical variables and Wilcoxon Rank Sum for quantitative variables. Spearman’s rank correlation coefficient (rho) was calculated to examine the correlation between % change in spine aBMD and % change in fracture rate during the first year of treatment in the 16 patients with complete BMD data.
Median (Range) Spine aBMD at Baseline*
Median (Range) Spine aBMD at One Year*
P - Value†
Median (Range) % Increase in Spine aBMD*
Median (Range) Fractures/Year at Baseline
Median (Range) Fractures/Year at One Year
P - Value†
Median (Range) % Decrease in Fracture Rate
Study Population (31)
0.42 (0.25 - 0.64)
0.54 (0.35 - 0.71)
0.002
23.2
(3.1 - 56.7)
1.3 (0 - 25)
0 (0 - 3)
<0.001
100 (-82.9 - 100)
Osteogenesis Imperfecta (21)
0.43 (0.25 - 0.64)
0.56 (0.35 - 0.71)
0.051
29.3 (8.3 - 36.2)
0.9 (0.2 - 25)
0 (0 - 3)
<0.001
100 (-82.9 - 100)
Non-Osteogenesis Imperfecta (10)
0.40 (0.38 - 0.53)
0.54 (0.46 - 0.68)
0.004
15.5 (3.1 - 56.7)
1.7 (0 - 12.2)
0 (0 - 2)
0.006
100 (-18 - 100)
Table 2: Change in Spine BMD and Fracture Rate by Diagnosis.
The study protocol was approved by the Administrative Panel on Human Subjects in Medical Research at Stanford University. Written, informed consent was obtained from parents or guardians, and assent was obtained from all children over the age of eight years. The researchers were at all times in compliance with the World Medical Association’s Declaration of Helsinki regarding the ethical conduct of research involving human subjects.
Results
Clinical characteristics of the 31 patients enrolled between 1998 and 2012 are summarized in Table 1. The age at initiation of pamidronate therapy ranged from 2.5 months to almost 16 years. At the time of study closure, median duration of treatment in our cohort was 39 months (range 6.5 to 164 months). The cohort was 52% male and ethnically diverse: Caucasian (48%), Asian (26%), Hispanic (19%), and African-American (6%). The majority of patients (68%) had a diagnosis of OI. Not all OI patients had been formally “typed” but they varied in severity from those presenting with fractures in infancy to some identified only as older children. Non-OI disorders included GIO (13%), IJO, cerebral palsy, Duchenne muscular dystrophy, metastatic osteosarcoma, congenital hypomyelination syndrome, and an unknown metabolic bone disease. Most patients were ambulatory with the exception of infants, one toddler with severe OI, and the patient with congenital hypomyelination syndrome. Demographic characteristics including age, sex, and ethnicity did not vary significantly between the diagnostic groups.
Study Population (31)
OI Patients (21)
Non-OI Patients (10)
P-Value†
Median Age in Years (Range)
8.0 (0.2-15.9)
8.5 (0.2-15.8)
7.6 (2.3-15.9)
0.500
Age at Enrollment (%)
<3 Years
3-12 Years
>12 Years
4 (13%)
21 (68%)
6 (19%)
3 (14%)
13 (62%)
5 (24%)
1 (10%)
8 (80%)
1 (10%)
0.843
Sex (%)
Female
Male
15 (48%)
16 (52%)
10 (48%)
11 (52%)
6 (60%)
4 (40%)
0.704
Ethnicity (%)
African-American
Asian
Caucasian
Hispanic
2 (6%)
8 (26%)
15 (48%)
6 (19%)
2 (9%)
4 (19%)
10 (48%)
5 (24%)
-
4 (40%)
5 (50%)
1 (1%)
0.527
Patients with Baseline Long Bone Fractures (%)
25 (81%)
20 (95%)
5 (50%)
0.007
Patients with Baseline Vertebral Fractures (%)
15 (48%)
9 (43%)
6 (60%)
0.458
Median # of Fractures at Baseline (Range)
Total
Long Bone
Vertebral
5 (0-18)
4 (0-18)
0 (0-11)
6 (2-18)
4 (0-18)
0 (0-4)
4.5 (0-11)
1 (0-7)
1.5 (0-11)
0.082
0.002
0.088
Median Spine aBMD Z-score at Baseline (Range)*
-2.8 (-8.3 to -0.6)
-2.6 (-5.7 to -0.6)
-2.8 (-8.3 to -2.0)
0.209
Table 1: Clinical Characteristics of Study Population.
Overall, 81% of the study population had a history of long bone fractures and 48% had sustained vertebral fractures at the time of study entry. A significantly greater proportion of patients in the OI group (95%) versus the non-OI group (50%) reported long bone fractures at study entry, whereas the proportion reporting baseline vertebral fractures (43% of OI and 60% of non-OI) did not differ significantly between groups. The number of historical long bone fractures in our study cohort ranged from 0 to 18, and historical vertebral fractures ranged from 0 to 11. Baseline spine aBMD Z-score ranged from -8.3 to -0.6 overall for the study population with a median of -2.8. Median values for baseline fractures and spine aBMD were similar in our two diagnostic groups, with the exception of significantly more long bone fractures among OI vs non-OI subjects. Base line vitamin D levels were >50 nmol/L (20ng/ml) for all patients except one who was treated with vitamin D supplementation concurrently with bisphosphonate therapy.
Changes in spine aBMD and fracture rate during the first year of therapy are summarized in Table 2. Patients in both diagnostic groups demonstrated significant improvement in spine aBMD and fracture rate over this time frame (p-values ranging <0.001 to 0.051). Median percent improvement in spine aBMD appeared greater in OI than non-OI patients (29.3% vs 15.5%), although this difference was not statistically significant due to small sample size (see Figure 1).
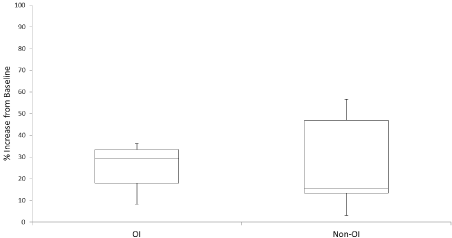
Figure 1: Change in Spine BMD in First Year of Therapy. Box-and-whiskers plot depicting the range, 1st and 3rd quartiles, and median values for % increase in
spine aBMD among OI and non-OI patients during first year of therapy. N = 16 (9 OI, 7 non-OI patients) with data available. P-value for statistical difference between
% change in OI vs non-OI patients (using Wilcoxon Rank Sum) is 0.280.
We explored the correlation between % change in spine aBMD and % change in fracture rate over the first year of treatment for the 16 patients with complete BMD data using Spearman’s rank correlation coefficient (rho). Rho was calculated at 0.13 (p-value of 0.32, 95% confidence interval of -0.32 to 1.00), indicating a poor correlation between the clinical outcome (fractures) and the change in densitometric parameters within our study population.
Twenty-two patients (71%) reported at least one side effect after the first infusion, with fever the most common complaint (16 patients or 73% of those reporting any symptom). Side effects were less common after subsequent infusions with 12 patients (39% of the study population) reporting an adverse reactions at any point during infusions 2-4, 16% during infusions 5-7, and only one patient (3%) reporting a side effect after infusion 7. Throughout the treatment period, the most common symptom was fever (experienced by 52% of the study population at some time), followed by myalgia or bone pain (35%), fatigue (32%), and gastrointestinal upset (16%). All symptoms were self-limited. Serum calcium, phosphorus, magnesium, creatinine, CBC and platelets remained normal throughout the treatment period. No patients experienced osteonecrosis of the jaw [19], atypical femur fracture [20], or clinically significant hypocalcemia.
Discussion
Recent reviews underscore the persistent knowledge gaps related to bisphosphonate therapy for both primary and secondary osteoporosis in childhood and adolescence [3,4,7]. There is still no consensus about the optimal agent, dose and duration of treatment for OP in pediatric patients, reflecting the lack of RCTs. Similarly,
there is no consensus around the best metric by which to monitor response to treatment.
In this prospective, longitudinal study of 31 pediatric patients with primary or secondary OP, low-dose pamidronate (4mg/kg/ year) was associated with gains in spinal aBMD and reduced fracture rates. We observed individual and group variability in the response to treatment. Although the median percent gain in spine aBMD was greater among children with OI than those with non-OI osteoporosis, this difference was not statistically significant perhaps due to our small sample size. Fracture rate decreased in both groups over the first year of therapy, and all patients reported a reduction in chronic bone pain and improvement in quality of life after initiation of treatment. We found the correlation between the % change in spine aBMD and % reduction in fractures over the first year of therapy to be weak in our cohort, suggesting that densitometry is an imperfect surrogate marker for the clinical response to pamidronate treatment. The therapy was well tolerated for up to 13.6 years with no serious adverse effects.
These data add to the small number of prior reports supporting the efficacy and safety of low-dose pamidronate. The magnitude of gain in aBMD for patients with OI or non-OI osteoporosis using low-dose therapy has been generally comparable to that seen with the higher dose protocol (9 mg/kg/year) [10,11,13]. Similar to the trend we observed, Steelman and colleagues observed that patients with OI had greater gains in aBMD than non-OI patients during lowdose pamidronate treatment [10]. Their report included data only for the first 6-22 months of therapy and did not analyze fracture rates by diagnosis. A second retrospective study compared the response to low-dose and high-dose pamidronate among children with secondary OP when treatment regimen was assigned at the discretion of the providers. Changes in aBMD and fracture rate in that study appeared to be equivalent between the two doses [13]. In the absence of a randomized, dose-response study, however, recommendations regarding the optimal dose of pamidronate are based upon expert opinion and experience rather than evidence-based.
Bone densitometry and biochemical markers of bone turnover have been used most commonly as outcome measures to assess drug efficacy because changes in these parameters can be detected more rapidly with smaller cohorts than clinical variables such as fractures [7]. However, these surrogate measures have proven to be imperfect correlates of the clinical response to pharmacologic therapy. In our study, we found a weak correlation between the % change in spine aBMD and change in fracture rate, which is supported by findings in multiple other studies of bisphosphonate efficacy. Studies in adults with osteoporosis have shown a reduction in fragility fractures even in subjects who failed to gain aBMD with bisphosphonate therapy [21]. Conversely, gains in aBMD do not ensure a significant improvement in clinical outcomes. One study of children with OI observed that spine aBMD increased significantly in those given oral alendronate compared to those given placebo whereas the reductions in bone pain and fractures did not differ significantly between the groups [8]. To date, there are no studies in pediatric patients to determine the magnitude of change in aBMD or the absolute aBMD value that predicts a reduction in fracture rates. In the absence of such data, stabilization of aBMD Z-score, increases in aBMD, and achievement of aBMD Z-scores >-2 have been proposed as reasonable benchmarks of a successful response to bisphosphonate therapy among pediatric patients [3]. The “gold standard” for assessing response to therapy in osteoporosis remains reduction in fragility fractures and bone pain.
We observed that pamidronate therapy reduced but did not eliminate all fractures in our patients, similar to the experiences reported by other investigators. The most extensive long-term study of children with OI treated with high-dose pamidronate or zoledronic acid (0.1 mg/kg/year) found considerable individual variability in the response to therapy. During a median treatment period of 14.8 years, five of 37 patients had more than 20 long-bone fractures of the lower extremity, while another five patients had fewer than five similar fractures [22]. These findings underscore the importance of setting realistic expectations about potential benefits of pharmacologic therapy with patients and their families before initiating treatment.
This study has several important limitations. The data were collected from a convenience sample of patients at a single medical center. All children were treated with the same pamidronate dose, thus precluding any conclusions about the optimal agent or dosing regimen. In the absence of an untreated control group, we cannot rule out the possibility of spontaneous improvement in bone strength, which has been reported in OI. We had incomplete spine aBMD data pretreatment and at follow-up due to metal implants, inclusion of younger patients, and non-adherence to the schedule for DXA scanning. Our cohort was not large enough to conduct meaningful DXA analyses after two and three years of therapy but fracture rates remained low in the study group (<0.5 fractures per person per year) throughout the second and third years of treatment. Our study was also not sufficiently powered to perform a multivariate regression analysis, which would allow us to evaluate associations between diagnostic group and our primary outcomes (changes in fracture rate and bone mineral density) while adjusting for differences in demographics, baseline fractures and baseline BMD. In addition, this was a non-blinded study and fracture data were based upon subjective recall by patients and parents, who often splinted painful areas without confirmatory x-rays. These factors could have led to an under- or over-estimate of treatment efficacy. Finally, we did not continue to follow patients after discontinuing pamidronate therapy to determine the length of protection against fracture post-treatment.
Despite these limitations, the observations from this prospective observational study provide additional support for the effectiveness and safety of long term low-dose pamidonate therapy. Children with primary or secondary OP treated from infancy through adolescence experienced a reduction in fracture frequency, and continuous treatment was well tolerated and effective for up to 13.6 years without clinical or densitometric evidence of osteopetrosis.
A multi-center RCT is needed to establish the optimal pharmacologic therapy for OP in pediatric patients. Intra venous zoledronic acid has replaced pamidronate as the preferred bisphosphonate in many pediatric bone centers because it can be administered in less than an hour on a less frequent basis every 3-12 months [3,22-25]. Because of its greater potency, however, safety monitoring for hypocalcemia, hypophosphatemia, hypomagnesemia and over-suppression of bone turnover is essential. Another promising agent is denosumab, a monoclonal antibody directed against RANKL which reduces osteoclast activity. This drug offers the advantage of subcutaneous route of delivery and more rapid reversibility of bone turnover suppression than the bisphosphonates [26]. These potential benefits must be weighed against risks associated with this drug. There have been reports of exaggerated bone resorption as the effects of denosumab wane. Marked hypercalcemia (from rapid bone turnover) has been reported in a pediatric patient [27]. Future pediatric studies are needed to evaluate the relative efficacy and safety of these and other agents. Ideally studies should be powered to assess fracture reduction, bone pain reduction, and mobility improvement as end points, given the imperfect correlation between DXA and these clinical outcomes [3]. Such research will require considerable collaboration and major financial support, but would provide the essential “gold standard” proof of optimal therapy for childhood osteoporosis that is currently lacking.
References
- Bachrach LK. Diagnosis and treatment of pediatric osteoporosis. Curr Opin Endocrinol Diabetes Obes. 2014; 21: 454-460.
- Boyce AM, Gafni RI. Approach to the child with fractures. J Clin Endocrinol Metab. 2011; 96: 1943-1952.
- Ward LM, Konji VN, Ma J. The management of osteoporosis in children. Osteoporos Int. 2016; 27: 2147-2179.
- Bachrach LK, Ward LM. Clinical review 1: Bisphosphonate use in childhood osteoporosis. J Clin Endocrinol Metab. 2009; 94: 400-409.
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001; 285: 785-795.
- Vahle JL, Sato M, Long GG, Young JK, Francis PC, Engelhardt JA, et al. Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1-34) for 2 years and relevance to human safety. Toxicol Pathol. 2002; 30: 312-321.
- Ward L, Tricco AC, Phuong P, Cranney A, Barrowman N, Gaboury I, et al. Bisphosphonate therapy for children and adolescents with secondary osteoporosis. Cochrane Database Syst Rev. 2007.
- Ward LM, Rauch F, Whyte MP, D'Astous J, Gates PE, Grogan D, et al. Alendronate for the treatment of pediatric osteogenesis imperfecta: a randomized placebo-controlled study. J Clin Endocrinol Metab. 2011; 96: 355-364.
- Glorieux FH, Bishop NJ, Plotkin H, Chabot G, Lanoue G, Travers R. Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. N Engl J Med. 1998; 339: 947-952.
- Steelman J, Zeitler P. Treatment of symptomatic pediatric osteoporosis with cyclic single-day intravenous pamidronate infusions. J Pediatr. 2003; 142: 417-423.
- Plotkin H, Coughlin S, Kreikemeier R, Heldt K, Bruzoni M, Lerner G. Low doses of pamidronate to treat osteopenia in children with severe cerebral palsy: a pilot study. Dev Med Child Neurol. 2006; 48: 709-712.
- Gandrud LM, Cheung JC, Daniels MW, Bachrach LK. Low-dose intravenous pamidronate reduces fractures in childhood osteoporosis. J Pediatr Endocrinol Metab. 2003; 16: 887-892.
- Martinez-Soto T, Pacaud D, Stephure D, Trussell R, Huang C. Treatment of symptomatic osteoporosis in children: a comparison of two pamidronate dosage regimens. J Pediatr Endocrinol Metab. 2011; 24: 271-274.
- Black DM, Rosen CJ. Clinical Practice. Postmenopausal Osteoporosis. N Engl J Med. 2016; 374: 254-262.
- Rauch F, Travers R, Plotkin H, Glorieux FH. The effects of intravenous pamidronate on the bone tissue of children and adolescents with osteogenesis imperfecta. J Clin Invest. 2002; 110: 1293-1299.
- Rauch F, Cornibert S, Cheung M, Glorieux FH. Long-bone changes after pamidronate discontinuation in children and adolescents with osteogenesis imperfecta. Bone. 2007; 40: 821-827.
- Rauch F, Plotkin H, Travers R, Zeitlin L, Glorieux FH. Osteogenesis imperfecta types I, III, and IV: effect of pamidronate therapy on bone and mineral metabolism. J Clin Endocrinol Metab. 2003; 88: 986-992.
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009; 42: 377-381.
- Christou J, Johnson AR, Hodgson TA. Bisphosphonate-related osteonecrosis of the jaws and its relevance to children--a review. Int J Paediatr Dent. 2013; 23: 330-337.
- Nicolaou N, Agrawal Y, Padman M, Fernandes JA, Bell MJ. Changing pattern of femoral fractures in osteogenesis imperfecta with prolonged use of bisphosphonates. J Child Orthop. 2012; 6: 21-27.
- Chesnut CH, Rosen CJ, Bone Quality Discussion G. Reconsidering the effects of antiresorptive therapies in reducing osteoporotic fracture. J Bone Miner Res. 2001; 16: 2163-2172.
- Palomo T, Fassier F, Ouellet J, Sato A, Montpetit K, Glorieux FH, et al. Intravenous Bisphosphonate Therapy of Young Children with Osteogenesis Imperfecta: Skeletal Findings During Follow Up Throughout the Growing Years. J Bone Miner Res. 2015; 30: 2150-2157.
- Simm PJ, Johannesen J, Briody J, McQuade M, Hsu B, Bridge C, et al. Zoledronic acid improves bone mineral density, reduces bone turnover and improves skeletal architecture over 2 years of treatment in children with secondary osteoporosis. Bone. 2011; 49: 939-943.
- Vuorimies I, Toiviainen-Salo S, Hero M, Makitie O. Zoledronic acid treatment in children with osteogenesis imperfecta. Horm Res Paediatr. 2011; 75: 346-353.
- Barros ER, Saraiva GL, de Oliveira TP, Lazaretti-Castro M. Safety and efficacy of a 1-year treatment with zoledronic acid compared with pamidronate in children with osteogenesis imperfecta. J Pediatr Endocrinol Metab. 2012; 25: 485-491.
- Semler O, Netzer C, Hoyer-Kuhn H, Becker J, Eysel P, Schoenau E. First use of the RANKL antibody denosumab in osteogenesis imperfecta type VI. J Musculoskelet Neuronal Interact. 2012; 12: 183-188.
- Setsu N, Kobayashi E, Asano N, Yasui N, Kawamoto H, Kawai A, et al. Severe hypercalcemia following denosumab treatment in a juvenile patient. J Bone Miner Metab. 2016; 34: 118-122.