
Review Article
J Pediatr & Child Health Care. 2024; 9(1): 1064.
Effectiveness of Immunotherapy in Preventing Childhood Asthma: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Suganya Nadarajan¹*; Shruthi Punnapu²
1Specialist Pediatrician, Mediclinic Parkview Hospital, Dubai, UAE
2Specialist Pediatrician, Mediclinic Parkview Jospital, Dubai, UAE
*Corresponding author: Suganya Nadarajan Specialist Pediatrician, Mediclinic Parkview Hospital, Dubai, UAE. Email: sugan214@gmail.com
Received: May 21, 2024 Accepted: June 11, 2024 Published: June 18, 2024
Abstract
Background: Childhood asthma is a significant public health concern, and despite advancements in traditional management strategies, there remains a need to explore alternative therapeutic approaches. One promising area of research is the use of immunotherapy, which aims to modulate the immune system’s response to specific allergens and reduce the severity of asthmatic symptoms.
Methods: This systematic review and meta-analysis evaluated the effectiveness of sublingual (SLIT) and subcutaneous (SCIT) immunotherapy in the management of childhood asthma. A comprehensive literature search was conducted, and Randomized Controlled Trials (RCTs) were included. The Quality Assessment tool (RoB 2) was used to assess the risk of bias, and a meta-analysis was performed to calculate the pooled Relative Risk (RR) and 95% Confidence Interval (CI) for the primary outcome of asthma incidence. We include all RCTs from inception till April 2024.
Results: 12 RCTs were included in our systematic review. The pooled analysis of 10 RCTs with 1,719 participants showed a significantly lower incidence of asthma in the immunotherapy group compared to the control group [RR = 0.58, 95% CI (0.48: 0.70), P < 0.00001]. Subgroup analyses revealed that both SLIT and SCIT were associated with a reduced risk of developing asthma in children with allergic diseases.
Conclusion: This systematic review and meta-analysis provide strong evidence that the use of immunotherapy, including both SLIT and SCIT, can be an effective strategy for preventing the development of asthma in children with allergic diseases. Further research is needed to strengthen the evidence and guide clinical decision-making in this important area of pediatric asthma management.
Introduction
Childhood asthma is a significant public health concern, affecting millions of children worldwide [1]. This chronic respiratory condition can have an impact on a child’s quality of life, physical activity, and overall well-being [2]. The causes of childhood asthma are multifactorial and involve a complex interplay of genetic predisposition and environmental factors [3,4]. Common triggers include allergens (such as pollen, dust mites, and pet dander), respiratory infections, air pollution, tobacco smoke exposure, and certain medications [5].
Childhood asthma is characterized by recurrent episodes of wheezing, coughing (particularly at night or early morning), shortness of breath, and chest tightness [6]. These symptoms can vary in severity and may be triggered or worsened by factors like exercise, allergens, or respiratory infections [7]. The prognosis of childhood asthma varies widely depending on factors such as the severity of symptoms, the effectiveness of treatment, and the individual’s response to management strategies [8]. With appropriate medical care and management of triggers, many children with asthma can lead normal and active lives without significant long-term complications [8].
Despite advancements in traditional asthma management strategies, such as inhaled corticosteroids and bronchodilators, there remains a need to explore alternative therapeutic approaches that can provide more effective and targeted treatment [9].
One promising area of research in the field of childhood asthma is the use of immunotherapy. Immunotherapy, also known as allergy or desensitization therapy, aims to modulate the immune system’s response to specific allergens, thereby reducing the severity of asthmatic symptoms and improving overall disease management [10]. This approach has been successfully employed in the treatment of various allergic conditions, including allergic rhinitis and bee venom hypersensitivity, and has garnered increasing attention as a potential strategy for managing childhood asthma [11].
The rationale behind the use of immunotherapy in childhood asthma is based on the understanding that many cases of childhood asthma are associated with underlying allergic sensitivities [12]. By exposing the immune system to gradually increasing doses of the offending allergens, immunotherapy can desensitize the body and reduce the inflammatory response that triggers asthmatic symptoms [13]. This can lead to improved symptom control, reduced reliance on rescue medications, and potentially a long-term positive impact on the natural history of the disease [14,15].
While the potential benefits of immunotherapy for childhood asthma are promising, the available evidence on its efficacy and safety has not been thoroughly synthesized.
This systematic review aims to critically evaluate the current literature on the use of immunotherapy in the management of childhood asthma, providing a comprehensive and up-to-date assessment of its effectiveness, and potential applications in clinical practice.
Methods
The meta-analysis followed the standards outlined in the Cochrane Handbook for Systematic Reviews and Meta-Analyses [16], and adhered to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [17].
Study Selection and Data Extraction
A systematic literature search was conducted to identify relevant Randomized Controlled Trials (RCTs) investigating Sublingual Immunotherapy (SLIT) and Subcutaneous Immunotherapy (SCIT) in pediatric populations with allergic diseases. The search encompassed major databases including PubMed, SCOPUS, web of science and Cochrane Library. The search strategy used a combination of relevant keywords and MeSH terms related to immunotherapy, allergic diseases, and pediatric populations. The search was performed in April 2024 and included studies published from their inception to this date.
Data extraction was performed independently by two reviewers using a standardized form. The extracted data included study characteristics (author, year, design), participant demographics (age, allergic disease), intervention details (type of immunotherapy, allergen used, treatment duration), control group details (placebo or standard care), and outcome measures related to asthma incidence, allergic symptoms, medication use, and lung function.
Quality Assessment
The Risk of Bias 2 (RoB 2) tool was utilized to assess the quality and risk of bias in the included RCTs [18]. This tool evaluates bias across multiple domains including randomization process, deviations from intended interventions, missing outcome data, measurement of outcomes, and selective reporting. Each study was independently assessed by two reviewers, and discrepancies were resolved through discussion or consultation with a third reviewer if needed.
Data Synthesis and Analysis
A meta-analysis was performed using Review Manager software (RevMan version 5.4). Pooled Relative Risks (RR) with 95% Confidence Intervals (CI) were calculated for primary outcome (asthma incidence). Subgroup analyses were conducted based on the route of administration (SLIT vs. SCIT) to explore the effects of different immunotherapy modalities.
Qualitative synthesis of study outcomes was conducted to summarize the diverse benefits of immunotherapy interventions observed across the included RCTs. Specific improvements in asthma prevalence, allergic symptoms, and other outcomes were highlighted based on the findings reported in each study.
Results
Our search initially identified 8600 references, out of which 1391 duplicates were excluded. Following screening of titles and abstracts, 24 studies met the eligibility criteria. Subsequent full-text screening led to the inclusion of 12 RCTs in this systematic review [14,15,19-28]. Among these, 10 RCTs were incorporated into the meta-analysis [14,15,19,20,22,24-28] (Figure 1).
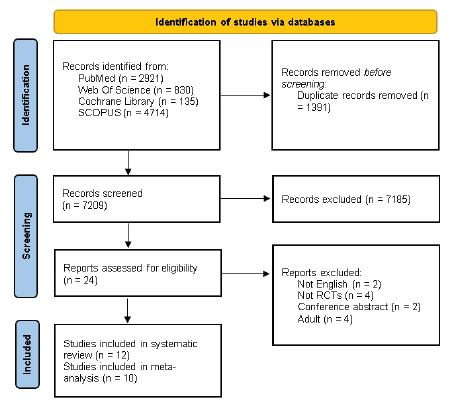
Figure 1: PRISMA flow diagram.
Included Studies Characteristics
The included RCTs investigated Sublingual Immunotherapy (SLIT) and Subcutaneous Immunotherapy (SCIT) in pediatric populations with allergic diseases. The studies included children across different age ranges, from infants aged 6–12 months to adolescents up to 17 years old, with various allergic diseases such as atopic dermatitis, rhinoconjunctivitis, and asthma. Sensitization profiles varied from no sensitization to mono- or poly-sensitization to specific allergens like house dust mite, grass, birch pollen, and food allergens. Intervention groups received either SLIT drops or tablets containing allergens such as house dust mite, grass pollen, or birch pollen, while control groups received placebos. Treatment durations ranged from 6 months to 3 years, with follow-up periods extending up to 7 years. Asthma outcomes were assessed based on symptoms, medication use, lung function, and response to bronchodilators, adhering to criteria such as GINA guidelines or specific symptom-based assessments depending on the study. Further details are shown in Table 1.
Study ID
Site
Design
Participant’s age
Allergic Disease(s)
Sensitization
Intervention group
Intervention details/Specifications
Comparator group
Therapy duration; follow-up
Asthma definition
Alviani et al. 2020
UK
RCT
Children (aged 6–12 months)
Healthy
No sensitization
N = 54. SLIT drops
SLIT drops containing house dust mite, administered at 2000 standard treatment units per day.
N = 53. Placebo
Therapy: 1 year; Follow-up: 5 years
Based on symptoms, medication, and lung function
Elsayed et al. 2022
Egypt
RCT
Children (aged 5–12 years)
Mild to moderate asthma
Mono- or poly-sensitized
N = 30. SLIT
SLIT drops prepared by study authors
N = 30. Placebo
Therapy: 6 months: Follow-up: 3 years
Based on GINA guidelines (intermittent or mild asthma).
Holt et al. 2013
USA
RCT
Children (12 months old)
Atopic dermatitis
Sensitization to =1 food allergen
N = 25. SLIT drops
SLIT drops containing a mixture of soluble allergens including HDM, cat, and timothy grass.
N = 25. Placebo
Therapy: 1 year; Follow-up: 3 years
Asthma symptoms.
Jacobsen et al. 2007
Multinational
RCT
Children (6–14 years old)
Rhinoconjunctivitis, mild seasonal asthma
Only sensitized to grass or birch
N = 79. SCIT
SCIT using grass and/or birch pollen extracts with a sample size of 79, reduced to 64 after follow-up.
N = 72. Control. Sample size after follow-up: 53
Therapy: 3 years; Follow-up: 7 years
Asthma symptoms and response to B2-agonists.
Kim et al. 2021
Korea
RCT
Children (6–15 years old)
Atopic asthma and allergic rhinitis
No details
N = 53. SCIT
SCIT using grass and/or birch pollen
N = 19. Control
Therapy: 3 years; Follow-up: 3 years
Asthma symptoms.
Marogna et al. 2008
Italy
RCT
Children (5–17 years old)
Rhinitis, intermittent asthma
Mono- or poly-sensitized
N = 144. SLIT
SLIT treatment using allergens of any type from Anallergo.
N = 72. Control
Therapy: 3 years; Follow-up: 0
Based on GINA guidelines (intermittent or mild asthma).
Moller et al. 1986
Sweden
RCT
Children (8–16 years old)
Rhinoconjunctivitis
Mono- or poly-sensitized
N = 16. SLIT tablets
SLIT tablets (capsules) containing birch pollen.
N = 16. Placebo
Therapy: 10 months; Follow-up: 0.
Lung symptoms.
Moller et al. 2002
Multinational
RCT
Children (6–14 years old)
Rhinoconjunctivitis, asthma
Only sensitized to grass or birch
N = 79. SCIT
SCIT using grass and/or birch pollen extracts with a sample size of 79.
N = 72. Control
Therapy: 3 years; Follow-up: 0
Asthma symptoms and use of B2-agonists.
Niggermann et al. 2006
Multinational
RCT
Children (6–14 years old)
Rhinoconjunctivitis, mild seasonal asthma
Only sensitized to grass or birch
N = 79. SCIT
SCIT using grass and/or birch pollen extracts with a sample size of 79, increased to 95 after follow-up.
N = 72. Control. Sample size after follow-up: 88.
Therapy: 3 years; Follow-up: 2 years
Asthma symptoms and response to B2-agonists.
Nolte et al. 2020
Multinational
RCT
Children (4–14 years old)
Rhinoconjunctivitis
Mono- or poly-sensitized
N = 513. SLIT
Once-daily treatment with the12 Amb a 1-Unit dose of ragweed SLIT-table
N = 512. Placebo
Therapy: 28 weeks; Follow-up: 28 weeks
History and beta-agonist
reversibility testNovembre et al. 2004
Italy
RCT
Children (5–14 years old)
Rhinoconjunctivitis
Only mono-sensitized
N = 54. SLIT drops
SLIT drops containing grass pollen with a sample size of 54, reduced to 47 after follow-up.
N = 59. Control. After follow-up: 50
Therapy: 3 years; Follow-up: 0.
Asthma symptoms and medication use.
Valovirta et al. 2018
Multinational
RCT
Children (5–12 years old)
Rhinoconjunctivitis
Mono- or poly-sensitized
N = 398. SLIT tablets
SLIT tablets containing grass pollen.
N = 414. Placebo
Therapy: 3 years; Follow-up: 2 years
Based on symptoms, medication, and lung function
Table 1: Summary of included studies.
Quality Assessment
The Risk of Bias 2 (RoB 2) assessment across the listed studies indicates mixed quality in terms of bias control. Studies like Moller et al. 1986, Nolte et al. 2020, and Valovirta et al. 2018 generally exhibit low bias risks across domains [19,21,22]. However, studies such as Elsayed et al. 2022, Novembre et al. 2004, and Marogna et al. 2008 show higher risks in specific bias domains, notably in randomization and deviations from intended interventions [14,24,26]. The rest of the studies were judged as having some concerns (Figure 2 & Figure 3).
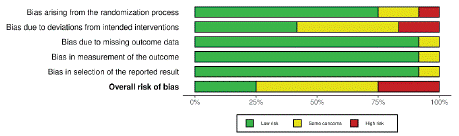
Figure 2: ROB-2 graph of the included studies.
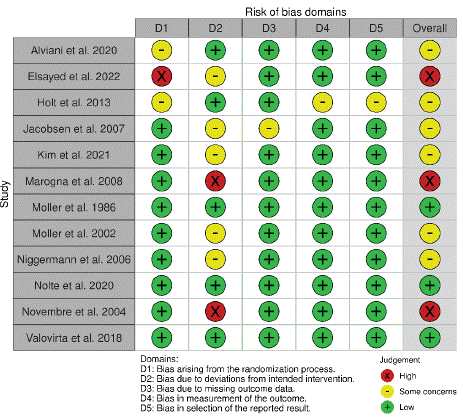
Figure 3: ROB-2 summary of the included studies.
Study Outcomes
Incidence of Asthma
The pooled analysis of 10 RCTs including 1719 participants showed a significant lower incidence of asthma in the immunotherapy group compared with control group as following [RR = 0.58, 95%CI (0.48: 0.70), P < 0.00001], and the data were homogenous (P = 0.25, I2 = 21%) (Figure 4).
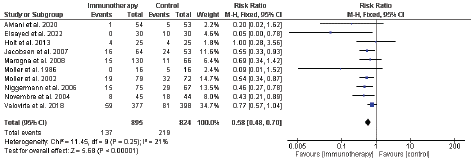
Figure 4: Forest plot for the incidence of childhood asthma.
Subgroup Analysis Based on Route of Administration
The pooled analysis of 7 RCTs including 1309 participants showed a significant lower incidence of asthma in the SLIT compared with the control group [RR = 0.62, 95%CI (0.48: 0.79), P = 0.0001], and the data were homogenous (P = 0.14, I2 = 38%).
Similarly, the pooled analysis of three RCTs including 410 participants showed a significant lower incidence of asthma in the SLIT compared with the control group [RR = 0.52, 95%CI (0.39: 0.69), P < 0.00001], and the data were homogenous (P = 0.87, I2 = 0%) (Figure 5).
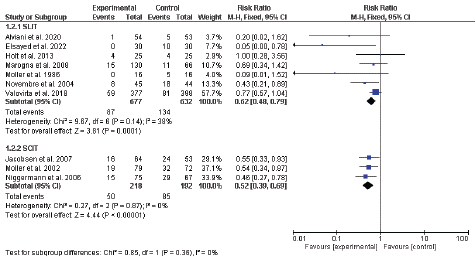
Figure 5: Forest plot for the incidence of childhood asthma.
Qualitative Synthesis
The collective evidence from the included RCTs highlights the diverse benefits of immunotherapy interventions, specifically SLIT and SCIT in pediatric populations with allergic diseases. Alviani et al. (2020) and Marogna et al. (2008) observed reductions in asthma prevalence and improvements in allergic outcomes like rhinitis and eczema with SLIT [14,28]. Elsayed et al. (2022) and Kim et al. (2021) demonstrated significant reductions in allergic inflammation markers, improvements in pulmonary function, and enhanced asthma control following SLIT and SCIT [23,26]. Jacobsen et al. (2007) and Niggermann et al. (2006) highlighted long-term benefits of SCIT in reducing rhinoconjunctivitis symptoms and preventing asthma development [15,27]. Additionally, Moller et al. (1986, 2002) reported decreased allergy symptoms and specific IgE levels with immunotherapy, indicative of its efficacy in reducing seasonal allergy symptoms and preventing asthma [19,25]. Furthermore, Nolte et al. (2020) and Valovirta et al. (2018) demonstrated significant improvements in symptom scores, bronchial hyperresponsiveness, and medication use with SLIT-tablets for ragweed and grass allergies, respectively [21,22].
Discussion
This systematic review and meta-analysis evaluated the effectiveness of immunotherapy, including sublingual (SLIT) and subcutaneous (SCIT) routes, in the management of childhood asthma. The analysis of 10 randomized controlled trials with 1,719 participants showed a significantly lower incidence of asthma in the immunotherapy group compared to the control group. Subgroup analyses based on the route of administration revealed that both SLIT and SCIT were associated with a reduced risk of developing asthma in children with allergic diseases.
The findings of this review suggest that immunotherapy, when used as an adjunct to standard asthma management, can provide substantial benefits in preventing the development of asthma in children with allergic conditions. The proposed mechanism involves the modulation of the immune system’s response to specific allergens, leading to reduced inflammation and improved symptom control. By targeting the underlying allergic drivers, immunotherapy may have a positive long-term impact on the natural history of the disease, potentially reducing the burden of childhood asthma.
Immunotherapy in childhood asthma involves administering controlled doses of specific allergens to modify the immune response [29]. This exposure aims to induce tolerance and shift immune reactions from allergy-promoting Th2 responses to regulatory T-cell responses [30]. Over time, this can reduce asthma symptoms by decreasing airway inflammation and hyperresponsiveness [30]. Immunotherapy helps desensitize the body to allergens, leading to improved respiratory health and potentially altering the course of the disease [30]. It offers a targeted approach to address the underlying immune dysfunction involved in childhood asthma, providing long-term benefits beyond symptomatic relief [22].
The results of this review are consistent with previous systematic reviews and meta-analyses that have investigated the role of immunotherapy in pediatric populations. A review by Farraia et al. 2022 also found that SLIT and SCIT were effective in reducing the risk of developing asthma in children with allergic rhinitis [31]. Similarly, a Cochrane review by Abramson et al. (2010) reported that immunotherapy was associated with a reduced risk of developing asthma allergic rhinitis [32].
The findings of this review have important clinical implications. The use of immunotherapy, particularly SLIT and SCIT, should be considered as a treatment option for children with allergic diseases, as it has the potential to prevent or delay the development of asthma. This is particularly relevant in children with a strong genetic predisposition or environmental exposures that increase their risk of developing asthma [33]. By addressing the underlying allergic triggers, immunotherapy may lead to improved asthma control, reduced reliance on rescue medications, and better overall quality of life for these children.
Clinicians should carefully assess the individual patient’s risk factors, sensitization profile, and preferences when considering immunotherapy as part of a comprehensive asthma management plan [34]. Appropriate patient selection, accurate allergen identification, and close monitoring of treatment response are crucial for optimizing the outcomes of immunotherapy in pediatric populations.
The key strengths of this review include the systematic and comprehensive search strategy and the use of well-established meta-analytic methods. The inclusion of only randomized controlled trials ensures a higher level of evidence and reduces the risk of bias. The subgroup analyses based on the route of administration (SLIT vs. SCIT) provide insights into the comparative effectiveness of different immunotherapy modalities.
However, the review also has some limitations. The included studies exhibited some variability in terms of participant characteristics, allergen profiles, and outcome assessments, which may have introduced heterogeneity. Additionally, the long-term sustainability of the observed benefits and the potential for disease-modifying effects require further investigation with extended follow-up periods. The overall quality of the included studies, as assessed by the RoB 2 tool, was mixed, with some studies showing higher risks of bias in specific domains.
This systematic review and meta-analysis provide strong evidence that the use of immunotherapy, including both SLIT and SCIT, can be an effective strategy for preventing the development of asthma in children with allergic diseases. The findings suggest that by targeting the underlying allergic mechanisms, immunotherapy can lead to improved asthma-related outcomes, reduced medication use, and potentially a long-term positive impact on the natural history of the disease.
Clinicians should consider incorporating immunotherapy as part of a comprehensive asthma management plan for children with allergic conditions, particularly those at high risk of developing asthma. Further research with larger sample sizes, longer follow-up periods, and a focus on disease-modifying effects of immunotherapy would help strengthen the evidence and guide clinical decision-making in this important area of pediatric asthma management.
Author Statements
Ethical Guidelines
Ethical approval is not required as there is no direct involvement with human subjects.
References
- Serebrisky D, Wiznia A. Pediatric Asthma: A Global Epidemic. Ann Glob Health. 2019; 85: 6.
- Jing Z, Wang X, Zhang P, Huang J, Jia Y, Zhang J, et al. Effects of physical activity on lung function and quality of life in asthmatic children: An updated systematic review and meta-analysis. Front Pediatr. 2023; 11: 1074429.
- Noutsios G, Floros J. Childhood asthma: causes, risks, and protective factors; a role of innate immunity. Swiss Med Wkly. 2014; 144: e14036.
- Pijnenburg MW, Frey U, De Jongste JC, Saglani S. Childhood asthma: pathogenesis and phenotypes. European Respiratory Journal. 2022; 59: 2100731.
- Gautier C, Charpin D. Environmental triggers and avoidance in the management of asthma. J Asthma Allergy. 2017; 10: 47–56.
- Lizzo JM, Cortes S. Pediatric Asthma. 2024.
- van Aalderen WM. Childhood Asthma: Diagnosis and Treatment. Scientifica (Cairo). 2012; 2012: 1–18.
- Martin J, Townshend J, Brodlie M. Diagnosis and management of asthma in children. BMJ Paediatr Open. 2022; 6: e001277.
- Agbetile J, Green R. New therapies and management strategies in the treatment of asthma: patient-focused developments. J Asthma Allergy. 2010; 4: 1-12.
- Akdis CA, Akdis M. Mechanisms of allergen-specific immunotherapy and immune tolerance to allergens. World Allergy Organization Journal. 2015; 8: 17.
- Kucuksezer UC, Ozdemir C, Akdis M, Akdis CA. Mechanisms of immune tolerance to allergens in children. Korean J Pediatr. 2013; 56: 505-513.
- Zhang W, Lin C, Sampath V, Nadeau K. Impact of allergen immunotherapy in allergic asthma. Immunotherapy. 2018; 10: 579–93.
- Cappella A, Durham S. Allergen immunotherapy for allergic respiratory diseases. Hum Vaccin Immunother. 2012; 8: 1499–512.
- Marogna M, Tomassetti D, Bernasconi A, Colombo F, Massolo A, Di Rienzo Businco A, et al. Preventive effects of sublingual immunotherapy in childhood: An open randomized controlled study. Annals of Allergy, Asthma and Immunology. 2008; 101: 206–11.
- Niggemann B, Jacobsen L, Dreborg S, Ferdousi HA, Halken S, Høst A, et al. Five-year follow-up on the PAT study: Specific immunotherapy and long-term prevention of asthma in children. Allergy: European Journal of Allergy and Clinical Immunology. 2006; 618: 55–9.
- Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019; 10: ED000142.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009; 62: e1–34.
- Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019; 366: l4898.
- Möller C, Dreborg S, Lanner Å, Björkstén B. Oral Immunotherapy of Children with Rhinoconjunctivitis due to Birch Pollen Allergy A Double Blind Study. Allergy. 1986; 41: 271–9.
- Patrick G Holt, Peter D Sly, Hugh A Sampson, Phil Robinson, Richard Loh, Henning Lowenstein, et al. Prophylactic use of sublingual allergen immunotherapy in high-risk children: A pilot study. J Allergy Clin Immunol. 2013; 132: 991-3.e1.
- Nolte H, Bernstein DI, Nelson HS, Ellis AK, Kleine-Tebbe J, Lu S. Efficacy and Safety of Ragweed SLIT-Tablet in Children with Allergic Rhinoconjunctivitis in a Randomized, Placebo-Controlled Trial. Journal of Allergy and Clinical Immunology: In Practice. 2020; 8: 2322-2331.e5.
- Valovirta E, Petersen TH, Piotrowska T, Laursen MK, Andersen JS, Sørensen HF, et al. Results from the 5-year SQ grass sublingual immunotherapy tablet asthma prevention (GAP) trial in children with grass pollen allergy. Journal of Allergy and Clinical Immunology. 2018; 141: 529-538.e13.
- Kim CK, Callaway Z, Park JS, Kwon E. Efficacy of subcutaneous immunotherapy for patients with asthma and allergic rhinitis in Korea: Effect on eosinophilic inflammation. Asia Pac Allergy. 2021; 11: e43.
- Novembre E, Galli E, Landi F, Caffarelli C, Pifferi M, De Marco E, et al. Coseasonal sublingual immunotherapy reduces the development of asthma in children with allergic rhinoconjunctivitis. Journal of Allergy and Clinical Immunology. 2004; 114: 851–7.
- Mller C, Dreborg S, Ferdousi HA, Halken S, Høst A, Jacobsen L, et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT-Study). Journal of Allergy and Clinical Immunology. 2002; 109: 251–6.
- Sarhan Elsayed S, Mohamed Shokry D, Mohamed El-Behedy R, Elsayed ElBadawy Ali N. Assessment of Efficacy of Sublingual Immunotherapy Compared to the Standard Treatment in Children with Bronchial Asthma in Zagazig University Hospitals. Vol. 89, The Egyptian Journal of Hospital Medicine. 2022; 89.
- Jacobsen L, Niggemann B, Dreborg S, Ferdousi HA, Halken S, Høst A, et al. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-Year follow-up on the PAT study. Allergy: European Journal of Allergy and Clinical Immunology. 2007; 62: 943–8.
- Alviani C, Roberts G, Mitchell F, Martin J, Zolkipli Z, Michaelis LJ, et al. Primary prevention of asthma in high-risk children using HDM SLIT: Assessment at age 6 years. Journal of Allergy and Clinical Immunology. 2020; 145: 1711–3.
- Miraglia Del Giudice M, Licari A, Brambilla I, Tosca M, Ciprandi G. Allergen Immunotherapy in Pediatric Asthma: A Pragmatic Point of View. Children. 2020; 7: 58.
- Smarr CB, Bryce PJ, Miller SD. Antigen-Specific Tolerance in Immunotherapy of Th2-Associated Allergic Diseases. Crit Rev Immunol. 2013; 33: 389–414.
- Farraia M, Paciência I, Castro Mendes F, Cavaleiro Rufo J, Shamji M, Agache I, et al. Allergen immunotherapy for asthma prevention: A systematic review and meta-analysis of randomized and non-randomized controlled studies. Allergy. 2022; 77: 1719–35.
- Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database of Systematic Reviews. 2010; 4: CD00186.
- Krusche J, Basse S, Schaub B. Role of early life immune regulation in asthma development. Semin Immunopathol. 2020; 42: 29–42.
- Cloutier MM, Baptist AP, Blake KV, Brooks EG, Bryant-Stephens T, DiMango E, et al. Focused Updates to the Asthma Management Guidelines: A Report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. Journal of Allergy and Clinical Immunology. 2020; 146: 1217–70.