Editorial
Austin J Pediatr. 2014;1(1): 1001.
Vitamin D-Deficiency Rickets - Where Do We Stand?
Milan Bayer*
Department of Pediatrics, Charles University in Prague, Faculty of Medicine in Hradec Králové, University Hospital Hradec Králové, Czech republic
*Corresponding author: Milan Bayer, Departmentc of Pediatrics, Charles University in Prague, Faculty of Medicine in Hradec Králové, University Hospital Hradec Králové, Czech Republic
Received: March 20, 2014; Accepted: April 06, 2014; Published: April 08, 2014
Vitamin D–binding protein belongs to prime albuminoidal proteins. It was documented that vitamin D was produced by ultraviolet (UV) irradiated phytoplankton and zooplankton already 500 million years ago. Vitamin D– receptor (VDR) has been detected in a wide range of primitive animals. In biological studies lamprey, zebra fish, frog, mouse, and human VDR´s were found to have similar ligand selectivity’s for vitamin D derivatives. Original role of active vitamin D (1alpha, 25–dihydroxyvitamin D3; calcitriol) was protective and immunological. VDR sensitivity to hepatotoxic secondary bile acids facilitated expression of cytochrom P450 enzymes detoxifying these acids in the liver and intestine. When animals left seas and colonized earth, the maintenance of strict mineral homeostasis became very important survival condition. At that time vitamin D and VDR involved in mineral metabolism. It is now evident that in human calcium absorption is highly vitamin D dependent during infancy until the end of puberty.
More than 90% of necessary vitamin D is formed in the skin from 7–dehydrocholesterol under the influence of UV light type B. During the winter, vitamin D composition in the skin is very low below 40 degrees and above 40 degrees. Line of latitude due to unfavorable sun location over the horizon in part of the year. It means that from May to September (southern hemisphere) and from October to April (northern hemisphere) UV exposition of skin in those areas is too low to cover common vitamin D requirements. Predecessors of human being lived in the middle Africa. The migration of populations out of the tropics consequently resulted in selective pressures favoring lighter skin in order to facilitate coetaneous previtamin D3 biosynthesis in regions far from the equator, under low UVB radiation. Four genes associated with this trait in Europeans were recently documented – KITLG, TYRP1, SLC24A5, and SLC45A2. The onset of the sweep shared by Europeans and East Asians at KITLG occurred about 30,000 years ago and the selective sweeps for the European–specific alleles at TYRP1, SLC24A5, and SLC45A2 started within the last 11,000–19,000 years. During the last ten thousand years none significant change in our genome appeared. But human populations went through large changes in their life style, especially in last several centuries.
Bone deformities in children corresponding to rickets were for the first time reported by roman physician Sopranos in 2nd century. Medical student David Whistler published his paper “De morbo puerileanglorum, quem patrio idiomatic indigene vacant the Rickets” in 1645. He presumed influence of alcohol during pregnancy in etiology of this illness. But only Francis Gilson’s description of rickets from 1650 is considered as official. He provided more detailed observation, recognized rickets as noninfectious disease and denatured it from scurvy. It was industry development with increasing air pollution in industrial areas, and child labor with their undernourishment and low exposition to UVB radiation which contributed to pathologists´ observation in second half of 19th century when some sign of rickets could be seen in 80–90% anatomized children. In 1920´s, studies in lion cubs, dogs, and rats showed the importance of cod liver oil in therapy of nutritional rickets. New anthracitic substance later termed vitamin D was described and distinguished from vitamin A. The efficacy of UV irradiation in prevention and treatment of rickets was established. Nevertheless, there were documented next to 14.000 deaths from rickets in The United States in period 1910–1961 and in 1940´s, rickets was still regarded as „probably the most common disease of early childhood.
Rickets is a disease characterized with insufficient and delayed mineralization of endochondral bone formation at the growth plates. Repeated decrease of calcemia results in secondary hyperparathyroidism. The main consequences of this situation are hyperphosphaturia, and stimulation of 1–alpha hydroxylase activity leading to increase of calcitriol levels, followed by bone tissue degradation and bone cells activity. Clinical manifestation as well as typical X–ray picture (Figure 1) of rickets on long bones appears. In case of untreated patient the 25–hydroxyvitamin D is run out and absolute or relative calcitriol deficiency appears. Calcium mobilization from bone becomes insufficient and hypocalcaemia and hypophosphatemia is going along with the effects of secondaryhyperparathyroidism in severe apparent rickets.
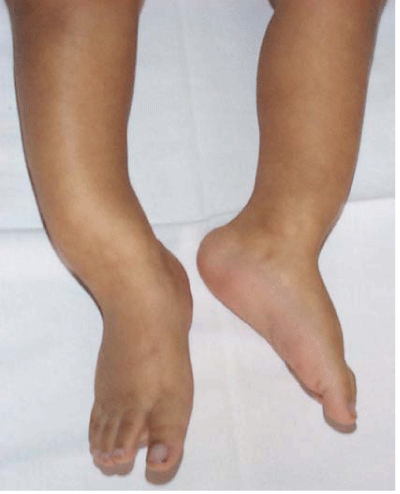
Figure 1: Vitamin D–deficiency rickets – bowing of legs – clinical manifestation.
Clinical manifestation of deficiency rickets depends on age. In infants could prevail symptoms of hypocalcaemia – anomic pauses, strider or tetany? Hypocalcaemia could be also possible cause of dilated cardiomyopathy and cardiac failure. Despite being a reversible condition, the prognosis depends on the severity and time of diagnosis. Typical skeletal symptoms dominate in toddlers (delayed fontanel closure, delayed cutting, metaphysical swelling, bowing of legs; waddling gate and leg pains). General symptoms – hypotonic, sweating, frequent respiratory inflammations is possible to find in any age.
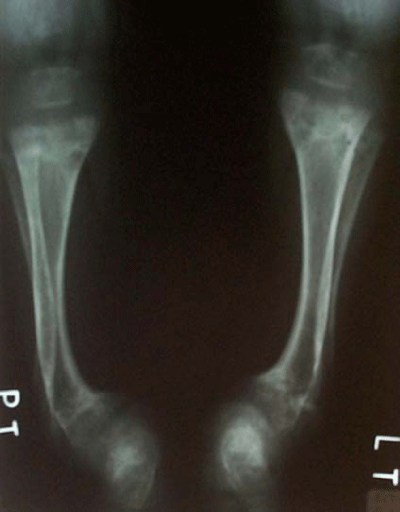
Elevated alkaline phosphates’ activity and typical findings on X–ray picture (Figure 2) (metaphyseal cupping, widening of the physics and irregularity on left forearm and wrist bones conventionally) are the most important laboratory diagnostic features.All patients have secondary hyperparathyroidism but hypocalcemia and hypophosphatemia is possible to observe only in 50%. Serum 25–hydroxyvitamin D levels are low. The high specificity as well as positive predictive value of alkaline phosphates to deficiency rickets allow to use it as cheap screening, especially in exclusively breast–fed children without vitamin D supplementation (age of 6–15months).
Figure 2: Vitamin D-deficiency rickets – deformities and metaphyseal cupping on X–ray.
Disease is successfully treated by cholecalciferol (vitamin D3) or ergocalciferol (vitamin D2) and appropriate calcium (carbonate or citrate) intake to prevent “hungry bone” syndrome.
Incidence of deficiency rickets decreased due to policy of vitamin D supplementation in early childhood as well as production of some food enriched with vitamin D (milk, yoghurts) in the second half of the last century. But the disease was not disappeared. Immigration of numerous groups of people with dark skin to latitude with lack of sun shine, some religious rules leading to low UVB exposition, and air pollution in large cities result in increased risk of vitamin D deficiency in North America and Europe. High risk group represent unsupplemented, full breast–fed children with dark skin. Toddlers with rickets are mostly unsupplemented obese children protected from the sun. They could have atypical muscular pain at the time of presentation. In Africa and some parts of Asia, calcium deficiency is the major cause of rickets due to inappropriate intake in food. Typical dietary calcium intakes in African children are about 200mg daily (approximately 20–28% of US RDAs for age).
Nutritional rickets has been described from at least 59 countries in the last 20 years.
Resurgence of rickets in the industrialized countries could represent some important warning. If there are children with vitamin D–deficiency rickets (it means very low blood 25–hydroxyvitamin D levels), much higher number of people of the same population could suffer from symptomless vitamin D hyposaturation. According with recent knowledge about vitamin D pleiotropic effects it means higher risk of autoimmune, oncological, and cardiovascular diseases. This fact should be taken into consideration in planning of prevention policy.