Case Report
Austin J Pediatr. 2014;1(1): 1002.
Calcifying Aponeurotic Fibroma of the Chest Wall in a 5-Month-Old Infant: A Case Report and Review of the Literature
Minoru Kuroiwa1*, Makoto Suzuki2, Shin-itu Hatakeyama3, Junko Hirato4 and Norio Suzuki2
1Department of Pediatric Surgery, Toho University Ohmori Medical Center, Japan
2Department of Surgery, Gunma University School of Medicine, Japan
3Department of Radiology, Gunma Children’s Medical Center, Japan
4Department of Pathology, Gunma University School of Medicine, Japan
*Corresponding author: Minoru Kuroiwa, Department of Pediatric Surgery, Toho University Ohmori Medical Center, 6-11-1 Omori-nishi Ota-ku, Tokyo 143-8541, Japan
Received: March 20, 2014; Accepted: April 06, 2014; Published: April 08, 2014
Abstract
A 5–month–old female infant was admitted to hospital because of a slowly growing solid mass in the right side of the chest. The mass was located on the right fifth rib and did not involve the skin. Ultrasound (US) revealed that the mass was echogenic with a central high echogenicity, suggesting calcification. Computed tomography (CT) and magnetic resonance imaging (MRI) showed that the calcifying tumor possibly originated from the periosteum of the rib. The tumor was successfully removed and histological diagnosed as a calcifying aponeurotic fibroma (CAF). The patient is free from the tumor 118 months after the surgery. Usually, a CAF develops in the distal extremities and often recurs following excision. To the best of our knowledge, there are no reports in the English literature of a CAF that originated from the chest wall. Herein, we review pediatric cases of CAF that occurred in uncommon sites such as the head, face, neck, and trunk, and discuss the clinical characteristics and treatment of CAF.
Keywords: Calcifying (juvenile) aponeurotic fibroma; Fibrous hamartoma in infancy.
Introduction
Calcifying aponeurotic fibroma (CAF) was first described by Keasbey in 1953 [1]. CAF is a slow–growing, painless soft tissue tumor usually located in the distal portion of extremities, especially the upper extremities such as the palms, fingers, and wrists [2], while the head, neck, and trunk are less frequently affected. Surgical removal is the treatment of choice, but CAF has a tendency to recur after surgery and may histologically be confused with fibrous soft tissue tumors. Here, we present a case of CAF originating from the chest wall. To our knowledge, only 14 pediatric cases of CAF originating from uncommon sites such as the face, head, neck, and trunk have been previously reported in the English literature.
Case report
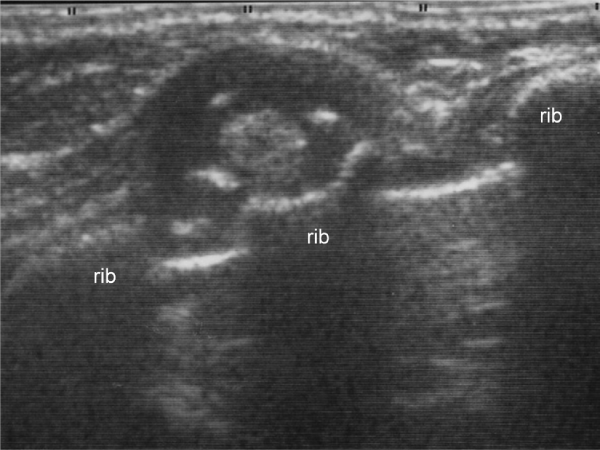
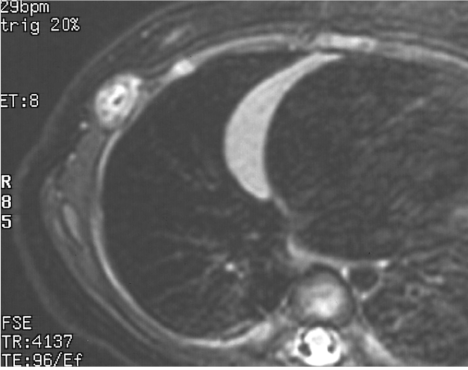
A 5–month–old female infant presented with a painless subcutaneous mass on the right lateral chest wall. She was admitted for surgical excision because the mass showed slow growth. Physical examination revealed a 10–mm, firm, round, non–movable mass on the right fifth rib. On X–ray examination, the tumor was detected as a protrusion of soft tissue with obscure cortex of the rib. Ultrasound (US) showed a solid tumor with internal high echogenicity, suggestive of calcification (Figure 1). A computed tomography (CT) scan revealed scattered high densities with possible cortical involvement of the rib; however, the soft tissue density of the marginal area in the tumor was indicative of a non–osseous origin of the tumor. On magnetic resonance imaging (MRI), the tumor had low signal intensity (SI) on T1–weighted images (T1–WI) and high SI on T2–WIcompared with that of muscle on T2–WI (Figure 2). Based on these imaging findings, the preoperative diagnosis was a fibrous soft tissue tumor with calcification.
Case
Author
(Year)
Age/Sex†††††††††††††† Location
Size (cm)
Recurrence
Follow-up
1
Keasbey
1953
6 y/M
Lumbar*
(-)
7 y
2
Rios-Dalenz
1965
15 y/M
Lumbar*††††††††††††††† 2.5
?
LTF
3
Goldman
1970
6 y/M
Neck
(-)
1 y
4
Rangwale
1988
8 y/F
Abdomen
3×2(-)
4 m
5
Murphy
1996
3 y/M
LS
(+), 3 times
23 y
6
Sharma
1998
5 y/F
Neck
(+)
22 m
7
Sharma
1998
3 y/M
LB
(-)
LTF
8
7 y/M
LB
(-)
LTF
9
7 y/F
LB
(-)
LTF
10
?/F
LB
(+)
Unknown**
11
3 y/M
LB
1.8×1.0†† (+), 4 times
17 y 7 m
12
8 y/M
LB
2×1.5††††† (-)
LTF
13
OruÁ
2007
3 y/M
Scalp
4×3†††††††† (-)
(+)
14
Arora
2010
2 y/M
Buttock
10×7†††††† (-)
3 y
15
Our case
2012
5 m/F
Chest
1.7×1.2††† (-)
9 y 10 m
Table 1: #
Figure 1: Ultrasound findings of the tumor. Ultrasound showing a solid tumor on the fifth rib with scattered high echogenicity, suggesting calcification
Figure 2: Magnetic Resonance Imaging of the chest. A tumor of unknown origin, characterized by low and high signal intensity (SI) on T2-–weighted images (T2–WI) and the thinner cortex of the rib.
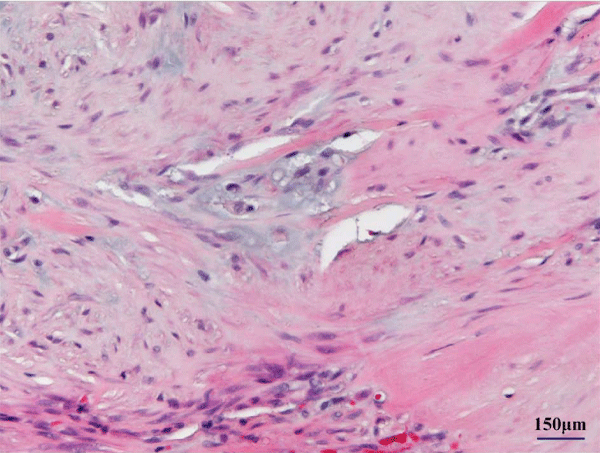
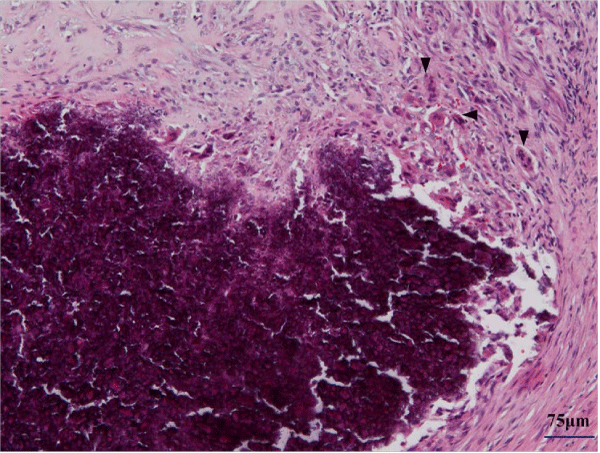
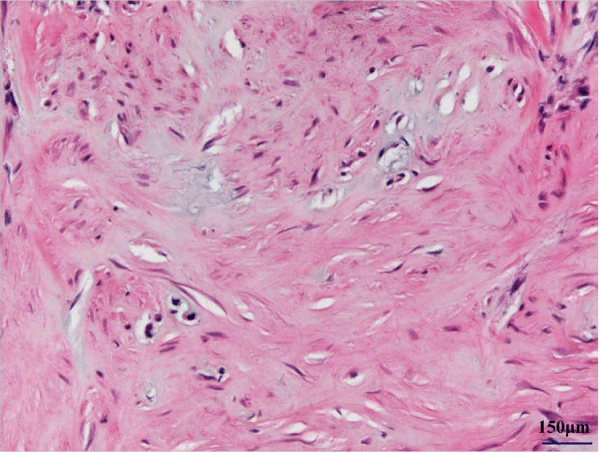
Although the tumor invaded the inter costal muscle and tightly adhered to the periosteum of the rib, it was totally excised at surgery. The rib on which the tumor had occurred caved in. The tumor was 17 × 12 mm in size. Cut sections revealed a firm, grayish–white nodule with a central gritty area. On histological examination, the tumor was composed of epithelioid–like fibroblasts with spindle nuclei and densecollagenous stroma (Figure 3a). Mitotic activity was inconspicuous, and there was massive calcification associated with multinucleated giant cells (Figure 3b) and cartilaginous metaplasia (Figure 3c). These findings made it easy to distinguish the tumor from infantile or juvenile forms of fibromatosis, fibrous hamartoma of infancy, and monophasic fibrous type of synovial sarcoma. Therefore, the histologic diagnosis of CAF was made. The patient was discharged onthe first postoperative day and has remained free from recurrence for 9 years and 10 months.
Figure 1: Epithelioid–like fibroblasts with chondroid differentiation.
Figure 4: Massive calcifications surrounded with multinucleated cells (arrow head).
Figure 5: Cartilaginous metaplasia and collagenous stroma.
Discussion
In 1953, Keasbey [1] first described CAF in the extremities of 4 children and termed it “juvenile aponeurotic fibroma.” In 1973, Iwasaki and Enjoji [3] coined the term “calcifying aponeurotic fibroma” because the tumor was also found in adult and elderly patients. CAF is more common in male patients. It usually occurs in the distal extremities such as the palms and soles of young children, and the head, neck, and trunk are less frequently affected [2,4]. CAFalmost always appears in the first decade of life, with peak incidence from ages 8–14 years; however, cases have been reported in neonates and seniors in their 60s [5]. We performed an extensive search of the English literature and were unable to find a similar case of CAF in the chest wall.
Clinically, CAF presents as a painless, slow–growing, freely movable, firm lesion. The lesion typically ranges in size from 1–3 cm, and is often present for years before surgery. It never adheres to the overlying skin but may be attached to or infiltrate into the surrounding fasciae, tendons, aponeuroses, and periosteum [2,4,6]. While the tumor is usually asymptomatic, cases of progressive local discomfort have been reported. It has been shown that the only associated disability was limitation of motion secondary to the presence of this firm, space–occupying lesion.
On radiographs, CAF is detected as a faint radiopaque soft tissue mass, with or without small foci of stippled calcification, and there is usually no associated osseous involvement [1,6]. Radiographic features are usually inconclusive, and a CT may be helpful in the detection of calcification in cases with osseous involvement. CT is possibly of great value in differentiating CAF from periosteal chondroma or panniculitis ossificans. Magnetic resonance imaging (MRI) usually shows that the tumor has low SI on T1– and T2–WI. Sundaram et al. [8] showed that all tumors with low SI on T2–WI in their study were fibrous ones and suggested that low SI on T2–WI was indicative of marked hypocellularity and abundant fibrous elements when compared with the findings obtained for tumors with high SI on T2–WI. In addition, T2–WI with fat saturation, a technique that needs no contrast medium, is helpful for determining the extent of a lesion [9]. However, it is impossible to determine if soft tissue tumors are benign or malignant based on physical or imaging findings, and biopsy or excisional biopsy must be performed for microscopic examination [10].
Keasbey et al. described 2 phases in the development of this tumor, namely, initial phase (diffuse or florid type tumors) and late phase (compact or demarcated type tumors) [1,11]. The former is common in infants and young children, and the tumor growth is diffuse and infiltrative, with a lack of calcification. In the latter, the tumor is anodular mass with calcification and cartilage formation and with a well–defined border. This phase is frequent in adolescents and adults. However, Enzinger et al. noted that in primary or recurrent form, lesions are usually infiltrative with no evidence of the changing phases [6]. They also noted that young patients, especially those under 5 years of age, have a high risk of recurrence, based on a review of the published work [6]. Histopathological characteristics of CAF include a highly cellular architecture composed of atypical fibroblast, foci of calcification, and lesions of chondroid differentiation. Usually, the differential diagnosis includes fibrous hamartoma of infancy, diffuse infantile fibromatosis, nodular fasciitis, or infantile fibrosarcoma as a soft–tissue mass [2,4]. If calcifications or osseous defects are present in addition to the soft–tissue mass, infantile fibrosarcoma, chondroma of soft parts, and synovial sarcoma should be considered. The highly cellular architecture, locally aggressive growth pattern, and tendency to recur may lead to a misdiagnosis of sarcoma, fibrosarcoma, and pseudosarcoma followed by unnecessary amputation [4,12].
CAF frequently involves tendons, nerves, or blood vessels. The rate of local recurrence after an initial surgery has been reported to be approximately 50%; however, there is no recordof malignant transformation, with the exception of 1 case [13], metastasis, or linkage with any associated diseases. Given the poor circumscription, infiltrative growth pattern, and the high recurrence rate of approximately 50%, CAF can be mistaken for a malignant soft–tissue tumor. Therefore, pathological examination is mandatory and careful attention to the morphologic features will aid in distinguishing CAF from other soft–tissue tumors. Functional deficits are usually iatrogenic rather than the result of tumor growth or invasion. Conservative surgical management is the treatment of choice. Local excision with subsequent re–excision, if necessary, is preferred to radical tumor resection because recurrent lesions resolve spontaneously and most functional disabilities result from extended surgical resection. Complete excision with a margin of normal tissue should be performed when it is feasible to excise the tumor without compromising functionality.
To the best of our knowledge, only 14 pediatric cases have been reported in the English literature regarding CAF tumor occurrence in uncommon sites (head, face, neck, and trunk) [4,5,14,16]. There have been no reports of CAF in the chest wall. Known uncommon tumor sites, including this case, were the back in 7 cases, lumbar in 3, neck in 2, abdomen in 1, buttock in 1, and chest in 1. Of 15 patients, 10 were male and 5 were female. Patient ages ranged from 5 months to 15 years. All tumors that were surgically treated showed persistent or slowly enlarging masses, and no patient reported other clinical symptoms such as discomfort, pain, or functional compromise. The excised tumors ranged from 1.7–7 cm in size. Follow–up information was available for 8 of the 13 patients, and the follow–up period ranged from 4 months to 23 years (276 months). All CAFs had benign characteristics; however, the tumor recurred in 4 patients, with a recurrence rate of 33.3% (4 of 12 patients), and 2 of them experienced recurrences 3 or more times. A total of 9 recurrences occurred in 13 patients, but there were no deaths related to CAF recurrence or surgery. The longest time interval of tumor persistence with multiple recurrences was over 23 years. Long–term follow–up is necessary for patients with CAF because recurrence can occur even 2 decades after the initial surgery.
In conclusion, the unusual features of the present case of CAF were the young age of the patient and the uncommon occurrence site. Given the high rate of local recurrence in CAF, complete excision with a margin of normal tissue is advisable when the excision can be performed without sacrificing functionally important structures. However, it should be kept in mind that functional deficit after surgery is related to wide surgical excision, and in recurrence, re–excising a tumor is preferred to performing an extensive surgical dissection.
References
- Klueh RL, Nelson AT. Ferritic/martensitic steels for next-generation reactors. J Nucl Mater. 2007; 371: 37-52.
- Klueh RL, Ehrlich K, Abe F. Ferritic/martensitic steels: promises and problems. J Nucl Mater. 1992; 191: 116-24.
- Klueh RL, Bloom EE. The development of ferritic steels for fast induced-radioactivity decay for fusion reactor applications. Nucl Eng Des Fusion. 1985; 2: 383-389.
- Miller MK, Kenik EA, Russell KF, Heatherly L, Hoelzer DT, Maziasz PJ. Atom probe tomography of nanoscale particles in ODS ferritic alloys. Mat Sci Eng A-Struct. 2003; 353: 140-145.
- Kurtz RJ, Alamo A, Lucon E, Huang Q, Jitsukawa S, Kimura A, et al. Recent progress toward development of reduced activation ferritic/martensitic steels for fusion structural applications. J Nucl Mater. 2009; 386-388: 411-417.
- Luzginova NV, Nolles HS, ten Pierick P, Bakker T, Mutnuru RK, Jong M, et al. Irradiation response of ODS Eurofer97 steel. J Nucl Mater. 2012; 428: 192-196.
- Rustomji CS, Frandsen CJ, Jin S, Tauber MJ . Dye-sensitized solar cell constructed with titanium mesh and 3-D array of TiO2 nanotubes. J Phys Chem B. 2010; 114: 14537-14543.
- Kang TS, Smith AP, Taylor BE, Durstock MF. Fabrication of highly-ordered TiO(2) nanotube arrays and their use in dye-sensitized solar cells. Nano Lett. 2009; 9: 601-606.
- Zhang YH, Xiao P, Zhou XY, Liu DW, Garcia BB, Cao GZ. Carbon monoxide annealed TiO2 nanotube array electrodes for efficient biosensor applications. J Mater Chem. 2009; 19: 948-953.
- Wang J, Lin ZQ. Anodic formation of ordered TiO2nanotube arrays: effects of electrolyte temperature and anodization potential. J Phys Chem C. 2009; 113: 4026-4030.
- Macak JM, Hildebrand H, Marten-Jahns U, Schmuki P. Mechanistic aspects and growth of large diameter self-organized TiO2 nanotubes. J Electroanal Chem. 2008; 621: 254-266.
- Ghicov A, Schmuki P. Self-ordering electrochemistry: a review on growth and functionality of TiO2 nanotubes and other self-aligned MO(x) structures. Chem Commun (Camb). 2009; 20: 2791-2808.
- Hoffmann S, Ostlund F, Michler J, Fan HJ, Zacharias M, Christiansen SH, Ballif C. Fracture strength and Young's modulus of ZnO nanowires. Nanotechnology. 2007; 18: 205503.
- Saraf G, Lu Y, Siegrist T. In-plane anisotropic strain in a-ZnO films grown on r-sapphire substrates. Appl Phys Lett. 2008; 93: 041903.
- Sun CL, Shi JA, Wang XD. Fundamental study of mechanical energy harvesting using piezoelectric nanostructures. J Appl Phys. 2010; 108: 034309.
- Zhang LL, Zhao XS. Carbon-based materials as supercapacitor electrodes. Chem Soc Rev. 2009; 38: 2520-2531.