Case Report
Austin J Pediatr. 2014;1(2): 1009.
Use of Adalimumab in a Patient with Juvenile Idiopathic Arthritis Refractory to Etanercept: a Case Report and Literature Review
Shih-Chieh Wei1, Chih-Fang Huang1, Shiau-Ru Chiou2 and Ho-Chang Kuo3*
1Department of Family Medicine, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Taiwan
2Department of Otolaryngology, Taipei Veterans General Hospital, Taiwan
3Department of Pediatrics, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Taiwan
*Corresponding author: Ho-Chang Kuo, Department of Pediatrics, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, No: 123, Da-Pei Rd., Niaosong, Kaohsiung, Taiwan
Received: June 13, 2014; Accepted: July 21, 2014; Published: July 26, 2014
Abstract
In this report, we present a child patient diagnosed with juvenile idiopathic arthritis (JIA) refractory to disease-modifying anti-rheumatic drugs (DMARDs), Methylprednisolone pulse therapy, and the TNF-a-inhibitor etanercept, with a significant improvement in hematologic abnormalities after the introduction of adalimumab.
Keywords: Juvenile idiopathic arthritis; Adalimumab; Etanercept
Introduction
Juvenile idiopathic arthritis (JIA) is the most commonly diagnosed rheumatoid disease in children, and it may lead to a distinct disease course and long-term complications. The conventional treatment of JIA includes non-steroidal anti-inflammatory drugs (NSAIDs), steroids, and disease-modifying anti-rheumatic drugs (DMARDs). However, the non-response rates to these agents may be up to 30% [1].
The introduction of biologic agents has greatly improved the clinical outcomes of patients with JIA. Etanercept, a TNF-a receptor fusion protein, was the first biologic medication approved by the U.S. Food and Drug Administration as a therapeutic agent for JIA. Another biologic agent adalimumab, a human monoclonal anti- TNF-a antibody, has become available for use in the treatment of JIA after proving its efficacy in a placebo-control withdrawal trial [2]. As new drugs are discovered, the completion of convincing clinical trials for quality, safety, and efficacy of these biologics remains a challenging but crucial task [3,4]. Proper selection of various biologics and shifting from one to another requires further exploration.
In this report, we present a patient with JIA of more than 4 years of duration, who presented to our outpatient department with hematologic abnormalities. The patient was initially started on etanercept because of previous poor responses to DMARDs and methylprednisolone (MP) pulse therapy. The patient was subsequently switched to adalimumab following unsatisfactory clinical results with etanercept. After the initiation of adalimumab, there was a dramatic improvement in hematologic abnormalities.
Case Presentation
A 14-year-old boy with a 4-year history of polyarticular JIA diagnosed in another medical institution, presented to our outpatient department because of leukocytosis, thrombocytosis, elevated C-reactive protein (CRP) level, and an elevated erythrocyte sedimentation rate (ESR). Despite taking prednisolone (10 mg/day) and methotrexate (5 mg/week), as prescribed by the other medical institution, the painful swelling of multiple joints was not relieved. The patient did not report any additional symptoms such as fever, cough, dyspnea, nasal congestion, rhinorrhea, nausea, vomit, diarrhea, dysuria, nor hematuria. No remarkable contact or travel history was reported.
According to the patient’s medical history, polyarticular type JIA was diagnosed at the age of 10 years. The involved joints included the bilateral knees, wrists, intercarpal, metacarpophalangeal, and multiple proximal and distal interphalangeal joints. Radiographic analysis performed in our hospital revealed diffuse osteopenia, joint space narrowing, subchondral erosion, and cortical irregularities over these joints.
A steroid (prednisolone 10 mg/day) and DMARD (methotrexate 5 mg/week) were administered for more than 4 years with inadequate clinical response. The patient was referred to our hospital from the other medical institution because of the incidental abnormal laboratory finding.
The initial laboratory data revealed severe leukocytosis (48,100/ μL), thrombocytosis (656,000/μL), and elevated CRP level (161.3 mg/L) and ESR (55 mm/h). Rheumatologic serology was negative for rheumatoid factor, anti-nuclear antibodies, and human leukocyte antigen-B27, and revealed a normal complement panel (C3, C4). A peripheral blood smears revealed neutrophilia, thrombocytosis, microcytic anemia, and a leukocyte alkaline phosphatase score of 239 points. There was no increase in radioactivity noted in a whole body gallium-67 scan. A tuberculosis skin test (PPD) was also negative.
After a series of diagnostic tests, chronic infection and hematologic disease were excluded. MP pulse therapy (30 mg/kg) was scheduled every 1 to 3 months for up to 10 courses over the following years. About 6 months after the initiation of MP pulse therapy, etanercept (0.4 mg/kg injected subcutaneously twice a week) was added to the treatment regimen, and maintained for about one and a half years. Methotrexate (7.5–10 mg/week [5–10 mg/m2/ week]), hydroxychloroquine (200 mg/week [6.5–8 mg/kg/day]), and prednisolone (10–15 mg/day [0.3–0.5 mg/kg/day]) were used concomitantly during treatment with the biological agent.
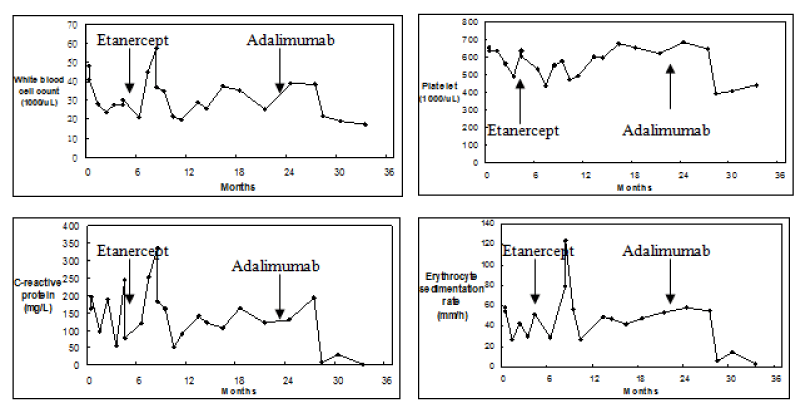
Despite the addition of etanercept, the patient’s leukocytosis, thrombocytosis, and elevation of inflammatory markers persisted. Symptoms of joint pain and swelling continued to fluctuate. Switching from etanercept to adalimumab was proposed then the patient was administered with adalimumab (40 mg injected subcutaneously every other week). Following the administration of adalimumab, the abnormal blood and biochemistry results gradually improved during the follow-up period (Figure 1). However, there was only a mild improvement in joint pain because of permanent bone deformity. The patient has been receiving adalimumab 1 year long and the treatment is still ongoing.
Figure 1: White blood cell (WBC) counts, Platelet counts, C-reactive protein levels, and Erythrocyte sedimentation rate of a patient with juvenile idiopathic arthritis treated with etanercept and adalimumab.
Discussion
Treatment of JIA is complex and may be challenging for practitioners. Etanercept has been demonstrated to be beneficial for patients with JIA, who were refractory to conventional agents, regardless of their disease onset [5], and adalimumab was shown to possess equivalent efficacy [6]. However, in light of a greater abundance of clinical experience regarding the use of etanercept, 90% of biologic-naive patients were administered etanercept rather than adalimumab. Etanercept has over 10 years’ data supporting its safety and efficacy, as well as considerable clinical experience, while adalimumab has been available for only 4 years. Based on this data, we pursued a similar treatment plan, and prescribed etanercept after several attempts of MP pulse therapy had failed.
Switching between different biologic agents for refractory JIA occurs frequently in clinical practice, but the safety needs further investigation. However, in a prospective observational study from the Dutch National ABC Register, second- and third-line biologic agents were usually found to be less effective than the first-line agents, especially when patients had failed first-line treatment owing to an insufficient clinical response [7]. However, another retrospective observational study conducted by the same institute recommended shifting from one biologic agent to another as part of a rational treatment plan [8]. Following these guidelines, we switched the patient from etanercept to adalimumab after a one-and-a-half-year refractory treatment period.
Adalimumab is a human recombinant immunoglobulin G1 anti-TNF monoclonal antibody, while etanercept is a recombinant human soluble TNF-a receptor fusion protein. As both biologic agents affect the same pathophysiologic pathway, they are thought to have similar side effects, including an increased risk of sepsis, opportunistic infections, tuberculosis, demyelinating diseases, and lupus-like reactions. However, adalimumab is theoretically believed to elicit stronger immune suppression due to its higher binding affinity [9]. Stronger immune suppression can lead to more serious side effects, which may help to explain why, even with similar efficacy, adalimumab was more prone to withdrawals related to adverse effects when compared with etanercept [10]. After weighing the benefits and risks, we believed that in serious JIA cases, patients might attain a higher profit and loss ratio when using adalimumab.
A recent report showed that adalimumab appears to be effective and safe in patients with JIA refractory to other anti-TNF-a agents [9]. Among previous literature, the American College Rheumatology Pediatric criteria (ACRpedi30) were mostly used to determine whether an improvement in symptoms was obtained. In our patient who had been diagnosed with JIA more than 4 years ago, with multiple permanent joint deformities, even though the disease was effectively controlled, the clinical symptoms of arthralgia and stiffness did not improve satisfactorily. In this case, we could only judge the disease status based on the hematologic report. Because clinical evaluation criteria are not suitable for every patient, a more comprehensive and complete assessment method is needed for patients with JIA.
Treatment efficacy for our patient emerged gradually. According to a prospective cohort study [11], the development of anti-adalimumab antibodies was an important factor in the treatment of rheumatoid arthritis, and was associated with a lower adalimumab concentration and a lower likelihood of minimal disease activity or clinical remission. About 28% of patients who received adalimumab develop anti-adalimumab antibodies, and two-thirds (67%) develop anti-adalimumab antibodies during the first 28 weeks of treatment. Because this period is shorter than the duration of treatment in our patient, we believe that our patient is negative for anti-adalimumab antibodies. Another single-arm, open-label, multicenter study [12] revealed that anti-adalimumab antibody-positive rates were numerically greater in JIA patients in Japan than in Europe or USA. However, the development of these antibodies was not associated with discontinuation of treatment or lack of clinical efficacy. Because the study was limited in size (25 cases) the data should be interpreted with caution. Schmeling et al. also showed adalimumab appears to be highly effective in biologic naïve and in biologic switcher children in a study with 289 cases of JIA. The treatment is safe and similar to other biologics used in JIA. Few patients (58/289) discontinued due to intolerance or inefficacy (13).
In conclusion, we reported the case of a patient with JIA refractory to DMARDs, MP pulse therapy, and the TNF-a-inhibitor etanercept, who showed clinical improvement following treatment with the TNF-a-inhibitor adalimumab in Taiwan. More importantly, this is the first case reported in the English literature of JIA that was refractory to etanercept, with a dramatic improvement in hematologic abnormalities after switching to adalimumab. Both etanercept and adalimumab play important roles in the treatment of JIA, and further controlled studies are needed to assist in determining appropriate treatment.
References
- Ruperto N, Murray KJ, Gerloni V, Wulffraat N, de Oliveira SK, Falcini F, et al. A randomized trial of parenteral methotrexate comparing an intermediate dose with a higher dose in children with juvenile idiopathic arthritis who failed to respond to standard doses of methotrexate. Arthritis Rheum. 2004; 50: 2191-2201.
- Lovell DJ, Ruperto N, Goodman S, Reiff A, Jung L, Jarosova K, et al. Adalimumab with or without methotrexate in juvenile rheumatoid arthritis. N Engl J Med. 2008; 359: 810-820.
- Kingsbury DJ, Quartier P, Karunaratne PM, Kalabic J, Kupper H. A152: safety and effectiveness of adalimumab in children with polyarticular juvenile idiopathic arthritis aged 2 to 4 years. Arthritis Rheumatol. 2014; 66 Suppl 11: S196-197.
- Brunner H, Ruperto N, Tzaribachev N, Horneff G, Wouters C, Panaviene V, et al. A148: a multi-center, double-blind, randomized-withdrawal trial of subcutaneous golimumab in pediatric patients with active polyarticular course juvenile idiopathic arthritis despite methotrexate therapy: week 48 results. Arthritis Rheumatol. 2014; 66 Suppl 11: S191-192.
- Lahdenne P, Vähäsalo P, Honkanen V. Infliximab or etanercept in the treatment of children with refractory juvenile idiopathic arthritis: an open label study. Ann Rheum Dis. 2003; 62: 245-247.
- Paediatric rheumatology: Choosing between etanercept and adalimumab for JIA. Nature reviews Rheumatology. 2013.
- Otten MH, Prince FH, Anink J, Ten Cate R, Hoppenreijs EP, Armbrust W, et al. Effectiveness and safety of a second and third biological agent after failing etanercept in juvenile idiopathic arthritis: results from the Dutch National ABC Register. Ann Rheum Dis. 2013; 72: 721-727.
- Tynjälä P, Vähäsalo P, Honkanen V, Lahdenne P. Drug survival of the first and second course of anti-tumour necrosis factor agents in juvenile idiopathic arthritis. Ann Rheum Dis. 2009; 68: 552-557.
- Katsicas MM, Russo RA. Use of adalimumab in patients with juvenile idiopathic arthritis refractory to etanercept and/or infliximab. Clin Rheumatol. 2009; 28: 985-988.
- Singh JA, Christensen R, Wells GA, Suarez-Almazor ME, Buchbinder R, Lopez-Olivo MA, et al. Biologics for rheumatoid arthritis: an overview of Cochrane reviews. Sao Paulo Med J. 2010; 128: 309-310.
- Bartelds GM, Krieckaert CL, Nurmohamed MT, van Schouwenburg PA, Lems WF, Twisk JW, et al. Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA. 2011; 305: 1460-1468.
- Imagawa T, Takei S, Umebayashi H, Yamaguchi K, Itoh Y, Kawai T, et al. Efficacy, pharmacokinetics, and safety of adalimumab in pediatric patients with juvenile idiopathic arthritis in Japan. Clinical rheumatology. 2012; 31: 1713-1721.
- Schmeling H, Minden K, Foeldvari I, Ganser G, Hospach T, Horneff G, et al. Efficacy and safety of Adalimumab as first and second used biologic agent in juvenile idiopathic arthritis - the German Biologics JIA Registry (BiKeR). Arthritis Rheumatol. 2014.