
Case Report
Austin Pediatr. 2016; 3(1): 1025.
Growing Teratoma Syndrome: Illustrative Case Review
Ahmed M* and Yedururi S
Department of Diagnostic Radiology, University of Texas MD Anderson Cancer Center, Houston, Texas, USA
Ahmed M, Department of Diagnostic Radiology, University of Texas MD Anderson Cancer Center, 1400 Pressler Street, Unit 1459, Houston, Texas, USA
Received: January 27, 2016; Accepted: February 10, 2016; Published: February 12, 2016
Abstract
We present two cases of Growing Teratoma Syndrome (GTS), one in a male patient with testicular mixed germ cell tumor and the second one in a female patient with ovarian mixed germ cell tumor. During or after chemotherapy, both patients were found to have enlarging masses, despite a significant decrease in or normalization of serum tumor markers. These enlarging masses were resected in one patient and pathological examination confirmed mature teratoma with no viable germ cell tumor. These findings are consistent with growing teratoma syndrome. It is important to recognize the possibility of a growing teratoma syndrome in patients with mixed germ cell tumor and growing metastatic masses on imaging studies despite a significant decrease/normalizing serum markers since additional chemotherapy would be ineffective and surgery is the recommended treatment.
Keywords: Growing teratoma syndrome; Mature teratoma
Abbreviations
CT: Computed Tomography; AFP: Alpha Feto-Protein; bHCG: Human Chorionic Gonadotropin; LDH: Lactate Dehydrogenase; MT: Mature Teratoma; GTS: Growing Teratoma Syndrome; CA- 125: Cancer Antigen-125; NSGCT: Non-Seminomatous Germ Cell Tumor; PNET: Primitive Neuroectodermal Tumor
Introduction
Growing Teratoma Syndrome (GTS) is an infrequent clinical condition seen in adolescents, men and women with metastatic germ cell tumors treated with chemotherapy [1-3]. It was first described by Logothetis et al., [1] in 1982 in patients with metastatic testicular mixed germ cell tumors in whom growing mature teratoma developed during chemotherapy. The defined criteria for diagnosis of growing teratoma syndrome include enlarging retroperitoneal or other metastatic masses during or after adjuvant systemic chemotherapy, normalization or near normalization of tumor markers and presence of only mature teratoma in the resected specimen [2-4]. The reported incidence of GTS in patients with testicular cancer at a single institute is 2.2% [2]. The incidence of malignant GTS after malignant ovarian GCT is unknown [3]. The purpose of this review is to illustrate growing teratoma syndrome in patients with testicular and ovarian mixed germ cell tumors and increase the awareness of this entity.
Case 1
A 20-year old male presented with lower back pain and increasing size of mass in left neck. Computed Tomography (CT) scans done at the time showed a massive left-sided neck mass extending into the chest, as well as bulky mediastinal and retroperitoneal masses (Figure 1a and 1b). A scrotal ultrasound performed at the same time demonstrated a 5cm complex mass in the right testes (Figure 1c). The patient underwent radical right inguinal orchiectomy and the pathology revealed: 55% embryonal carcinoma, 20% yolk sac, 20% teratoma, 5% choriocarcinoma. Pre-op lab values were: Alpha Feto- Protein (AFP) 14,601; Human Chorionic Gonadotropin (bHCG) 11,788; and lactate dehydrogenase (LDH) 922.
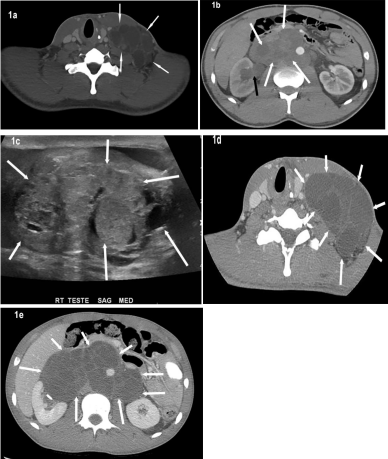
Figure 1: Axial CT neck (a) and axial CT abdomen (b) illustrate large,
confluent, multi-lobulated heterogeneous but predominantly hypodense
metastatic lymphadenopathy in the left neck and retroperitoneum (white
arrows). The retroperitoneal lymphadenopathy is obstructing the right distal
ureter (not shown) that is resulting in right hydronephrosis (black arrow). A
scrotal ultrasound (c) at the same time illustrates a complex heterogeneous,
complex solid and cystic right testicular mass (arrows). Post chemotherapy
CT neck (d) and abdomen (e) shows interval enlargement of the metastatic
neck and retroperitoneal lymphadenopathy (arrows). Note is made of
decreased density of these enlarged confluent nodes compared to the base
line imaging studies.
The patient then received chemotherapy with bleomycin, etoposide and cisplatin. Post-chemotherapy scans demonstrated interval increase in size of the left neck mass and the bulky retroperitoneal mass (Figures 1d and 1e). Post-therapy bHCG and AFP lab values were negligible and LDH level was within normal limits. The patient subsequently underwent resection of disease in the neck and retroperitoneum; pathology was consistent with metastatic Mature Teratoma (MT) without the presence of viable tumor elements. Despite enlargement of the masses after treatment with chemotherapy, lab markers remained low/normal and upon resection, the pathology was consistent with mature teratoma; this constellation of findings is consistent with Growing Teratoma Syndrome (GTS).
Case 2
A 16-year old female presented with abdominal pain and was initially treated at an outside facility. Initial CT images showed large abdominal mass with cystic and solid components (Figure 2a). AFP level was 207. She underwent exploratory laparotomy and right salpingo-oophorectomy which revealed mixed germ cell tumor comprising of immature teratoma grade 3 (immature teratoma 95% with endodermal sinus/yolk sac tumor 5%). Pelvic washings revealed malignant immature teratoma cells.
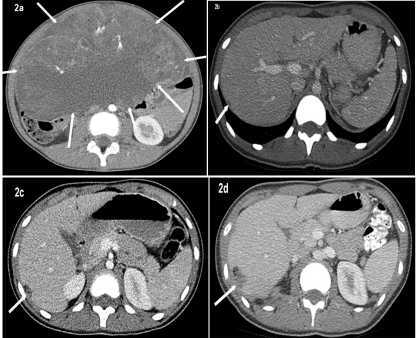
Figure 2: Axial CT abdomen (a) at the time of diagnosis and before surgery
demonstrates large abdominal mass with cystic and solid component (arrows)
pathology proven to be mixed germ cell tumor after surgical excision. Axial CT
abdomen performed one year post surgery shows a small right perihepatic
implant (b). Axial CT abdomen performed 2 years after surgery (c) shows
interval enlargement of right perihepatic implant. Subsequent CT abdomen
performed 3 years after surgery (2d) shows further enlargement in size of
right perihepatic implant that now shows both soft tissue and fatty elements.
She then received 3 cycles of chemotherapy. Subsequent CT scans were done for surveillance which demonstrated slowly growing peritoneal implants over the course of 3 years (Figures 2b-d). She presented to our hospital for second opinion regarding enlarging intra-abdominal masses. Lab values remained normal at this point: AFP 1.2; bHCG < 1; CA-125 18.6; normal liver functions tests. Surgical excision was recommended and the patient went back to her local physicians for further treatment and management.
Discussion
Both cases illustrate growing metastatic masses with decreasing/ normalizing tumor markers. Radiologists, oncologists, urologists and gynecologists should consider the possibility of growing teratoma syndrome in this clinical scenario. Failure to recognize growing teratoma leads to a misdiagnosis of progressive disease potentially resulting in more but ineffective chemotherapy. Pathological examination of the primary tumor revealed mixed germ cell tumors in both patients. Both patients underwent chemotherapy; for the first patient, the masses grew during treatment, and for the second patient the masses grew after the completion of treatment. The pathology of our first patient’s resected masses post-chemotherapy showed mature teratomatous elements and no evidence of viable germ cell tumor.
Growing Teratoma Syndrome (GTS) has been described as enlarging retroperitoneal or other metastatic Mature Teratoma (MT) masses during systemic chemotherapy for testicular or ovarian mixed germ cell tumors [1-5]. It is important to recognize that these residual masses encountered following chemotherapy for mixed germ cell tumors are not always indicative of residual active disease [1]. GTS should be suspected when a metastatic mass grows in the presence of normalized serum tumor markers [2]. Moskovich et al., found certain CT characteristics of these growing tumors after the onset of chemotherapy, including: better definition of the masses with increased density relative to surrounding tissues; fatty and cystic changes within the masses; as well as development of internal calcifications [6].
Two hypotheses have been proposed for the pathogenesis of GTS: differentiation of malignant cells into mature teratoma; and selective chemotherapy- induced destruction of components other than the mature teratomatous elements [4]. Based on literature review and observations made from their series, Andre at al., suggest that factors predicting the development of GTS include: the presence of MT in primary NSGCT, absence of metastatic tumor size reduction during chemotherapy, and the presence of MT in residual masses after chemotherapy [4].
Andre et al reported a 3% rate of malignant transformation, in a series of 30 patients with testicular mixed germ cell tumors with growing teratoma syndrome treated with surgical resection. Complete resection was reported in 80% of patients [4]. Malignant transformation into NSGCT and sarcoma has also been reported [7,8]. Therefore, it is important for patients with NSGCT who undergo chemotherapy to be followed by imaging regularly to monitor subtle changes in tumor size [2]. It is recommended that patients with GTS undergo complete resection [1-4,9,10].
Conclusion
Growing Teratoma Syndrome is seen in patients with metastatic testicular and ovarian mixed germ cell tumors. We have described and illustrated growing teratoma syndrome in testicular and ovarian mixed germ cell tumors. In our first patient, the masses grew despite receiving chemotherapy and tumor markers were normal, consistent with GTS. In our second patient, masses grew slowly over a period of 4 years after chemotherapy was discontinued, and tumor markers were normal. Based on recommendations of the current literature available, chemotherapy for GTS is ineffective, and surgery would be the treatment of choice.
References
- Logothetis CJ, Samuels ML, Trindade A, Johnson DE. The growing teratoma syndrome. Cancer. 1982; 50: 1629-1635.
- Spiess PE, Kassouf W, Brown GA, Kamat AM, Liu P, Gomez JA, Tu SM. Surgical management of growing teratoma syndrome: the M. D. Anderson cancer center experience. J Urol. 2007; 177: 1330-1334.
- Byrd K, Stany MP, Herbold NC, Leath CA 3rd, Hamilton CA. Growing teratoma syndrome: Brief communication and algorithm for management. Aust N Z J Obstet Gynaecol. 2013; 53: 318-321.
- André F, Fizazi K, Culine S, Droz J, Taupin P, Lhommé C, Terrier-Lacombe M. The growing teratoma syndrome: results of therapy and long-term follow-up of 33 patients. Eur J Cancer. 2000; 36: 1389-1394.
- Tongaonkar HB, Deshmane VH, Dalal AV, Kulkarni JN, Kamat MR. Growing teratoma syndrome. J Surg Oncol. 1994; 55: 56-60.
- Moskovic E, Jobling T, Fisher C, Wiltshaw E, Parsons C. Retroconversion of immature teratoma of the ovary: CT appearances. Clin Radiol. 1991; 43: 402-408.
- Ahmed T, Bosl GJ, Hajdu SI. Teratoma with malignant transformation in germ cell tumors in men. Cancer. 1985; 56: 860-863.
- Hughes DF, Allen DC, O'Neill JJ. Angiosarcoma arising in a testicular teratoma. Histopathology. 1991; 18: 81-83.
- Gelderman WA, Scraffordt Koops H, Sleijfer DT, Oosterhuis JW, Oldhoff J. Late recurrence of mature teratoma in nonseminomatous testicular tumors after PVB chemotherapy and surgery. Urology. 1989; 33: 10-14.
- Tait D, Peckham MJ, Hendry WF, Goldstraw P. Post-chemotherapy surgery in advanced non-seminomatous germ-cell testicular tumours: the significance of histology with particular reference to differentiated (mature) teratoma. Br J Cancer. 1984; 50: 601-609.