
Case Report
Austin Pediatr. 2016; 3(2): 1033.
Toxic Epidermal Necrolysis (TEN) Induced by a Combination of Phenytoin and Oxcarbazepine
Ochoa MC1*, Ramirez LDH2, Morales FCA3 and Valle LJG1
1Department of Pediatrics, Regional General Hospital #1 (IMSS), Sonora Delegation, Sonora, Mexico
2Department of Family Medicine, Family Medicine Unit #1 (IMSS), Sonora Delegation, Sonora, Mexico
3Department of Dermatology, Regional General Hospital #1 (IMSS), Sonora Delegation, Sonora, Mexico
*Corresponding author: Maria Citlaly Ochoa, Department of Pediatrics, Regional General Hospital #1 (IMSS), Sonora Delegation, Sonora, México, Colonia Centro, Cd. Obregon, Sonora, Mexico
Received: June 04, 2016; Accepted: June 13, 2016; Published: June 15, 2016
Abstract
Toxic Epidermal Necrolysis (TEN) or Lyell syndrome is an acute systemic inflammation that involves skin, mucous membranes, respiratory epithelia and intestinal. It is regarded as final stage of Stevens - Johnson syndrome (SJS); both entities are part of same pathophysiological and immunological process, differing only by extent of the body surface (BS) area affected. We report an 8 years old female which present a severe phenytoin and oxcarbazepine adverse reaction coursing with all clinical stages of SJS-TEN responding favorably to treatment with intravenous immunoglobulin (IGIV), without use of steroids.
Keywords: Toxic Epidermal Necrolysis; Oxcarbazepine; Phenytoin
Introduction
Typical case of TEN described by Lyell, is characterized by sudden onset of 39-40 °C fever, fatigue, headache, sore throat, nausea, vomiting, muscle and joint pains [1]. Key feature is the subsequent appearance of a papular, erythematous skin lesion as target, painful, initially on face and trunk, which then spreads to the extremities. Vesicle-bullous lesions also appear in nasal, oral, vulvovaginal, anorectal and urethral mucosa, with pseudo membrane formation and compromising important functions as feeding and urination are [2].
Because of great similarity between clinic of SJS and TEN, Bastuji conducted a study in 1993 in which defined criteria for classification of both diseases, determining as SJS cases with less epidermal commitment of 10% BS and as TEN cases with more than 30% of BS affected. Cases between 10 and 30% of BS affected would be established as a superposition of both pathologies [1]. Annual risk is 0.4 to 2 cases per million for TEN and mortality can be as high as 70%. An estimated 20% of all cases are pediatric patients [3]. There are more than 220 medications associated with SJS/TEN, most common are: anticonvulsants 35.1%, antibiotics 33.3% and Non-Steroid Anti- Inflammatory Drugs 24.6% [4].
Case Presentation
Female patient 8 years old, native and resident of Cd. Obregon, Sonora, Mexico, with 25 kilograms of weight and 120 centimeters in height, both according to age. She began his condition a month ago with seizures characterized by initial cry, sucking movements and generalized stiffness, followed by generalized tonic-clonic movements and last approximately three minutes, not related to hyperthermia. She is admitted to emergency department where crisis was controlled, was kept under observation for six hours; the absence of alarm data was discharged with phenytoin (8 mg/kg/day) as treatment.
Eleven days after starting phenytoin treatment was assessed by neurology who diagnosed partial simple seizures secondarily generalized. Start treatment with oxcarbazepine (15mg/kg/day) without suspending phenytoin by high risk of seizure to new monthly assessment. Four days after starting oxcarbazepine has generalized rash, itching, hives and foreign body sensation in oropharynx, go to emergency room where was treated with antihistamines and systemic steroids for a non-specific allergic reaction (Figure 1), had improvement and was discharged. Twelve hours later was readmitted to emergency for exacerbation of rash, pruritic, hyporexia, malaise, labial mucosa redness, conjunctival hyperemic and bleeding lip lesions.
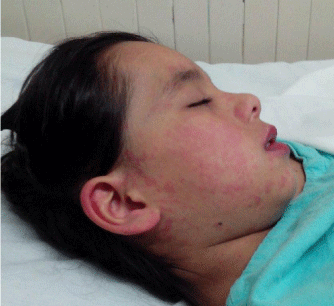
Figure 1: Early skin lesions (Day 1).
Patient was assessed by dermatology who refers to as a severe adverse reaction to medication. She was admitted to department of pediatrics for observation, systemic steroids and anticonvulsants were suspended, and it is added to management antihistamines and topic colloid therapy. In first 24 hours presented clinical deterioration with toxic appearance, mouth and lips edema, purulent discharge from conjunctive, increase rash maculopapular, few vesicles are added with a condition of approximately 9% of BS (Figure 2), pruritic erythema also appears in genital and perianal region. Diagnosis of Stevens-Johnson syndrome is established and treatment is initiated with intravenous fluids, intravenous immunoglobulin (0.4gr/kg/day) for 5 days, paracetamol (15mg/kg/dose) as antipyretic, hydroxyzine (10mg/day) and topic antibiotic (mupirocin 2%), remains under observation at possibility of progression to toxic epidermal necrolysis.
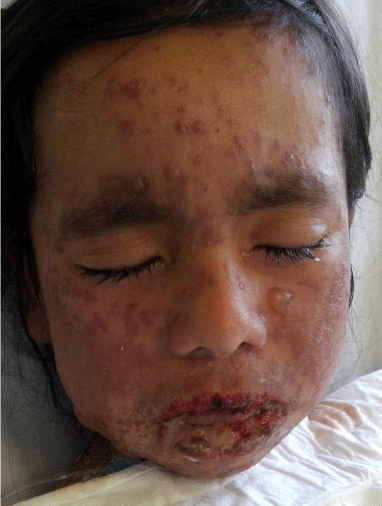
Figure 2: Confluent vesicles with injured oral mucosa (Day 3).
The laboratory showed: hemoglobin 13.8g/dl, hematocrit 43%, White Blood Cell #12400, Lymphocytes #6710, Neutrophils #4620, Eosinophils #368, Platelets 269.000, erythrocyte sedimentation rate6mm/h, C-reactive protein 0.31, prothrombin time 17.0sec, plasma thromboplastin time 24.3 sec. Urinalysis: normal. Electrolytes: sodium 131meq, potassium 3.4meq, chlorine 96meq, calcium 8.5meq, magnesium 1.7meq, phosphate 3.2meq.
At 48 hours appear confluent vesicles with sloughing of oral cavity, lips, cheeks, ears, chest, back and arms; bleeding lips with fissures and facial edema. Due to torpid evolution was assessment by intensive care who refers stable without criteria for admission to pediatric intensive care unit, a central venous catheter for parenteral feeding is placed due to oral intolerance. Currently with a BS affected 20% considered transitional stage in SJS/TEN. In third and fourth days of internment, an important confluence of blisters was observed with detachment of epidermis mainly on the face, chest, abdomen and arms with positive Nikolsky sign (Figure 3), calculating 35% of BS affected, diagnosis of TEN was established. Clindamycin (40mg/kg/day) was started at high risk of infection. Patient was valued for ophthalmology who relates with clear cornea and anterior segment without cellularity, began topical treatment for keratitis with tobramycin, chloramphenicol and hypromellose. By genital condition was assessed by gynecological who recommended topical treatment with mupirocin 2% combined with zinc oxide. Skin biopsy confirmed structural alterations as a severe form of erythema multiforme type TEN. Blood, skin, throat and eye cultures were negative.
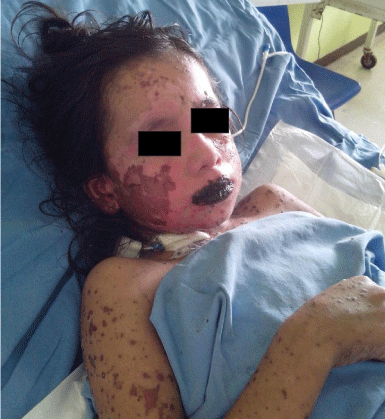
Figure 3: Positive Nikolsky sign (Day 4).
There was improvement evident at six days; on fifth day of treatment with immunoglobulin inflammatory response was limited, began reabsorption of transudate fluid of skin lesions and re-epithelialization of denuded areas. On day nine presented improvement of oral lesions and therefore parenteral nutrition was no longer necessary. Patient was discharged 19 days after admission in good conditions, with re-epithelialization of lesion areas, with hypopigmentation of affected areas, erosions and fragile lips but without involvement of oral cavity. Two months after acute event there is total recovery of skin and mucous (Figure 4) with hyper pigmented lesions as sequelae (Figure 5).

Figure 4: Two months after acute event.
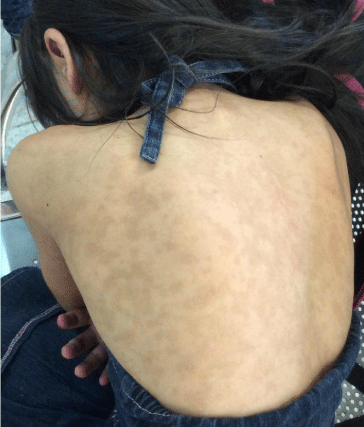
Figure 5: Hyperpigmented lesions as sequelae.
Discussion
The causal immunologic mechanism of SJS/TEN is a delayed cellular response that leads to apoptosis of keratinocytes, caused by a cytolytic protein called granulysin found in granules of CD8 and NK lymphocytes with perforin and granzyme B; these molecules are elevated in blisters TEN. The granulysin is secreted with a perforin, which allows to enter keratinocyte and cause cell death by damage to cell and mitochondrial membrane [5]. SJS/TEN is an acute inflammatory process; in which three phases (that crossed patient in this case) are distinguished. The first phase (acute), involves signs and nonspecific symptoms with early skin lesions. In second phase release long epidermal areas with positive Nikolsky sign. Third phase include sequels; being the most frequent, dry eyes, corneal ulcers, keratitis and skin hyper or hypopigmentation [6].
Patient in this case was in first two weeks of treatment with two anticonvulsants, which had a high risk of severe reaction to medication as described by Rzany in a study of SJS/TEN risk with several anticonvulsant drugs, reported a relative risk of 120 for carbamazepine, 91 phenytoin, 57 phenobarbital, and 24 for valproate in first five weeks of treatment [7]. Oxcarbazepine, a drug involved in this case was synthesized in 1966, approving its use as an anticonvulsant in Denmark in 1990, European countries in 1999 and US in 2000. Patients usually develop a hypersensitivity reaction to drug between week two and 12 after starting treatment [8]. In clinical case our patient develops the illness four days after start oxcarbazepine and 15 days after phenytoin, so she was within high risk period for SJS/TEN.
Pathological studies have shown that SJS and TEN are variants of same disease. These anatomopathological changes include sub epidermal edema and epidermolysis [9] as indicated by biopsy performed for diagnostic certainty. Conventional treatment consists of supportive care and treatment of complications. Although steroid use is mentioned, this treatment has not shown efficacy in all studies, there are even reports of increased susceptibility to infections as well as morbidity and mortality. Considering TEN immunological basis has been proposed use of IVIG as part of treatment in pediatric population with satisfactory results in several studies. Some effects of IVIG are major neutralization, rapid catabolism and suppression of antibody production, limitation of complement system and modulation of cell proliferation and apoptosis. Recommended dose in world literature is from 0.2 to 1 g/kg/day for three or four days [10].
Conclusion
In case of our patient, it was decided to manage with monitoring, administration of fluids and electrolytes, nutritional support (parenteral nutrition), prophylactic antibiotic topical and systemic (clindamycin and mupirocin) general skin care, anticoagulant therapy (enoxaparin), pain control (buprenorphine) without steroids and IVIG 0.4g/kg/day for five days, with favorable results at fifth day after initiation of treatment. We recommend use of IGIV since in most studies and in this case, effect is beneficial. Steroid use is discussed and controversial therefore it is not recommended; even they are associated with serious complications which this case was treated without steroids with satisfactory answer.
References
- Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau JC. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993; 129: 92-96.
- Roujeau JC, Stern RS. Severe adverse cutaneous reactions to drugs. N Engl J Med. 1994; 331: 1272-1285.
- Sotelo-Cruz N. [Stevens-Johnson syndrome and toxic epidermal necrolysis in children]. Gac Med Mex. 2012; 148: 265-275.
- Sharma VK, Sethuraman G, Minz A. Stevens Johnson syndrome, toxic epidermal necrolysis and SJS-TEN overlap: a retrospective study of causative drugs and clinical outcome. Indian J Dermatol Venereol Leprol. 2008; 74: 238-240.
- Chung WH, Hung SI, Yang JY, Su SC, Huang SP, Wei CY, et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med. 2008; 14: 1343-1350.
- Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010; 5: 39.
- Rzany B, Correia O, Kelly JP, Naldi L, Auquier A, Stern R. Risk of Stevens- Johnson syndrome and toxic epidermal necrolysis during first weeks of antiepileptic therapy: a case-control study. Study Group of the International Case Control Study on Severe Cutaneous Adverse Reactions. Lancet. 1999; 353: 2190-2194.
- Pirmohamed M, Lin K, Chadwick D, Park BK. TNFalpha promoter region gene polymorphisms in carbamazepine-hypersensitive patients. Neurology. 2001; 56: 890-896.
- Laguna C, Martín B, Torrijos A. Stevens-Johnson Syndrome and Toxic epidermal necrolysis: clinical experience and review. Actas Dermosifiliogr. [Internet]. 2006; 97: 177-185.
- Hoyos-Bachiloglua R, Andresen M, González S, Molgó M, Carreo N. Use of intravenous human immunoglobulin in patients with toxic epidermal necrolysis and Stevens-Johnson syndrome overlaying toxic epidermal necrolysis. Rev Méd Chile. [Internet]. 2009; 137: 383-389.