
Special Article – Pediatric Case Reports
Austin Pediatr. 2016; 3(3): 1039.
Peritonitis due to Staphylococcus Aureus: An Unusual Complication of Pediatric Renal Abscess
Fuchs I1,2*, Zodikov V3, Golan D4 and Einhorn M5
1Clalit Health Services, Southern District Infectious Disease Unit, Beer Sheva, Israel
2Faculty of Health Sciences, Ben Gurion University of the Negev, Israel
3Department of Radiology, Soroka University Health Center, Beer Sheva, Israel
4Pediatric Intensive Care Unit, Soroka University Health Center, Beer Sheva, Israel
5Pediatric Infectious Diseases Unit, Soroka University Health Center, Beer Sheva, Israel
*Corresponding author: Inbal Fuchs, Clalit Health Services, Southern District Infectious Disease Unit, Beer Sheva, Israel
Received: August 02, 2016; Accepted: September 08, 2016; Published: September 09, 2016
Abstract
Staphylococcus aureus causes intra-abdominal infections primarily due to hematogenous seeding. Abscesses in retroperitoneal organs, and specifically renal abscesses are rare complications of staphylococcal bacteremia in children as compared to osteomyelitis, or septic arthritis. We report a case of a ruptured staphylococcal renal abscess in a child without renal anomalies that presented as clinical peritonitis in order to underscore this potentially elusive diagnosis.
Keywords: Staphylococcus aureus; Peritonitis; Renal abscess
Introduction
Retroperitoneal abscesses present a diagnostic challenge in young children because symptoms are often indolent and poorly localized manifesting a wide spectrum of presentations from fever and flank pain and limp to overwhelming sepsis [1]. In a study spanning 10 years in a tertiary center, 45 pediatric patients were identified with Computerized Tomograpghy (CT)-proven renal abscess. Of note, 43% of the patients had known vesico-uretral reflux [2]. Other risk factors associated with renal abscesses are damaged kidneys, diabetes mellitus, immunocompromised status and renal calculi [3,4].
Primary renal abscesses which present as corticomedullary involvement on imaging, are most commonly associated with ascending urinary tract infection, caused by gram negative organisms [5]. In contrast, the renal “carbuncles” in children with a healthy urinary tract are thought to result from bacteremia from a primary focus of infection elsewhere and are caused mainly by Staphylococcus aureus [4,5]. A meticulous history taking might reveal a source of infection such as a skin wound, that occurred 1-8 weeks before the abscess formation in the kidney, but many times a port of entry is not apparent [5]. We present a patient with a rare complication of a staphylococcal renal abscess.
Case Presentation
A previously healthy one year old male was admitted to the hospital with complaints of a three day fever, watery diarrhea up to 6 times a day and multiple vomiting episodes. There was no history of trauma. He was born at term after a normal pregnancy. Before the current admission, he received three doses of amoxicillin due to fever, without improvement. Upon admission the child had a 38.8 c temperature, pulse of 186 beats per minute, a respiratory rate of 40 breaths per minute and 96% oxygen saturation in room air. On physical examination he was severely dehydrated. Laboratory tests showed hemoglobin 10 mg/dl, leukocytes 37x103 cells/mm3 with 13% stab forms, elevated urea 73mg/dl (age adjusted norm 17-43) and creatinine 0.75mg/dl (age-adjusted norm 0.2-0.4). Stool was watery with no leukocytes.
He was treated with fluids and ceftriaxone in the pediatric ward with a presumptive diagnosis of occult bacteremia. The following day, the child was admitted to the pediatric intensive care unit as a result of deteriorating renal function which manifested as oliguria, weight gain of 1.4 kg and peripheral edema. His respiratory rate decreased to 15 per minute and saturation dropped to 78.6% in room air. Blood pressure dropped to 95/54mm Hg. Laboratory examination showed hemoglobin 7.9mg/dl, a rising peripheral leucocyte count, thrombocytopenia of 75x103. Schistocytes were seen on peripheral smear. Impending renal failure due to Hemolytic-Uremic syndrome was suspected and emergent peritoneal dialysis was attempted. Upon introduction of the dialysis catheter, a cloudy peritoneal aspirate was observed. The fluid contained 13.6x103cells, of which 90% were polymorphonuclear cells. Blood, stool, and urine cultures were negative. Empiric treatment was initiated with ceftriaxone and metronidazole on the suspicion of a perforated viscus. The next day the culture from the peritoneal fluid was positive for oxacillin sensitive Staphylococcus aureus. Therapy was changed to cefazoline and gentamicin. An abdominal CT demonstrated a laceration in the upper pole of the right kidney which transversed the cortex extending to a high-density fluid area involving the Morrison pouch and compressing the kidney (Figure 1). CT-guided percutaneous drainage was performed and 3ml of yellow pus was aspirated. Staphylococcus aureus with the same sensitivity as from the peritoneal fluid was cultured from the exudate. On day 7 of admission, blood cultures were sterile, trans-thoracic echo was negative for vegetations, and bone scan was negative for osteomyelitis. However, the patient remained febrile. A second aspiration was performed and Staphylococcus aureus grew from the drain that was placed during the procedure. The third CT guided aspiration performed on day 18 was sterile, after which the patient became afebrile. The patient was discharged from the hospital in good condition after 21 days of intravenous antibiotic therapy with instructions to continue oral cephalexin for 10 more days. Follow-up Dimercaptosuccinic acid (DMSA) isotope scan after four months was recommended by nephrological consult to rule out a renal scar. Immunologic consultation was obtained because of an invasive staphylococcal infection at an early age, and neutrophile function studies including super-oxide generation, chemotaxis and phagocytosis were normal. Blood immunoglobulin levels were normal as well.
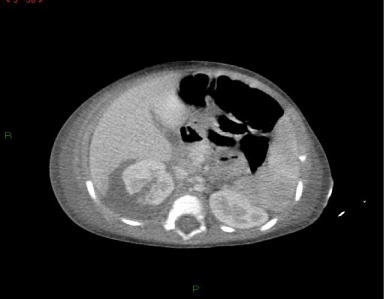
Figure 1: Abdominal computed tomography, childhood protocol, shows a
right renal abscess with rupture into the perirenal space with involvement of
the Morrison pouch.
Discussion
The three pathological mechanisms that explain the etiology of renal abscess in children are
a) proximity of the kidney to an infected area
b) ascending infection due to stasis of infected urine
c) hematogenous spread [1,6].
A study using animal models illustrated that Staphylococcus aureus causes deep seated infection by hematogenous seeding. Experimentally, when staphylococci were inoculated directly into six rat bladders, a renal parenchymal infection developed in the right kidney of only one rat. In contrast, a renal infection rate of 100% was achieved when the same innoculum of Staphylococcus aureus was injected intravenously [7].
We cannot discern without a doubt if the peritonitis diagnosed in our case resulted from a secondary focus of seeding or was secondary to abscess rupture. It is known that once the renal cortical parenchyma is infected, several interconnecting abscesses form and coalesce forming a fluid-filled mass which progresses to rupture to the perinephric space in 10% of cases [5]. We speculate that the inflammatory process from the perinephric space eroded the peritoneum which is delicate in this age-group, extending from its retroperitoneal focus into the peritoneal space.
We found only two reports of peritonitis in children with a perforated staphylococcal pyogenic retroperitoneal abscess. Both described ruptured psoas abscesses-one induced by a rectal exam and one that occurred spontaneously [7,8].
Additionally, two reports of an undiagnosed renal abscess complicated by generalized bacterial peritonitis in were published involving adults, one which was managed surgically, and the other with a proven staphylococcal etiology which was managed by percutaneous drainage and antibiotics [11,12]. Management of retroperitoneal abscess involves imaging, drainage, and directing appropriate antimicrobial therapy against potential pathogens [9]. Imaging of the retroperitoneum is necessary for localizing the lesion and ruling out viscus perforation. Both Computerized Tomography (CT) and ultrasound can be performed. Of note, CT can demonstrate collapsed bowel loops that are often not evident on ultrasound examination and safeguard percutaneous aspiration [10]. Drainage of the abscess is recommended in all children to reduce the risk of kidney damage or loss [1]. Open surgical drainage is rarely necessary unless multiple or vascular abscesses are present or the patient remains unstable [1,9,10].
In a patient with no predisposing factors, no evidence of urinary tract infection, and imaging which rules out bowel perforation, a staphylococcal etiology should be entertained in considering empiric therapy. The child we presented had both clinical and laboratory signs of sepsis induced by peritonitis, and therefore empiric antimicrobial therapy was directed towards bowel flora and changed accordingly when culture results returned [10].
According to the guidelines published by the Infectious Diseases Society of America (IDSA), antimicrobial therapy of established intra-abdominal infection should be limited to 4-7 days, unless it is difficult to achieve adequate source control [13]. Longer durations of therapy have not been associated with improved outcome. In the case presented, since the exudate continued to produce positive cultures, treatment was extended to three weeks parenterally and ten more days orally. There is no evidence that supports this duration and the decision was made in conjunction with an infectious disease consult.
Conclusion
Renal abscesses are diagnostic challenges in previously healthy children. We presented a child with suspected rupture of a renal staphylococcal abscess which manifested as peritonitis. A good outcome was obtained with percutaneous drainage of the peritoneal fluid as well as the abscess collection in conjunction with an extended regimen of an antistaphylococcal antibiotic with no need for open surgical intervention.
References
- Angel C, Shu T, Green J, Orihuela E, Rodriquez G, Hendrick E. Renal and peri-renal abscesses in children: proposed physio-pathologic mechanisms and treatment algorithm. Pediatr Surg Int. 2003; 19: 35-39.
- Cheng CH, Tsai MH, Su LH, Wang CR, Lo WC, Tsau YK, et al. Renal abscess in children: a 10-year clinical and radiologic experience in a tertiary medical center. Pediatr Infect Dis J. 2008; 27: 1025-1027
- Baradkar VP, Mathur M, Kumar S. Renal and perinephric abscess due to Staphylococcus aureus. Indian Journal of Pathology and Microbiology. 2009; 52: 440-441.
- Brook I. Microbiology of retroperitoneal abscesses in children. J Med Microbiol. 1999; 48: 697-700.
- Dembry LM, Andriole VT. Renal and perirenal abscesses. Infect Dis clin North Am. 1997; 11: 663-680.
- Dougherty FE, Gottlieb RP, Gross GW, Denison MR. Neonatal renal abscess caused by staphylococcus aureus. Pediatr Infect Dis J. 1991; 10: 463-466.
- Livne M, Serour F, Aladjem M, Vinograd I. General peritonitis induced by rectal examination: an unusual complication of primary psoas abscess. Eur J Pediatr Surg. 1994; 4: 186-187.
- Kleiner O, Cohen Z, Barki Y, Mares AJ. Unusual presentation of psoas abscess in a child. Journal of Pediatric Surgery. 2001; 12: 1859-1860.
- Gandon Y, Mueller PR, Ferrucci JT. Abscess and intra-abdominal fluid collections. Diagnosis and percutaneous drainage. J Radiol. 1989; 70: 235- 247.
- Diament MJ, Stanley P, Kangarloo H, Donaldson JS. Percutaneous aspiration and catheter drainage of abscesses. The Journal of Pediatrics. 1986; 108: 204-208.
- Sule AZ. Undiagnosed renal abscess presenting as acute bacterial peritonitis: case report. East Afr Med J. 2001; 78: 500-501.
- Michel P, Pagliano G. Peritonitis caused by rupture of a retroperitoneal abscess. J Chir. 1993; 130: 240-246.
- Solomkin JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJ, Baron EJ, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010; 50: 133-164.