
Special Article – Cerebral Palsy
Austin Pediatr. 2016; 3(4): 1043.
Trends in Perinatal Death and Brain Damage: A Regional Population-Based Study in Southern Japan, 1998-2012
Yamashita R, Kodama Y*, Sameshima H, Doi K, Michikata K, Kaneko M and Ikenoue T
Department of Obstetrics and Gynecology and Perinatal Center, Faculty of Medicine, University of Miyazaki, Japana
*Corresponding author: Yuki Kodama, Department of Obstetrics and Gynecology and Perinatal Center, Faculty of Medicine, University of Miyazaki, 5200 Kihara, Kiyotake, Miyazaki 889-1692, Japan
Received: September 28, 2016; Accepted: October 18, 2016; Published: October 20, 2016
Abstract
Assessing overall and gestational age-specific trends in perinatal mortality and cerebral palsy in Miyazaki, both neonatal death and cerebral palsy in term infants were significantly reduced for 15 years. This might be contributed by educational and referral system in perinatal medicine.
Keywords: Population-based study; Cerebral palsy; Perinatal mortality rate; Stillbirth; Neonatal death
Introduction
The prevalence of cerebral palsy (CP) has not changed over the past 50 years despite a 6-fold increase in cesarean sections [1]. The widespread use of electronic fetal heart rate (FHR) monitoring has not led to a decrease in the incidence of CP [2,3]. Clark et al. [4] concluded that CP was unpreventable considering the current status of our technology.
The prevalence of brain damage in preterm infants increased during the 1970s and 1980s as a result of their increased survival due to improvements in perinatal and neonatal management [5-8]. However, other researchers expressed different views in different periods in various countries [9-12]. In our previous study [13], preterm infants accounted for over 60% of all the infants with brain damage registered in the study region.
It is important to elucidate the causes and background of such infants with neurological damage and such information may potentially hold the key to decreasing perinatal mortality and brain damage.
Therefore, we performed a longitude study to see trends in perinatal death and brain damage by using a regional populationbased study from 1998 to 2012.
Materials and Methods
In 1998, we initiated a regional population-based study focusing on perinatal death and neurological damage occurring in Miyazaki Prefecture, which has 10,000 deliveries annually and a population of 1 million. Details of our work have been reported previously [13-19].
The Miyazaki Perinatal peer-review audit conference is held twice a year, where perinatal and neonatal specialists from eight perinatal centers discuss the clinical factors associated with perinatal deaths and the neurological complications of each case. Pediatric neurologists also participate in the conference. Our criteria for registering high-risk infants with neurological damage are listed in Table 1. The definition for hypoxia to cause CP is listed in Table 2, modified from the international consensus statement on CP [20]. Congenital abnormalities include chromosomal disorders, neurological anomalies, myopathies, metabolic diseases, hydrops fetalis, known anomaly syndromes, and intrauterine exposure to teratogenic substances.
1
Umbilical arterial pH < 7.0 or base deficit > 12 mmol/l.
2
Abnormal neurological findings during the neonatal period.
a. Seizure activity
b. Hypertonia or hypotonia
c. Abnormal reflex.
d. Irritability or hyperexcitability
e. Poor sucking and swallowing reflexes
f. Shallow, irregular respirations.
g. Apnea (not caused by prematurity)
3
Abnormal neurological images during the neonatal period.
a. Intraventricular hemorrhage (grades 3-4)
b. Periventricular leukomalacia
c. Hydrocephalus
d. Congenital CNS anomalies
e. Hypoxic-ischemic encephalopathy
4
Congenital infection tnat may cause neurological damage.
5
Severe IUGR (< 3SD)
IUGR: Intrauterine growth restriction.
Table 1: Inclusion criteria for the neurological high-risk infants.
1 Evidence of metabolic acidosis (Umbilical arterial pH < 7.0 and base deficit > 12 mmol/l)
2 Early onset of severe or moderate neonatal encephalopathy
3 A sentinel (signal) hypoxic event occurring immediately before or during labor
4 A sudden, rapid and sustained deterioration of the fetal hypoxic sentinel event where the pattern was previously normal
5 Apgar scores of 0-6 for longer than 5 minutes
6 Early evidence of multisystem involvement
7 Imaging evidence of acute hypoxic-ischemic encephalopathy
#1 and/or #2; essential criteria, without #1, fit at least 3 of #3-7.
Modified from international consensus statement [20].
Table 2: The definition of hypoxia to cause cerebral palsy.
Infants were classified into five groups according to gestational age as follows: term (>37 weeks), late preterm (34-36 weeks), moderately preterm (28-33 weeks), very preterm (27-25 weeks), and extremely preterm (22-24 weeks). The infant survival rate and prevalence of neurological damage were compared among three 5-year periods, namely, 1998-2002, 2003-2007, and 2008-2012.
Using these registered cases, we investigated the prevalence of CP, clinical background, and pregnancy disorders. Perinatal deaths were defined as stillbirth occurring at >22 weeks of gestation and neonatal deaths at < 28 days of age. The study protocol was approved by the institutional ethics committee of the Faculty of Medicine, University of Miyazaki.
The chi-squared and Fisher’s exact tests were used to compare proportions, and significance was taken as p < 0.05.
Results
Of the 156,766 deliveries in Miyazaki Prefecture from 1998 to 2012, 337 (0.21%) were stillbirths, 198 (0.13%) were neonatal deaths, and 312 (0.20%) had high risk of poor neurological outcome.
Overall trends
The perinatal data for Miyazaki Prefecture are shown in Figure 1. The total number of live births was approximately 10,000 per year, with a slight decrease noted over the 15 years. The preterm birth rate increased from 5.3% in 2000 to 6.3% in 2010-2012, while the perinatal death rate significantly decreased from 6.5 to 3.0 per 1,000 births. The total prevalence of CP was 1.0-2.0 per 1,000 births with no change over the study period. The prevalence of CP in term infants appeared to be decreasing, whereas that in preterm appeared increasing.
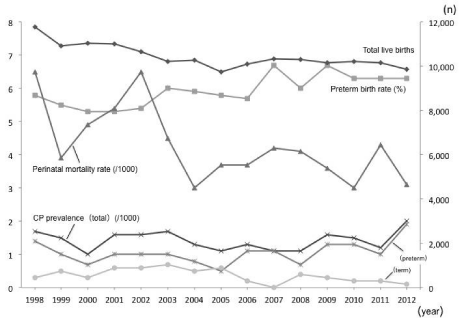
Figure 1: Trends in perinatal data in Miyazaki Prefecture (1998-2012).
Perinatal deaths
As shown in Table 3, perinatal deaths (including stillbirths and neonatal deaths) decreased from 219 in 1998-2002 to 151 in 2008- 2012 (p <0.01, chi-squared test). These findings were due to the remarkable decrease in neonatal deaths (from 91 in 1998-2002 to 45 in 2008-2012, p=0.0006). Neonatal mortality significantly decreased in term infants (>37 weeks) from the period 1998-2002 to the period 2003-2007 and 2008-2012 (p=0.038, respectively). A significant decrease was also noted for extremely preterm infants (22-24 weeks) when comparing the neonatal mortality in 2003-2007 and 2008-2012 (p=0.045).
Cerebral palsy
During the 15-year study period, the number of registered CP cases was 312 out of 156,766 live births (0.20%). These included extremely preterm (22-25 weeks) infants (n = 45), very preterm (25- 27 weeks) infants (n = 34), moderately preterm (28-33 weeks) infants (n = 81), late preterm (34-36 weeks) infants (n = 40), and term (>37 weeks) infants (n = 112). Thus, 64% of CP cases were among the preterm infants, while 36% were among the term infants. The number of live births and prevalence of CP in each group is shown in Figure 2. In term infants, the prevalence of CP was 0.4 per 1,000 live births, while in late-preterm infants, it rose up to 3.0 per 1,000. It increased exponentially to 271 per 1,000 in extremely preterm infants.
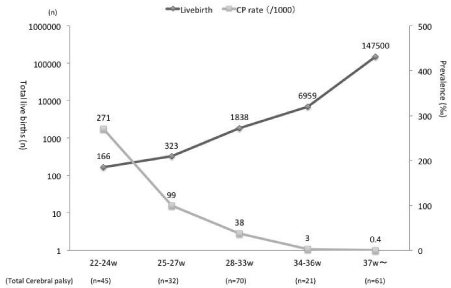
Figure 2: Prevalence of brain damage in each gestational age group, excluding congenital malformation (1998-2012).
The backgrounds of the infants with CP are shown in Figure 3. In preterm infants, the major factors were periventricular leukomalacia (26%), prematurity (23%) such as intraventricular hemorrhage, necrotizing enterocolitis, and the presence of a congenital anomaly (18%), while in term infants, the most common factor was the presence of a congenital anomaly (45%), followed by hypoxia (33%).
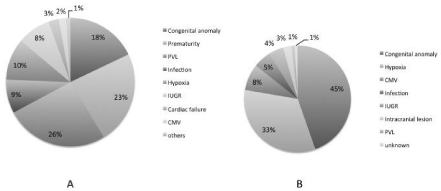
Figure 3: Background of brain damage (1998-2012) by A, preterm infant (n = 200); and B, term infant (n = 112).
The trends for all CP evaluated for the 5-year intervals indicated no significant changes (Table 3). However, in term infants, the number of CP cases decreased from 44 in 1998-2002 to 22 in 2008- 2012 (p =0.019, Fisher’s exact test). Similarly, in extremely preterm infants, it decreased from 20 in 1998-2002 to 8 in 2008-2012, without significant difference (p =0.065). After excluding the cases of congenital anomaly, the number of CP in each time interval of 1998- 2002, 2003-2007, and 2008-2012 was 29, 20, and 13, respectively.
Period
p value
1) 1998-2002
2) 2003-2007
3) 2008-2012
1) vs 2)
2) vs 3)
1) vs 3)
Total live births
Perinatal deaths
55,421
219
50,656
165
50,689
151
0.059
0.427
0.007
Stllbirth
22 to 24 wk
25 to 27 wk
28 to 33 wk
34 to 36 wk
37 wk <
Total
25
19
34
19
31
128
18
10
32
16
27
103
13
11
21
24
37
106
0.439
0.152
0.905
0.809
0.855
0.355
0.368
0.828
0.130
0.207
0.212
0.839
0.094
0.223
0.154
0.291
0.273
0.449
Neonatal death
22 to 24 wk
25 to 27 wk
28 to 33 wk
34 to 36 wk
37 wk <
Total
16
12
18
8
37
91
18
7
13
5
19
62
7
6
9
4
19
45
0.664
0.354
0.517
0.694
0.038
0.073
0.045
0.999
0.521
0.990
0.873
0.099
0.095
0.322
0.190
0.476
0.038
0.0006
Cerebral palsy
22 to 24 wk
25 to 27 wk
28 to 33 wk
34 to 36 wk
37 wk <
(37 wk < (excluding anomaly))
Total
20
11
30
11
44
29
116
17
9
20
12
46
20
104
8
14
31
17
22
13
92
0.826
0.982
0.272
0.829
0.595
0.331
0.889
0.109
0.405
0.124
0.354
0.004
0.222
0.388
0.065
0.410
0.633
0.17
0.019
0.029
0.306
Table 3: Stillbirth, neonatal death and brain damage in each gestational age group of 5-year intervals.
There was a significant decrease from 1998-2002 to 2008-2012 (p =0.029). And the occurrence of hypoxia in term infants in each interval decreased significantly from 18 in 1998-2002 to 6 in 2008- 2012 (p <0.05) (Figure 4).
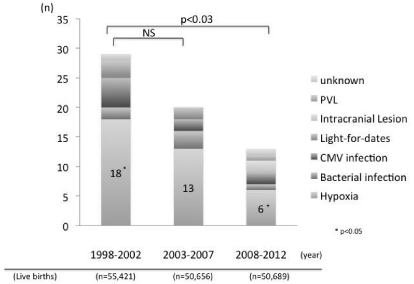
Figure 4: Brain damage in term infants in a 5-year interval, excluding congenital anomalies (1998-2012).
Discussion
Some population-based studies have investigated perinatal death and CP in different regions over different time periods [9-12]. Over the past 50 years, the preterm mortality rate, especially in very low birth weight infants have shown a remarkable reduction [6-12]. Given such improved survival rates in preterm or very low birth weight infants, the prevalence of CP was noted to increase during the 1970s and 1980s [6-8]. However, in another study area, it was noted that improvements in the survival of preterm infants did not increase the prevalence of CP [9-11].
In our regional population-based study encompassing 15 years, the perinatal mortality has improved significantly with improving neonatal mortality rate. This observation is compatible with previous reports [5,8,10-12].
We observed significant reductions in the rate of death and CP in term infants. These reductions contributed to the decrease in total neonatal mortality and prevalence of CP. Hypoxia was the second major factor that accounted for one-third of all CP cases in term infants. A reduction in the occurrence of hypoxia related CP in term infants thus had a significant impact on the prevalence of CP.
A possible explanation for the improvements in mortality and prevalence of CP in term infants is the wide spread use of FHR monitoring and some outreach educations in our district. For example, we previously reported that there were 1,312 referral cases from primary physicians to perinatal centers for fetal indications, those were preterm labor (37%), non reassuring fetal status on FHR monitoring (35%), and preterm premature rupture of membranes (26%) [21]. Thus, referral cases due to abnormal FHR findings are relatively frequent in our district. We speculated that maternal transfer was performed during the early stages of abnormal FHR patterns and the fetuses were delivered early enough to prevent severe acidosis with adequate support for neonatal resuscitation.
Another clinical trial in our local district was the centralization of FHR monitoring with the help of specialists in the secondary centers to interpret results and determine clinical actions on a 24-h basis [22]. In that study, out of 9,139 deliveries over three-year period, FHR centralization system showed that the incidence of acidemia was significantly decreased (from 0.47% to 0.11%, p= 0.028) without a increase in cesarean birth rate due to nonreassuring FHR patterns.
The educational courses of neonatal cardiopulmonary resuscitation for obstetricians, midwives, and nurses might have also helped to achieve better outcomes for asphyxiated newborns in our district.
The US National Institute of Child Health and Human Development (NICHD) advocated the importance of late-preterm infants in 2005 [23]. Petrini JR et al. [24] reported that late-preterm infants were 3.39 times more likely to have CP than term infants. Although short-term outcomes in preterm infants appear to have improved in recent years, long-term outcomes remain unfavorable. Our data also showed that the CP prevalence of late-preterm was more than 7 times higher than that of term.
This study has several limitations. First, we mainly followed highrisk infants in the neonatal period in order to investigate neurological complications. Such an approach involves the risk of underreporting due to an incomplete dataset. In this study, we could follow the neurological development, as evaluated by pediatricians, in 90% of the high-risk infants until the age of 2 years or older. We also did not fully include neonates, who appeared normal at birth but developed neurological damage later, as reported in the Dublin study [3]. Despite these limitations, strength of our study is that our registration system was previously determined to cover 96% (136/142) of infants with neurological damage in our population [17].
The trends observed in the present study indicate that perinatal death and the prevalence of CP in Miyazaki Prefecture from 1998 through 2012 decreased significantly in term structively normal infants. This speculated to be the establishment of the perinatal referral system, the educational system for interpretations of FHR tracings, and timely provision of necessary medical treatment.
Based on these findings, we should work out countermeasures as gestational ages in order to prevent brain damage. And still there is a room to reduce not only perinatal death but also CP. Future studies regarding the effects on the outcome should be evaluated without congenital abnormalities, which accounts for high percentage of brain damage especially in term infants.
Acknowledgements
This study was supported by the Japan Association of Obstetricians and Gynecologists (JAOG) Ogyaa Donation Foundation (2012-14).
We would like to thank the following members of the Miyazaki Perinatal Data Group for assisting in data collection: Miyazaki City Hospital, Miyazaki Prefectural Hospital (in Nobeoka, Nichinan, and Miyazaki), National Miyakonojo Hospital, Fujimoto-Hayasuzu Hospital, and Koga Hospital.
References
- MacLennan AH, Thompson SC, Gecz J. Cerebral palsy: causes, pathways, and the role of genetic variants. Am J Obstet Gynecol. 2015; 213: 779-788.
- MacDonald D, Grant A, Sheridan- Pereira M, Boylan P, Chalmers I. The Dublin randomized controlled trial of intrapartum fetal heart rate monitoring. Am J Obstet Gynecol. 1985; 152: 524-539.
- Grant A, O’Brien N, Joy MT, Hennessy E, MacDonald D. Cerebral palsy among children born during Dublin randomized trial of intrapartum monitoring. Lancet. 1989; 8674: 1233-1236.
- Clark SL, Hankins GDV. Temporal and demographic trends in cerebral palsy – Fact and fiction. Am J Obstet Gynecol. 2003; 188: 628- 633.
- Stanley FJ, Watson L. Trends in perinatal mortality and cerebral palsy in Western Australia, 1967 to 1985. BMJ. 1992; 304: 1658-1663.
- Reid SM, Carlin JB, Reddihough DS. Rates of cerebral palsy in Victoria Australia, 1970 to 2004: has there been a change?. Dev Med Child Neurol. 2011; 53: 907-912.
- Winter S, Autry A, Boyle C, Yeargin-Allsopp M. Trends in the prevalence of cerebral palsy in a population-based study. Pediatrics. 2002; 110: 1220-1225.
- Suzuki J, Ito M. Incidence patterns of cerebral palsy in Shiga Prefecture, Japan, 1977-1991. Brain Dev. 2002; 24: 39-48.
- Van Naarden Braun K, Doernberg N, Schieve L, Christensen D, Goodman A, Yeargin-Allsopp M. Birth prevalence of cerebral palsy: A population-based study. Pediatrics. 2016; 137: 1-9.
- Himmelmann K, Hagberg G, Beckung E, Hagberg B, Uvebrant P. The changing panorama of cerebral palsy in Sweden. X. Prevalence and origin in the birth-year period 1995-1998. ActaPeaediatr. 2005; 94: 287-294.
- Himmelmann K, Hagberg G, Uvebrant P. The changing panorama of cerebral palsy in Sweden. X. Prevalence and origin in the birth-year period 1999-2002. ActaPeaediatr. 2010; 99: 1337-1343.
- Himmelmann K, Uvebrant P. The changing panorama of cerebral palsy in Sweden. XI. Changing patterns in the birth-year period 2003-2006. ActaPeaediatr. 2014; 103: 618-624.
- Kodama Y, Sameshima H, Ikenoue T. Temporal trends in perinatal mortality and cerebral palsy: A population-based study in southern Japan. Brain & Development. 2016; 38: 386-391.
- Sameshima H, Ikenoue T; Miyazaki Perinatal Data Group. Risk factors for perinatal deaths in Southern Japan: population-based analysis from 1988 to 2005. Early Hum Dev. 2008; 84: 319-323.
- Kodama Y, Sameshima H, Ikenoue T. Regional population-based study on pregnancy outcomes in women with diabetes mellitus in Japan. J Obstet Gynecol Res. 2007; 33: 45-48.
- Sameshima H, Kodama Y, Kaneko M, Ikenoue T; Miyazaki Perinatal Data Group. Deaths of early-onset, invasive sepsis in full-term infants in Miyazaki: Nine cases from a regional population-based analysis from 1998-2006. Jpn J Infect Dis. 2008; 61: 400-401.
- Kodama Y, Sameshima H, Ikeda T, Ikenoue T. Intrapartum fetal heart rate patterns in infants (>/=34 weeks) with poor neurological outcome. Early Hum Dev. 2009; 85: 235-238.
- Doi K, Sameshima H, Kodama Y, Furukawa S, Kaneko M, Ikenoue T. Perinatal death and neurological damage as a sequential chain of poor outcome. J Matern Fetal Neonatal Med. 2012; 25: 706-709.
- Kodama Y, Sameshima H, Yamashita R, Oohashi M, Ikenoue T. Intrapartum fetal heart rate patterns preceding terminal bradycardia in infants (> 34 weeks) with poor neurological outcome: A population-based study in Japan. J Obstet Gynecol Res. 2015; 41: 1738-1743.
- MacLennan A. A template for defining a causal relation between acute intrapartum events and cerebral palsy. BMJ. 1999; 319: 1054-1059.
- Ikenoue T. Development of perinatal case system and clinical research (in Japanese). ActaObstGynaec JPN. 2008; 60: 1605-1610.
- Michikata K, Sameshima H, Urabe H, Tokunaga S, Kodama Y, Ikenoue T. The regional centralization of electric fetal heart rate monitoring and its impact on neonatal acidemia and cesarean birth rate. Journal of Pregnancy vol. 2016; Article ID 3658527.
- Raju T.N.K., Higgins RD, Stark AR,Leveno KJ. Optimizing care and outcome for late-preterm (near-term) infants: A summary of the workshop sponsored by the National Institute of Child Health and Human Development. Pediatrics. 2006; 118: 1207-1214.
- Petrini JR, Dias T, McCommick MC, Massolo ML, Green NS, Escobar GJ. Increased risk of adverse neurological development for late preterm infants. J Pediatr. 2009; 154: 169-176.