
Research Article
Austin Pediatr. 2017; 4(2): 1056.
Sleep, Fatigue and Neurodevelopmental Outcomes in Pediatric Sickle Cell Disease
Rogers VE¹* and Lance EI²
¹School of Nursing Department of Family & Community Health, University of Maryland Baltimore, USA
²Department of Neurology, Kennedy Krieger Institute, Baltimore, USA
*Corresponding author: Rogers VE, School of Nursing Department of Family & Community Health, Baltimore, USA
Received: May 12, 2017; Accepted: June 13, 2017; Published: June 20, 2017
Abstract
Background: Children with sickle cell disease (SCD) experience neurodevelopmental decline over time. They also tend to have short duration, poor quality sleep and elevated fatigue levels. This study aimed to describe sleep and fatigue in children and adolescents with SCD and their association with neurodevelopmental measures.
Methods: Participants included 19 children and adolescents with SCD, aged 7-18 years, recruited from a tertiary care SCD clinic. The majority were referred for neurodevelopmental testing due to academic or behavioral difficulties. Parents completed the Behavior Rating Inventory of Executive Function (BRIEF). Participants completed the Wide Range Achievement Test (WRAT) and wore an actigraph for one week. Both completed the PedsQL Multidimensional Fatigue scale.
Results: Short duration or poor quality sleep were identified in every participant. Fatigue was common. Eighteen participants had at least one abnormal result of the six neurodevelopmental subscales administered. No sleep measure was associated with any neurodevelopmental measure. Greater parent-proxy reported cognitive fatigue was associated with lower WRAT reading (p=0.041), spelling (p=0.002) and math (p=0.010) scores, and with poorer scores on two BRIEF subscales. Greater reported parent-proxy general fatigue was associated with elevated scores on all three BRIEF subscales.
Conclusion: Fatigue more importantly than sleep appears to be related to deficits in academic skills and executive function in a clinical sample at risk for neurodevelopmental problems. Larger studies are needed to better define the effects of sleep and fatigue on specific aspects of cognition and behavior in children and adolescents with SCD across early development.
Keywords: Sickle Cell Disease; Children; Adolescents; Sleep; Fatigue; Cognition
Abbreviations
BRIEF: Behavioral Rating Inventory Of Executive Function; FI: Fragmentation Index; Hb: Hemoglobin; MRI: Magnetic Resonance Imaging; SCD: Sickle Cell Disease; SE: Sleep Efficiency (%); SOL: Sleep Onset Latency (Min); TCD: Transcranial Doppler Ultrasonography; TST: Total Sleep Time (Min); WASO: Wake After Sleep Time (Min); WRAT-4: Wide Range Achievement Test–Version 4
Introduction
Sickle cell disease (SCD) is a group of genetic disorders characterized by the presence of abnormal hemoglobin S, which has a propensity to polymerize, or sickle, under certain conditions. This process leads to anemia with accompanying decreased oxygen delivery, and tissue hypoxemia due to vaso-occlusion. Multiple genotypes of SCD have been identified, with homozygous hemoglobin (Hb) SS and HbSβ0 thalassemia generally demonstrating more severe phenotypes, while HbSC and HbS-β+ thalassemia generally have milder presentations. However, these pathobiological processes occur in all individuals with SCD regardless of disease severity [1]. In turn, they contribute to ongoing, progressive organ damage [2], a high risk for stroke and silent cerebral infarct [3,4] and progressive decrements in cognition and behavior. The term neurodevelopmental functioning is used in this in this article to refer to the way the brain and nervous system affect learning, cognition and behavior.
Neurodevelopmental impairment is common in children with SCD compared to their siblings and healthy peers [5,6]. It presents in a number of domains such as academic achievement, intelligence, executive function and behavior. Impairment begins in infancy [7] and can progress over time. While the aforementioned SCD-related sequelae are significant contributors to this decline, deficits cannot be attributed exclusively to these events. Family and environmental factors such as poverty, stress, maternal education, parenting skills and the home environment [8] have also been shown to impact the development of children with SCD. Fatigue, too, can impact cognitive development [9] and has additionally been associated with disrupted sleep in children with SCD [10].
Sleep is a less commonly considered variable that may impact pediatric neurodevelopment. Research in the general pediatric population suggests that short duration and poor quality sleep contribute to neurodevelopmental impairment [11]. Disturbances in sleep duration and quality are common in children with SCD due to sleep-interfering disease symptoms such as pain and enuresis [12,13]. Little is known about neurodevelopmental risks posed by sleep problems in children with SCD, yet it is conceivable that the impairment they cause might be even greater than seen in typically developing children due to their additional underlying neurological vulnerability [14,15]. Given the high prevalence of sleep disturbances in children with SCD, if short duration or poor quality sleep are found to be risk factors for neurodevelopmental impairment, a majority of these children could be affected. Minimizing adverse neurodevelopmental outcomes is imperative to helping children with SCD achieve optimal health, quality of life, and academic and personal potential.
There were two aims to this study. The first aim was to describe the sleep of children with SCD who underwent neurodevelopmental testing and compare it to the sleep of typically developing children without SCD reported in the literature and research-based sleep recommendations [16-19]. Given the association between fatigue and sleep, and the potential for both to affect cognitive development, the second aim was to explore associations between sleep, fatigue and neurodevelopmental outcomes. It was hypothesized that shorter duration and poorer quality sleep, and greater levels of fatigue, would be associated with greater neurodevelopmental deficits.
Materials and Methods
Procedures
Participants were recruited from a tertiary care SCD clinic between September 2013 and April 2015. Recruitment occurred only during the school year to standardize the sleep schedule. Data were not collected during periods of transition that affect sleep, such as the first and last two weeks of the school year, major holidays, and the week following the daylight savings time change. Baseline evaluation included questionnaires, neurodevelopmental testing if not completed previously for clinical reasons, and training on use of the actigraph and sleep diary. This was followed by seven consecutive days of wearing the actigraph at home, and concurrently completing a sleep diary. Families received compensation for participation.
Participants
Children and adolescents aged 7-18 years with any type of SCD were recruited. Participants were specifically recruited, where possible, if they had been referred for neurodevelopmental testing due to academic or behavioral difficulties. All children had either undergone recent clinically indicated formal neurodevelopmental assessment or were willing to undergo assessment as part of study participation. Exclusion criteria were major psychiatric illness, neurological, or neuromuscular disorder severe enough to disrupt regular school attendance, and pregnancy.
This study was approved by the Institutional Review Boards of Johns Hopkins Hospital and the University of Maryland, Baltimore Human Research Protections Office. Prior to participation, parents provided written informed consent for children less than 18 years of age, and children 7-17 years provided written assent. Participants 18 years old self-consented.
Measures
Information was collected by parent report and from the medical record, and included age, sex, race, SCD genotype, results of neuroimaging studies including brain magnetic resonance imaging (MRI) and transcranial Doppler (TCD) velocities, and current use of hydroxyl urea or chronic blood transfusion.
Sleep was measured with an actigraph (Actiwatch2, Philips Respironics, and Bend, OR), a wrist-worn battery operated device that measures movement and translates it into measures of sleep and wake. It also contains a light meter that continuously measures light exposure. Actigraphy has demonstrated validity as an objective measure of sleep [20,21]. The actigraph was worn on the nondominant wrist continuously for seven days and nights, a period of time that produces the most reliable measures of sleep minutes in children [22]. Data were collected in 60 second epochs, and sleep and wake were calculated using the medium sensitivity (default) threshold. Actigraphs were not removed for bathing.
Sleep parameters collected for this study were defined as follows. Total sleep time (TST) was minutes of sleep between nocturnal sleep onset and morning awakening. Total nap time included the number of minutes of any daytime sleep. Sleep onset latency (SOL) was the number of minutes from bedtime to sleep onset. Sleep efficiency (SE) was the total minutes of sleep divided by total minutes from sleep onset to sleep offset, as a percent. Wake after sleep onset (WASO) was the number of minutes of wake between sleep onset and sleep offset. Finally, the sleep fragmentation index (FI) was defined as the sum of percent mobile and percent immobile (no movement) bouts of less than one-minute duration to the number of immobile bouts for the given interval. The FI is an index of restlessness during sleep, where a higher score indicates greater sleep disruption. An event marker on the actigraph was pressed at bedtime and wake time each day to mark the sleep period.
A sleep diary was kept concurrently with actigraphy. Parents or participants recorded bedtimes, wake times, actigraph off-wrist times, and presence of pain and pain medication use during the previous night. Sleep diary data, event markers, and light measurements recorded by the actigraph were used to manually edit sleep periods in order to optimize the scoring and interpretation of sleep [23].
Quality of life related to fatigue was measured using the PedsQL™ Multidimensional Fatigue Scale developed by Dr. James W. Varni [24]. This is an 18-item child/adolescent report and parent-proxy report of child fatigue that yields three 6-item subscale scores: general fatigue, sleep/rest fatigue, and cognitive fatigue. Responses are rated on a 5-point Likert scale from 0 (never) to 4 (almost always). Items are then scored or reverse-scored such that lower scores indicated greater fatigue, and linearly transformed to a 0-100 scale. This scale has been validated in children with SCD [25,26], and yielded Cronbach’s alphas for the child self-report and parent-proxy report fatigue subscales of, respectively, 0.84 and 0.93 for general fatigue, 0.77 and 0.90 for sleep/ rest fatigue and 0.84 and 0.97 for cognitive fatigue. Scales with alphas of =0.70 are recommended for group comparisons [27].
The Wide Range Achievement Test-Version 4 (WRAT-4) is a measure of academic performance validated in a sample of over 15,000 people aged 5-94 years in the U.S. Reported scores are standardized to a mean (SD) of 100 (15), with scores <90 classified as below average [28]. Three subscales of the WRAT-4 were completed by participants: word reading, spelling, and math computation.
The Behavioral Rating Inventory of Executive Function (BRIEF) [29] is an 86-item parent-report assessment of executive function behaviors of children and adolescents ages 5–18 years. The scale was normed on data from 1419 parents from a representative distribution of socioeconomic statuses [29]. Scores are reported as t-scores normed at a mean (SD) of 50 (10), with higher scores indicating higher dysfunction. T-scores of =65 were classified as ‘elevated’ [30]. Three subscales of the BRIEF were measured: behavioral regulation index (ability to shift cognitive set and modulate behavior and emotions), metacognition index (ability to plan, organize, initiate, self-monitor and sustain working memory) and global executive composite (overall executive function).
Statistical analysis
Descriptive statistics were used to describe the sample and their sleep, fatigue and neurodevelopmental test scores. Actigraphic sleep parameters were compared descriptively to published sleep parameters of healthy children and research-based sleep recommendations [16-19]. Paired t-tests were used to test for differences between parent-proxy and self-reported fatigue subscales. Spearman correlation was used to test associations between sleep, fatigue and neurodevelopmental scores. WRAT-4 scores were dichotomized into ‘average or above average’ and ‘below average’ (standardized score <90). BRIEF scores were dichotomized into ‘normal’ or ‘elevated’ (t-score =65) for descriptive purposes. Analyses were carried out using IBM SPSS Statistics, Version 21. Statistical significance was a p-value <0.05.
Results
Nineteen children and adolescents with SCD and their parent participated in the study. Sample descriptors are detailed in Table 1. Details regarding enrollment rates and reasons for nonparticipation were not recorded; however, failing to keep scheduled study appointments was common, with eight children who qualified and were scheduled for participation failing to keep their appointment. Mean lag time between neurodevelopmental testing and the start of the study for 12 patients who had previously undergone clinically indicated testing was 30.7±39.0 days (range 0-131 days). The remaining 7 participants were evaluated on the first day of the study. All testing was performed in a private room in an outpatient clinic by one of the authors (EIL), a neurodevelopmental physician who works with children with SCD. Three of ten participants who had brain MRIs had evidence of overt stroke and one had a silent cerebral infarct.
Variable
N (%) or Mean ± SD
Sex, female
12 (63.2)
Age, y
12.1 ± 3.4
BMI percentile
56.8 ± 28.5
Race
African American
17 (89.5)
Multiracial
2 (10.5)
Sickle cell genotype
SS
10 (52.6)
SC
4 (21.1)
S-β thalassemia
5 (26.4)
Hydroxyurea
6 (31.6)
Chronic transfusions
4 (21.1)
Brain MRI (abnormal), n=10
4
Stroke risk by TCD, n=10
Normal
8
Conditional
1
Abnormal
1
BMI: Body Mass Index (kg/m2); MRI: Magnetic Resonance Imaging of the Brain; TCD: Transcranial Doppler.
Table 1: Description of the sample, N=19.
Sleep
Seventeen participants recorded all seven days of actigraphy. One child recorded three days and then discontinued the study due to discomfort with wearing the actigraph. One adolescent demonstrated very fragmented sleep early in the recording week and unknown to the investigators, was admitted to the hospital for management of a pain crisis during the study. Although this participant was not included in analyses related to sleep, his actogram is depicted in Figure 1 to demonstrate the sleep problems faced by many children with SCD who experience frequent pain. The actogram of an adolescent participant with a more typical sleep pattern is also depicted for comparison.
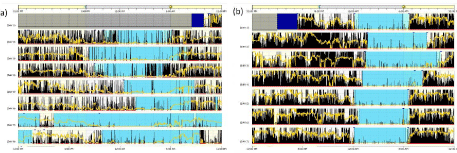
Figure 1: Actogram comparison of two participants. Actogram (a) is a 15 year old before and during a pain crisis requiring hospitalization in study day 4. Note
high activity during sleep period prior to onset of pain crisis. After hospital admission and adequate pain management, activity during sleep decreases, while sleep
is extended. Actogram (b) is a 13 year old with a more typical adolescent sleep/wake pattern. Despite somewhat irregular and late bedtimes, healthy wake and
sleep activity is suggested by high activity during the day, and low activity during sleep. Each row represents a 24-h period. Black lines indicate activity, with higher
amplitude and density indicating more vigorous activity. Yellow line indicates light exposure. Blue areas indicate rest (lighter blue) or sleep (darker blue). Purple (b)
indicates areas excluded from analysis (e.g. watch off).
Sleep variables measured by actigraphy are presented in Table 2. There was substantial day-to-day variability across the seven days of actigraphy measurement for all sleep measures (Figure 2), suggesting dysregulated sleep/wake cycles due to disease-related disturbances such as pain, or poor sleep hygiene, or both. Mean TST for participants was just over 7 hours, well below the 9-11 hours of sleep for school-aged children or the 8-10 hours of sleep for adolescents recommended by the National Sleep Foundation [31]. No participant met the recommendations for adequate sleep duration. Mean nap time was 15.8 minutes, with 13 participants (72.2%) napping at least on some days during the study week. Generally, time spent napping in school-aged children and older is near zero. SOL, which is generally less than 20 minutes in healthy children and adolescents [17,19], was nearly 50% longer for participants in this study, at 29.6 minutes. Seven participants (38.9%) achieved an average SOL of less than 20 minutes. SE, which is normally 85% or greater, averaged 79.9%, with only four participants (22.2%) achieving a mean SE of 85% or greater. WASO is generally short in children, not usually exceeding 15 minutes, but in this sample, it averaged 63.0 minutes. No participant averaged less than 34.7 minutes of WASO during the study, with one 15 year old averaging 124 minutes, and one 10 year old averaging 113 minutes of WASO. No normative references were found for the FI, but one study [32] reported a mean FI of 21.2 (1.0) in children 2-18 years of age with primary snoring (snoring without sleep apnea), while the FI of children with moderate-severe obstructive sleep apnea was 27.9 (2.4). Thus, the fragmentation of sleep in children with SCD appears to exceed even that of children with moderate-severe sleep apnea.
Sleep measures
Mean (SD)
Did not meet recommendations or reference values, N (%)
Total (nocturnal) sleep time, hrs
7.05 (0.8)
18 (100)
Total nap time, min
15.8 (20.7)
13 (72.2)
Sleep onset latency, min
29.6 (23.0)
11 (61.1)
Sleep efficiency, %
79.9 (6.9)
14 (77.8)
Wake after sleep onset, min
63.0 (24.9)
18 (100)
Fragmentation Index, %
32.6 (8.9)
NA
Sleep recommendations were based on the following citations (Gaina, Sekine, Chen, Hamanishi, & Kagamimori, 2004; McLaughlin Crabtree & Williams, 2009; Paruthi et al., 2016; Scholle, Wiater, & Scholle, 2011). NA, guidelines or healthy group comparisons not available.
Table 2: Actigraphic sleep measures in children with sickle cell disease, n=18.
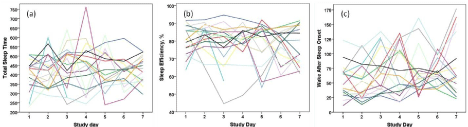
Figure 2: Day–to-day variability of sleep across 7 days in children and adolescents with sickle cell disease. (a) Total sleep time, min., (b) Sleep efficiency,%, (c)
Wake after sleep onset, min.
Fatigue
Fatigue scores (Table 3) suggested a high level of fatigue among the sample. Compared to healthy control children from a validation study [26], participants in this study scored lower (reported greater fatigue) by an average of 24 points (difference range 16.1-29.5 points lower) on fatigue subscales, both by child report and parent-proxy report. Children self-reported higher fatigue levels than did their parents on all subscales. However, this difference was significant only for sleep/rest fatigue (p=0.001), and showed a trend toward a difference for cognitive fatigue (p=0.094). Cronbach’s alpha for selfand parent-proxy reported fatigue measures in the present study were, respectively, 0.77 and 0.80 for general fatigue, 0.76 and 0.80 for sleep/rest fatigue, and 0.80 and 0.948 for cognitive fatigue.
Fatigue subscale
Parent report
Participant report
t-value
Mean (SD)
Mean (SD)
General fatigue
64.1 (22.6)
61.0 (19.0)
0.6
Cognitive fatigue
63.7 (28.0)
52.9 (22.4)
1.77a
Sleep/Rest fatigue
71.3 (19.0)
52.2 (22.7)
3.87b
ap=0.094, bp=0.001. Test of significant difference is paired t-test. Fatigue measured with the PedsQL™ Multidimensional Fatigue Scale.
Table 3: Fatigue ratings by parent-proxy and participant report, n=18.
Neurodevelopmental outcomes
The WRAT-4 was completed by 18 children and the BRIEF by 17 children. Mean scores on neurodevelopmental tests were within the normal range for all but the WRAT-4 math computation subscale (Table 4). Of the 16 participants who completed all six subscales, however, only one scored in the ‘average or above average’ range on all subscales. Ten participants scored below average on the WRAT- 4 math computation subscale, and eight were below average on the WRAT-4 word reading and WRAT-4 spelling subscales. Eight participants had elevated scores on the BRIEF behavioral regulation index and BRIEF global executive composite subscales, and six had elevated scores on the BRIEF metacognition index subscale.
Scales
Mean (SD)
Range
Outside of normal range, N (%)
WRAT-4 Word Reading (n=18)
90.8 (11.7)
73-117
8 (42.1)
WRAT-4 Spelling (n=18)
92.8 (12.7)
78-118
8 (42.1)
WRAT-4 Math Computation (n=18)
88.5 (13.5)
61-108
10 (56.2)
BRIEF Behavioral Regulation Index (n=17)
59.8 (17.6)
36-96
8 (42.1)
BRIEF Metacognition Index (n=17)
61.0 (13.7)
30-84
6 (31.6)
BRIEF Global Executive Composite (n=17)
61.4 (15.5)
32-93
8 (42.1)
BRIEF: Behavior Rating Inventory of Executive Function (elevated t-score = 65); WRAT-4: Wide Range Achievement Test–Version 4 (below average standardized score <90).
Table 4: Mean scores on cognitive and behavioral measures.
Relationships between sleep, fatigue and neurodevelopmental measures
No sleep measure was significantly correlated with any neurodevelopmental outcome. Fatigue and sleep measures showed a limited relationship, with two significant associations between parent-proxy sleep/rest fatigue and SE (rho= -0.634, p=0.006) and parent-proxy sleep/rest fatigue and FI (rho=0.532, p=0.028). However, several parent-proxy fatigue measures were significantly correlated with neurodevelopmental test scores (Table 5). Greater general fatigue was significantly associated with elevated (worse) BRIEF behavioral regulation index (p=0.031), metacognition index (p=0.002) and global executive composite (p=0.006) scores. Greater cognitive fatigue was significantly associated with lower (worse) WRAT-4 word reading (p=0.041), spelling (p=0.002) and math (p=0.010) scores; and with elevated BRIEF metacognition index (p=0.015) and global executive composite (p=0.028) scores. No selfreported fatigue score was associated with any neurodevelopmental subscale score.
PedsQL Multidimensional Fatigue Scale
WRAT-4 Word Reading
WRAT-4 Spelling
WRAT-4
BRIEF Behavioral Regulation Index
BRIEF Metacognition Index
BRIEF Global Executive Composite
Math Computation
Parent-proxy report
General Fatigue
0.041
0.24
-0.027
-0.539a
-0.705b
-0.656b
Sleep/Rest Fatigue
0.075
0.403
0.051
-0.375
-0.413
-0.371
Cognitive Fatigue
0.500a
0.691b
0.608b
-0.424
-0.594a
-0.548a
Participant report
General Fatigue
-0.173
0.217
-0.116
-0.099
0.101
-0.012
Sleep/Rest Fatigue
-0.295
-0.101
-0.424
0.1
0.186
0.148
Cognitive Fatigue
0.161
0.191
0.2
-0.06
-0.11
-0.14
ap<0.05, bp= 0.01. BRIEF: Behavioral Rating of Executive Function; WRAT-4: Wide Range Achievement Test–Version 4. Bolded values are significant correlations.
Table 5: Spearman correlations between fatigue and neurodevelopmental scores.
Discussion
Children with SCD are at increased risk for neurological complications, including overt and silent stroke, vaso-occlusive events and hypoxemia due to severe anemia [33,34]. These complications affect higher-level cognitive functions. Children with SCD also experience more sleep disturbances [12,35] and fatigue [26] than typically developing children. Sleep problems, including short duration and poor quality sleep, have been associated with lower academic achievement, cognition and behavioral performance in healthy children. Poor sleep is common in children with SCD, and is frequently associated with SCD-related sequelae. This was aptly demonstrated by our participant who developed a pain crisis during data collection, wherein his sleep was very fragmented prior to and during the onset of acute pain, and was no less influenced, albeit in the opposite direction, by administration of opioids to manage pain. However, sleep problems have been little studied with regard to their impact on neurodevelopmental functioning in SCD.
We identified evidence of disturbed sleep in a clinical sample of children and adolescents with SCD. This included shorter than recommended nocturnal sleep duration, and prolonged SOL and WASO, which resulted in low SE. Thus by all measures, sleep disturbances in our sample, compared to published samples of healthy children were severe, in agreement with the findings of other studies [12,13]. Contrary to our hypothesis and to research findings of children and adolescents in the general population, however, we found no significant relationships between sleep and neurodevelopmental measures. This is in contrast to the one study to date that has reported on the association between neurodevelopmental measures and sleep in children with SCD. In this study, Butt, et al. [36] found that children aged 6-12 years with a history of sleep problems, defined as trouble falling or staying asleep, restless sleep or prior surgery for sleep apnea, scored significantly lower on a test of working memory than children without sleep problems. They further found that children who had a history of stroke, pain and sleep problems performed 1.5 SD below children without these problems, although this trend was not significant.
Reasons for the relationship between short duration or poor quality sleep and neurodevelopmental functioning are just beginning to be understood, and several theories have been proposed. Among them is the hypothesis that sleep has a role in reactivating traces of neuronal activity patterns developed during wake, thereby promoting the encoding of learned information and consolidation of memory [37,38]. Inadequate sleep, then, would decrease retention and processing of information learned during the prior wake period. Another theory suggests that sleep might play a role in providing an environment conducive to the reprocessing of emotional experiences, and in resetting of the neuronal systems involved in the regulation of affect. In this case, inadequate sleep could lead to the development of dysfunctional behaviors [39]. Although no unifying theory has yet to emerge, there is growing evidence that sleep plays an active role in the neuronal processing of information learned during wakefulness and that inadequate sleep causes this process to become dysregulated [11].
In addition to sleep problems, we also found high fatigue levels in our sample. Compared to healthy control children from a validation study of the PedsQL™ Multidimensional Fatigue Scale [26], participants in our study reported greater fatigue by an average of 24 points on fatigue scores both by participant and parent-proxy report. Interestingly, compared to children’s self-reported fatigue, we found that parents underestimated fatigue in their children. As we did not study very young children, it is possible that our school-aged sample was largely beyond the need for close oversight of daily activities and that parents were not observing their children in situations where fatigue may be the greatest, for example in school or while playing with peers. It is also possible that in day-to-day living with a child having a chronic illness, bothersome symptoms like fatigue become the norm for their child and its impact is underrated by parents.
We did find several associations between fatigue and neurodevelopmental test scores. Greater parent-proxy reported cognitive fatigue was associated with lower WRAT-4 reading, spelling, and math scores. All BRIEF subscales were associated with at least some parent-proxy fatigue subscales, such that greater fatigue was associated with greater executive dysfunction. This is in agreement with a recent study in which fatigue in children with SCD was found to be associated with lower working memory, executive function and higher levels of internalizing symptoms [9]. The relationship between fatigue and neurodevelopmental functioning is unclear. In SCD, it could be related to elevated levels of pro-inflammatory markers caused by vascular endothelial disruption and vaso-occlusion, stress response due to pain, or decreases oxygen delivery to the tissues, all of which ultimately affect brain integrity and consequently cognition, while also increasing fatigue [40]. Fatigue has also been associated with disrupted sleep in children with SCD [10]. Although we found limited association between sleep and fatigue in this study, it is possible that inadequate or poor quality sleep contributes to greater fatigue, and that fatigue consequently impacts neurodevelopmental functioning. A larger sample is required to fully test this hypothesis.
Limitations and Future Directions
As this was a pilot study, the results of this study are exploratory and should be interpreted in light of several limitations. Our sample was small, and as such, we were limited in our ability to statistically control for the multiple factors likely to confound the relationship between sleep and neurodevelopmental functioning, such as pain, SCD type, neurological sequelae, socioeconomic status and fatigue. Nearly two-thirds of the sample was recruited through a clinic to which children with cognitive or behavioral difficulties were referred for testing. This was by design, as our interest was in determining whether, in children with SCD who had neurodevelopmental difficulties, there were associated sleep problems. Nevertheless, sampling biased the neurodevelopmental test results toward being more likely to below normal or abnormal than might be seen in the population of children with SCD. Our use of actigraphy as a measure of sleep may also have affected our results. Actigraphy is a sensitive measure of sleep continuity and quality, but has low specificity for wakefulness. Thus in our sample, which demonstrated a high degree of wakefulness, actigraphy may have overestimated sleep variables such as TST and napping and underestimated SOL, SE and WASO, potentially changing their relationship with neurodevelopmental outcomes. We studied a sample with a broad range of ages. Both sleep and neurodevelopmental functioning change across childhood, thus important age-related changes in these measures and their relationship may have been missed in the reporting of group means.
Studies in children without SCD have demonstrated domainspecific more frequently than global cognitive and behavioral deficits related to short duration or poor quality sleep. There may be different aspects of cognition and behavior that we did not test that might have shown different relationships to sleep and fatigue than what we found. Larger, age-specific studies using a variety of neurodevelopmental measures should be carried out to better evaluate the effects of sleep and fatigue on neurodevelopmental performance across the developmental trajectory of children with SCD, to determine whether sensitivities to decrements in sleep and fatigue match the neurodevelopmental profiles of typically developing children who experience similar decrements, and to determine which measures are the most robust in detecting domain-specific cognitive and behavioral differences.
Conclusion
In conclusion, neither sleep duration nor sleep quality was associated with any neurodevelopmental outcome. Parent-proxy reported general and cognitive fatigue scores measured with the PedsQL Multidimensional Fatigue Scale were associated with multiple subscales of the WRAT-4 and BRIEF–measures of academic achievement and executive functioning.
References
- Schnog JJ, Lard LR, Rojer RA, Van der Dijs FP, Muskiet FA, Duits AJ. New concepts in assessing sickle cell disease severity. Am J Hematol. 1998; 58: 61-66.
- Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: A 4-decade observational study of 1056 patients. Medicine. 2005; 84: 363-376.
- Kwiatkowski JL, Zimmerman RA, Pollock AN, Seto W, Smith-Whitley K, Shults J, et al. Silent infarcts in young children with sickle cell disease. Br J Haematol. 2009; 146: 300-305.
- Quinn CT, Shull EP, Ahmad N, Lee NJ, Rogers ZR, Buchanan GR. Prognostic significance of early vaso-occlusive complications in children with sickle cell anemia. Blood. 2007; 109: 40-45.
- Epping AS, Myrvik MP, Newby RF, Panepinto JA, Brandow AM, Scott JP. Academic attainment findings in children with sickle cell disease. J Sch Health. 2013; 83: 548-553.
- Ezenwosu O, Emodi I, Ikefuna A, Chukwu B. Academic performance and intelligence scores of primary school-aged children with sickle cell anemia. Pediatr Hematol Oncol. 2013; 30: 733-741.
- Hogan AM, Pit-ten Cate IM, Vargha-Khadem F, Prengler M, Kirkham FJ. Physiological correlates of intellectual function in children with sickle cell disease: Hypoxaemia, hyperaemia and brain infarction. Dev Sci. 2006; 9: 379-387.
- Fields ME, Hoyt-Drazen C, Abel R, Rodeghier MJ, Yarboi JM, Compas BE, et al. A pilot study of parent education intervention improves early childhood development among toddlers with sickle cell disease. Pediatr Blood Cancer. 2016; 63: 2131-2138.
- Anderson LM, Allen TM, Thornburg CD, Bonner MJ. Fatigue in children with sickle cell disease: Association with neurocognitive and social-emotional functioning and quality of life. J Pediatr Hematol Oncol. 2015; 37: 584-589.
- Ameringer S, Elswick RK, Jr Smith W. Fatigue in adolescents and young adults with sickle cell disease: Biological and behavioral correlates and health-related quality of life. J Pediatr Oncol Nurs. 2014; 31: 6-17.
- Astill RG, Van der Heijden KB, Van Ijzendoorn MH, Van Someren EJ. Sleep, cognition, and behavioral problems in school-age children: A century of research meta-analyzed. Psychol Bull. 2012; 138: 1109-1138.
- Daniel LC, Grant M, Kothare SV, Dampier C, Barakat LP. Sleep patterns in pediatric sickle cell disease. Pediatr Blood Cancer. 2010; 55: 501-507.
- Graves JK, Jacob E. Pain, coping, and sleep in children and adolescents with sickle cell disease. J Child Adol Psychiatr Nurs. 2014; 27: 109-120.
- Iampietro M, Giovannetti T, Tarazi R. Hypoxia and inflammation in children with sickle cell disease: Implications for hippocampal functioning and episodic memory. Neuropsychol Rev. 2014; 24: 252-265.
- King AA, Strouse JJ, Rodeghier MJ, Compas BE, Casella JF, McKinstry MJ, et al. Parent education and biologic factors influence on cognition in sickle cell anemia. Am J Hematol. 2014; 89: 162-167.
- Gaina A, Sekine M, Chen X, Hamanishi S, Kagamimori S. Sleep parameters recorded by actiwatch in elementary school children and junior high school adolescents. Schooldays vs. weekends. Sleep and Hypnosis. 2004; 6: 55-66.
- McLaughlin Crabtree V, Williams NA. Normal sleep in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2009; 18: 799-811.
- Paruthi S, Brooks LJ, D’Ambrosio C, Hall W, Kotagal S, Lloyd RM, et al. Recommended amount of sleep for pediatric populations: A consensus statement of the American Academy of Sleep Medicine. J Clin Sleep Med. 2016; 15: 785-786.
- Scholle S, Beyer U, Bernhard M, Eichholz S, Erler T, Graness P, et al. Normative values of polysomnographic parameters in childhood and adolescence: quantitative sleep parameters. Sleep Med. 2011; 12: 542-549.
- Meltzer LJ, Montgomery-Downs HE, Insana SP, Walsh CM. Use of actigraphy for assessment in pediatric sleep research. Sleep Med Rev. 2012; 16: 463- 475.
- Van de Water ATM, Holmes A, Hurley DA. Objective measurements of sleep for non-laboratory settings as alternatives to polysomnography–a systematic review. J Sleep Res. 2011; 20: 183-200.
- Acebo C, Sadeh A, Seifer R, Tzischinsky O, Wolfson AR, Hafer A, et al. Estimating sleep patterns with activity monitoring in children and adolescents: How many nights are necessary for reliable measures? Sleep. 1999; 22: 95- 103.
- Littner M, Kushida CA, Anderson WM, Bailey D, Berry RB, Davila DG, et al. Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: an update for 2002. Sleep. 2003; 26: 337-341.
- Varni JW, Seid M, Kurtin PS. PedsQL 4.0: Reliability and validity of the Pediatric Quality of Life Inventory Version 4.0 generic core scales in health and patient populations. Med Care. 2001; 39: 800-812.
- Dampier C, Lieff S, LeBeau P, Rhee S, McMurray M, Rogers Z, et al. Healthrelated quality of life in children with sickle cell disease: A report from the Comprehensive Sickle Cell Centers Clinical Trial Consortium. Pediatr Blood Cancer. 2010; 55: 485-494.
- Panepinto JA, Torres S, Bendo CB, McCavit TL, Dinu B, Sherman-Bien S, et al. PedsQLTM Multidimensional Fatigue Scale in sickle cell disease: Feasibility, reliability, and validity. Pediatr Blood Cancer. 2014; 61: 171-177.
- Nunnally JC, Bernstein IR. Psychometric testing. New York: McGraw-Hill; 1994.
- Wilkinson GS, Robertson GJ. Wide Range Achievement Test 4 (WRAT4). Lutz, FL: Psychological Assessment Resources, Inc. 2005.
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior rating inventory of executive function. Child Neuropsychol. 2000; 6: 235-238.
- Isquith PK, Gioia GA, PAR Staff. Behavior Rating Inventory of Executive Function. BRIEF Interpretive Report. Lutz, FL. 2008.
- Hirschkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, et al. The National Sleep Foundation’s sleep time duration recommendations: Methodology and results summary. Sleep Health. 2015; 1: 40-43.
- O’Driscoll DM, Foster AM, Davey MJ, Nixon GM, Horne RS. Can actigraphy measure sleep fragmentation in children? Arch Dis Child. 2010; 95: 1031- 1033.
- DeBaun MR, Sarnaik SA, Rodeghier MJ, Minniti CP, Howard TH, Iyer RV, et al. Associated risk factors for silent cerebral infarcts in sickle cell anemia: Low baseline hemoglobin, sex, and relative high systolic blood pressure. Blood. 2012; 119: 3684-3690.
- Routhieaux J, Sarcone S, Stegenga K. Neurocognitive sequelae of sickle cell disease: Current issues and future directions. J Pediatr Oncol Nurs. 2005; 22: 160-167.
- Rosen CL, Debaun MR, Strunk RC, Redline S, Seicean S, Craven DI, et al. Obstructive sleep apnea and sickle cell anemia. Pediatrics. 2014; 134: 273-281.
- Butt SM, Goldstein AL, Goldman ML, Alvarez O, Armstrong FD. Sleep problems in pediatric sickle cell disease and the impact on cognition. Am J Hematol. 2011; 86: 42.
- Sutherland GR, McNaughton B. Memory trace reactivation in hippocampal and neocortical neuronal ensembles. Curr Opin Neurobiol. 2000; 10: 180- 186.
- Hoffman KL, McNaughton BL. Coordinated reactivation of distributed memory traces in primate neocortex. Science. 2002; 297: 2070-2073.
- Eisenberg N, Cumberland A, Spinrad TL, Fabes RA, Shepard SA, Reiser M, et al. The relations of regulation and emotionality to children’s externalizing and internalizing problem behavior. Child Dev. 2001; 72: 1112-1134.
- Ameringer S, Smith WR. Emerging biobehavioral factors of fatigue in sickle cell disease. J Nurs Scholarsh. 2011; 43: 22-29.