
Review Article
Austin Pediatr. 2019; 6(1): 1067.
High Fat Diet and Childhood Diseases Maternal High Fat Diets and Offspring Diseases
Song B1,2, Zheng C1,2, Zhong Y1,2, Yan Z1, Duan Y1* and Deng J2
1Laboratory of Animal Nutritional Physiology and Metabolic Process, Institute of Subtropical Agriculture Chinese Academy of Sciences; Key Laboratory of Agroecological Processes in Subtropical Region; Hunan Provincial Engineering Research Center for Healthy Livestock and Poultry Production; Scientific Observing and Experimental Station of Animal Nutrition and Feed Science in South-Central, Ministry of Agriculture, China
2Guangdong Provincial Key Laboratory of Animal Nutrition Regulation, South China Agricultural University, Guangzhou, China
*Corresponding author: Yehui Duan, Laboratory of Animal Nutritional Physiology and Metabolic Process, Institute of Subtropical Agriculture Chinese Academy of Sciences; Key Laboratory of Agro-ecological Processes in Subtropical Region; Hunan Provincial Engineering Research Center for Healthy Livestock and Poultry Production; Scientific Observing and Experimental Station of Animal Nutrition and Feed Science in South- Central, Ministry of Agriculture, Changsha China Jinping Deng, Guangdong Provincial Key Laboratory of Animal Nutrition Regulation, South China Agricultural University, Guangzhou, China
Received: March 13, 2019; Accepted: April 18, 2019; Published: April 25, 2019
Abstract
Maternal High Fat Diets (HFDs) together with obesity represents a special problem that can lead to poor fetal development, resulting in harmful, persistent effects on offspring, including predisposition on obesity and its associated metabolic disorders as well as certain types of cancer. However, the mechanisms underpinning these programming effects induced by maternal HFDs and/or obesity remain poorly defined. Given the increasing number of obese women entering pregnancy and the current obesity epidemic, there is an urgent need to gain more insights into possible underlying mechanisms and to develop effective therapeutic strategies.
Keywords: Maternal high fat diets; Offspring disease; Mechanisms
Introduction
Obesity has become a significant threat for global public health, predisposing individuals to diseases such as type 2 Diabetes Mellitus (T2DM), Cardiovascular Disease (CVD), and certain types of cancer [1,2]. Surprisingly, the proportion of obese women of reproductive age is as high as 34% [3]. Increasing evidence from animal models have suggested that consumption of High Fat Diets (HFDs) during pregnancy exposes the fetus to an inflammatory environment during development. This inflammatory environment has long-term consequences for offspring, predisposing or “programming” them to the development of metabolic disorders in adulthood independent of adult environmental factors [4-6]. In this context, a comprehensive understanding of the pathogenesis of maternal HFD-driven metabolic disorders may help to reduce the disease burden worldwide. This review will elaborate on the precise pathology and etiology of metabolic disorders induced by maternal HFDs (Figure 1), and will provide a summary of potential treatments to manage these diseases and cancer.
Developmental programming by maternal HFDs
It is now recognized that maternal HFD consumption (even without maternal obesity) affects adversely fetal development, which has long-term adverse outcomes for the offspring health in later life even independent of postnatal nutrition [7]. In this section, we will discuss how maternal HFD-induced obesity or maternal HFD per se affects the health of progeny. The ultimate aim is to identify potential targets, which may be amenable to prevention or early intervention in order to improve the health of this and future generations.
Obesity, insulin resistance, and diabetes
Maternal consumption of HFDs or maternal obesity is associated with the development of offspring adiposity and insulin resistance. Indeed, pups (babies) born to mothers with diabetes or consuming HFD during pregnancy and lactation are at increased risk of glucose intolerance and diabetes in adult life [8,9]. Further evidence to support this effect of maternal obesity comes from recent studies, in which increased fat mass was observed in adult wild-type progeny of obese and insulin resistant heterozygous leptin receptor-deficient mice, thus leading to the development of offspring adiposity [10,11]. Nonobese rats fed a HFD during pregnancy and suckling induced increased body fat mass and insulin resistance in the offspring, supporting the effect of maternal HFD per se [12-14]. Moreover, evidence from rodent models suggests that maternal HFD promotes the onset of T2DM in offspring [15].
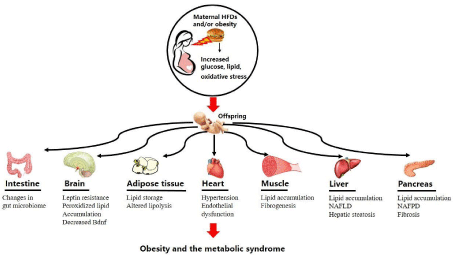
Figure 1: Basic schematic outlining consequences of maternal high fat diets on the health and well-being of offspring.
Several mechanistic pathways, including alterations in the offspring muscle, may effectively impair glucose metabolism and result in the development of insulin resistance and diabetes in the offspring once they reach adulthood. Skeletal muscle, the principal site responsible for insulin-stimulated utilization of glucose and Fatty Acids (FAs), is affected by maternal obesity. Fetal development of skeletal muscle implicates myogenesis, adipogenesis, and fibrogenesis, all of which are derived from Mesenchymal Stem Cells (MSCs) [16]. An increasing body of evidence demonstrated that in response to maternal obesity, fetal skeletal muscle exhibited increased intramuscular fat and reduced myogenesis, along with altered AMP-activated Protein Kinase (AMPK) signaling and increased expression of inflammatory markers [17-19]. In addition, fetal skeletal muscle exhibited increased collagen content in response to maternal obesity, indicating increased fibrogenesis in fetal muscle [20]. All of these alterations in fetal skeletal muscle suggest that MSCs commitment shifts from myogenesis to adipogenesis and fibrogenesis, thus impairing skeletal muscle physiological functions, such as reduction in oxidative capacity [21] and muscle force [22]. Notably, this shift of MSCs commitment may be mediated by maternal obesity-induced chronic inflammation through three major mechanisms: down regulation of wingless and int (WNT)/β-catenin, inhibition of AMPK signaling, and induction of epigenetic modifications [16,18]. As a result, elevated intramuscular fat and inflammatory signaling in offspring muscle may correlate with increases in adipogenesis and insulin resistance, predisposing offspring to later-life obesity and diabetes [19].
Intestinal diseases
Numerous evidence has linked maternal HFD consumption to intestinal development and health in offspring [23]. Maternal HFDs (60% energy from fat) for 8 weeks can elicit many structural and functional adaptations in the intestine of offspring, including increased intestinal permeability and gut inflammation as well as decreased villus to crypt ratio and goblet cells density in the ileum [24]. These alterations increase gene expression of proinflammatory cytokines in offspring intestine and may translate into increased susceptibility to Inflammatory Bowel Diseases (IBD) and other associated diseases in offspring. One recent study showed that maternal and postnatal HFDs accelerated the onset of ileitis in the distal ileum of offspring TNF?ARE/WT mice, a genetically susceptible model for CD-like ileitis [25]. Similarly, using a dextran sulfate sodium-induced colitis mouse model, previous studies found that maternal consumption of HFDs (60% energy from fat) during gestation and lactation predisposes female offspring to a higher susceptibility to develop IBD and related inflammatory gut diseases [23]. The increased gut inflammation and colitis may be associated with elevated production of proinflammatory cytokines such as interleukin (IL)-1β, IL-6, and IL-17, reduced AMPK signaling, and amplified NF-κB signaling cascade in the offspring [23].
Although conflicting, the underlying mechanism for the impacts of maternal intake of a HFD on the intestinal development and function in offspring may involve the TGF-β signaling pathway and Jak-STAT signaling pathway [26]. Another possible mechanism may be perturbation of gut microbiome induced by a maternal HFD in gestation [27]. In response to a maternal HFD, shifts in gut microbial composition of the mother can be transferred to the offspring and influence its gut microbiome [27-29]. Moreover, alterations in the bacterial composition of offspring are associated with the maternal consumption of nutrients, especially a HFD, independent of maternal body mass [27,30-33]. Along this line, maternal HFD per se but not maternal obesity can restructure the offspring’s intestinal microbiome, which in turn influences intestinal maintenance of metabolic health [34]. Further investigations are warranted to confirm this hypothesis.
Cardiovascular disease
There is compelling evidence showing that a maternal HFD during pregnancy and/or suckling is related to increased risk of CVD in the offspring. For instance, in a study of 37,709 subjects, maternal obesity was related to elevated mortality from cardiovascular events in adult offspring [35]. Similar observations were made in animal studies where using an integrated approach of determining both cardiac structure and molecular markers of cardiac hypertrophy, cardiac hypertrophy and dysfunction was observed in the offspring, even in the absence of any change in its body weight and adiposity at 12 weeks of age [36]. In addition, other studies reported that increased systolic blood pressure and endothelial dysfunction were observed in offsprings, who were exposed to maternal pre-pregnancy obesity/overweight [12,37-39]. Although poorly defined, mechanisms implicated in the programming of offspring CVD may include increased inflammation, oxidative stress, lipotoxicity, and epigenetics [40]. In support, elevated oxidative stress was observed in the offspring heart due to maternal obesity, accompanied by activation of p38 and JNK and downregulation of cardioprotective AMPK expression [41]. The combination of oxidative stress and inflammation may have an additive effect. Studies have shown that inflammation is a risk factor for cardiac fibrosis in fetal sheep offspring [20]. The roles of lipotoxicity and epigenetics in developing offspring CVD have also been described in numerous animal models. Interested readers can refer to [42-44] for further reading.
Liver disease
Non-Alcoholic Fatty Liver Disease (NAFLD) is regarded as the commonest cause of chronic liver disease. Developmental programming has been shown to be implicated in the pathogenesis of NAFLD and chronic liver disease. In support, after 3 months of exposure to maternal obesity and a postnatal obesogenic diet, offspring exhibited increased adiposity, hepatic Triglyceride (TG) content and upregulation of tumor necrosis factor (TNF)-α, IL-6, and alpha smooth muscle actin, indicative of liver injury and fibrosis [45]. Upon further investigation, the same study found a more-profound evidence of hepatosteatosis and a more-robust NAFLD phenotype with hepatic fibrosis at 12 months [45]. Intriguingly, offspring, which were born to lean dams but were suckled by obese dams, exhibited an exaggerated NAFLD phenotype, accompanied by elevated body weight, elevated concentrations of insulin, leptin, aspartate transaminase, IL-6, TNF-α, liver TGs, steatosis, hepatic fibrogenesis, renal norepinephrine, and liver α1-D plus β1-adrenoceptors, indicative of activation of Sympathetic Nervous System (SNS) [46]. SNS activation promotes fibrosis progression via the actions of norepinephrine [47]. These data suggest that exposure to maternal obesity during pregnancy and lactation programs development of a NAFLD phenotype. The mechanisms may involve alterations of hypothalamic appetite nuclei signaling by neonatal adipose tissue derived leptin and maternal breast milk [46], and disturbance of the hepatic innate immune system with increased Kupffer cell numbers and reduced NKT cell populations [45].
Brain health and function
Maternal nutritional status during gestation exerts crucial roles in modulating fetal brain formation and development, with long-lasting consequences on its function, such as memory, learning, and brain senescence. When pregnant rats were exposed to a HFD beginning with gestational day 5, their fetuses displayed enhanced proliferation of neural progenitors (indicative of decreased neurogenesis) within the hypothalamus at embryonic day 14 [48]. In agreement, other lines of evidence demonstrated that long-lasting (until postnatal day P70) decreased neurogenesis in the dentate gyrus was observed in pups from mothers fed a HFD (57.5% fat with mainly lard) for 6 weeks prior to and during gestation as well as during lactation [49]. Neonatal brain development may also be affected in suckling pups exposed to a maternal HFD [50]. In addition, maternal exposure to a HFD (60% calories from fat) for 10 weeks prior to and during gestation altered fetal hippocampal development at embryonic day 17, as shown by region-specific alterations in proliferation of neural precursors, reduced apoptosis, and by reduced neuronal differentiation within the dentate gyrus [51]. Altogether, maternal exposure to a HFD can affect prenatal and postnatal brain development.
Several mechanisms may be responsible for adverse effects of a maternal HFD on brain. Peroxidized lipid accumulation in the dentate gyrus may be associated with alterations in hippocampal neurogenesis [49]. Decreased levels of brain-derived neurotrophic factor in the cortex and hippocampus may be related to reduced hippocampal spatial learning performance and to alterations in discrimination reversal [52-54]. Another hypothesized mechanism implicated in the dietary modulation of hippocampal development is associated with the leptin receptor [55], which plays a key role in facilitating memory and learning [56]. The expression of leptin receptor in the hypothalamus and liver is reduced in response to obesity [57].
Pancreatic cancer
The prevalence of pancreatic cancer in developed countries is rising at an alarming rate and may parallel rising rates of obesity and dysmetabolism [58]. Intriguingly, the phenomenal rises in the rates of pancreatic cancer may be attributed to transgenerational amplification of obesity through epigenetic mechanisms [59], a possible link being Non-Alcoholic Fatty Pancreas Disease (NAFPD) [60]. In parallel with increases in body weight, TG content in pancreas tissue was dramatically elevated in offspring of maternal obesity. Meanwhile, pancreatic expression of fibrogenic markers TGF-β and collagen type 1-α2 genes was greatly upregulated in offspring of maternal obesity, accompanied by increases in the response of night-time systolic blood pressure and systolic blood pressure to restraint. Therefore, a fatty pancreas with induced fibrogenesis developed in offspring when exposed to an obesogenic environment, indicating a dysmetabolic and NAFPD phenotype [60]. Therefore, maternal obesity-induced pancreatic cancer in offspring may result from pancreatic fat accumulation and fibrosis in offspring.
Breast cancer
Exposure (in utero and lactation) to maternal HFD predisposed female progeny to elevated risk for breast cancer [61]. Previous studies found that maternal consumption of HFD (45% kcal from fat), beginning at weaning (postnatal day 21) and maintained on the same diet 12 weeks prior to mating and throughout pregnancy and lactation, alters mother’s metabolism and systemic oxidative status, leading to systemic alterations in female offspring in the absence of dramatic weight gains. On one hand, maternal HFD elevated IL-6 levels and oxidative stress status, both of which could directly contribute to the development of breast cancer. In addition, maternal HFD promoted hyperinsulinemia and suppression of PTEN (a tumor suppressor) expression/function, both of which increased breast cancer risk via upregulation of Insulin Receptor Substrate-1 (IRS-1) expression [61]. Other studies also demonstrated increased risk for breast cancer in the offspring of dams-fed HFD [62]. These data have important implications for developing novel strategies for the prevention of maternal HFD-induced breast cancer.
Potential treatment options
Given the close relationship between maternal HFDs and the offspring health, it is of great importance for obese mothers to eat balanced meals and to reduce their body weight. In this regard, diet reversal from HFDs to control diets during pregnancy resulted in improvements in fetal hepatic triglycerides, normalization of the melanocortin levels, and partial normalization of the expression of gluconeogenic enzymes [43]. Similar results were obtained in male offspring of obese rats, in which dietary intervention prior to pregnancy reversed metabolic programming of offspring [63]. In addition, interventions with exendin-4, folic acid, and leptin in the early phases of developmental plasticity have been reported to alleviate or reverse some of the effects related to developmental programming [64-66]. In addition, exercise exerts beneficial effects in obesity-prone offspring of undernourished mothers [67,68]. In support, children had reduced birth weight and exhibited improved metabolic profiles with greater insulin sensitivity and improved lipid profile when their mothers reduced their body weight (36 ± 1.8%) [69].
Conclusions and Perspectives for Future Studies
Overall, many animal and human studies have indicated that maternal HFDs and/or obesity negatively affect offspring health, which has profound implications for public health policy. Based upon evidence to date, we suggest that intake of SFA should be avoided, and clear guidelines for fat intake (just like trace elements iron and folic acid) should be established for mothers in pregnancy, thus improving body health status. However, lots of questions remained to be addressed. For instance, when is the appropriate time for obese woman to lose weight when planning pregnancy, and how should they manage their weight when pregnant? In addition, further studies are urgently warranted to identify appropriate interventions to reduce the risks of these complications in the offspring.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31802077) and the science and technology project of Guangxi Province (2018JJB130239).
References
- Stevens GA, Singh GM, Lu Y, Danaei G, Lin JK, Finucane MM, et al. Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating G: National, regional, and global trends in adult overweight and obesity prevalences. Popul Health Metr. 2012; 10: 22.
- Calle EE, Kaaks R. Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004; 4: 579-591.
- Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among us adults, 1999-2010. Jama-J Am Med Assoc. 2012; 307: 491-497.
- Alfaradhi MZ, Ozanne SE. Developmental programming in response to maternal overnutrition. Front Genet. 2011; 2: 27.
- Madan JC, Davis JM, Craig WY, Collins M, Allan W, Quinn R, et al. Maternal obesity and markers of inflammation in pregnancy. Cytokine. 2009; 47: 61-64.
- Chechi K, Cheema SK. Maternal diet rich in saturated fats has deleterious effects on plasma lipids of mice. Experimental & Clinical Cardiology. 2006; 11: 129-135.
- Ashino NG, Saito KN, Souza FD, Nakutz FS, Roman EA, Velloso LA, et al. Maternal high-fat feeding through pregnancy and lactation predisposes mouse offspring to molecular insulin resistance and fatty liver. J Nutr Biochem. 2012; 23: 341-348.
- Srinivasan M, Katewa SD, Palaniyappan A, Pandya JD, Patel MS. Maternal high-fat diet consumption results in fetal malprogramming predisposing to the onset of metabolic syndrome-like phenotype in adulthood. Am J Physiol. 2006; 291: 792-799.
- Gunderson EP. Breastfeeding after gestational diabetes pregnancy: subsequent obesity and type 2 diabetes in women and their offspring. Diabetes Care. 2007; 30: S161-S168.
- Lambin S, van Bree R, Caluwaerts S, Vercruysse L, Vergote I, Verhaeghe J. Adipose tissue in offspring of Lepr(db/+) mice: early-life environment vs. genotype. Am J Physiol Endocrinol Metab. 2007; 292: 262-271.
- Yamashita H, Shao J, Qiao L, Pagliassotti M, Friedman JE. Effect of spontaneous gestational diabetes on fetal and postnatal hepatic insulin resistance in Lepr(db/+) mice. Pediatr Res. 2003; 53: 411-418.
- Khan IY, Dekou V, Douglas G, Jensen R, Hanson MA, Poston L, et al. A high-fat diet during rat pregnancy or suckling induces cardiovascular dysfunction in adult offspring. Am J Physio-Reg I. 2005; 288: R127-R133.
- Gregersen S, Dyrskog SEU, Storlien LH, Hermansen K. Comparison of a high saturated fat diet with a high carbohydrate diet during pregnancy and lactation: effects on insulin sensitivity in offspring of rats. Metabolism. 2005; 54: 1316-1322.
- Taylor PD, McConnell J, Khan IY, Holemans K, Lawrence KM, Asare-Anane H, et al. Impaired glucose homeostasis and mitochondrial abnormalities in offspring of rats fed a fat-rich diet in pregnancy. Am J Physio-Reg I. 2005; 288: R134-R139.
- Ohta T, Toriniwa Y, Ryumon N, Inaba N, Hirao T, Yamanaka S, et al. Maternal high-fat diet promotes onset of diabetes in rat offspring. Anim Sci J. 2017; 88: 149-155.
- Du M, Yan X, Tong JF, Zhao JX, Zhu MJ. Maternal obesity, inflammation, and fetal skeletal muscle development. Biol Reprod. 2010; 82: 4-12.
- Zhu MJ, Han B, Tong J, Ma C, Kimzey JM, Underwood KR, et al. AMP-activated protein kinase signalling pathways are down regulated and skeletal muscle development impaired in fetuses of obese, over-nourished sheep. J Physiol-London. 2008; 586: 2651-2664.
- Tong JF, Yan X, Zhu MJ, Ford SP, Nathanielsz PW, Du M. Maternal obesity downregulates myogenesis and beta-catenin signaling in fetal skeletal muscle. Am J Physiol Endocrinol Metab. 2009; 296: E917-E924.
- Yan X, Zhu MJ, Xu W, Tong JF, Ford SP, Nathanielsz PW, et al. Up-regulation of Toll-like receptor 4/nuclear factor-kappaB signaling is associated with enhanced adipogenesis and insulin resistance in fetal skeletal muscle of obese sheep at late gestation. Endocrinology. 2010; 151: 380-387.
- Huang Y, Yan X, Zhao JX, Zhu MJ, McCormick RJ, Ford SP, et al. Maternal obesity induces fibrosis in fetal myocardium of sheep. Am J Physiol Endocrinol Metab. 2010; 299: E968-E975.
- Zhu MJ, Ford SP, Means WJ, Hess BW, Nathanielsz PW, Du M. Maternal nutrient restriction affects properties of skeletal muscle in offspring. J Physiol-London. 2006; 575: 241-250.
- Bayol SA, Macharia R, Farrington SJ, Simbi BH, Stickland NC. Evidence that a maternal "junk food" diet during pregnancy and lactation can reduce muscle force in offspring. Eur J Nutr. 2009; 48: 62-65.
- Bibi S, Kang Y, Du M, Zhu MJ. Maternal high-fat diet consumption enhances offspring susceptibility to DSS-induced colitis in mice. Obesity (Silver Spring). 2017; 25: 901-908.
- Xue Y, Wang H, Du M, Zhu MJ. Maternal obesity induces gut inflammation and impairs gut epithelial barrier function in nonobese diabetic mice. J Nutr Biochem. 2014; 25: 758-764.
- Gruber L, Hemmerling J, Schuppel V, Muller M, Boekschoten MV, Haller D. Maternal high-fat diet accelerates development of crohn's disease-like Ileitis in TNF Delta ARE/WT offspring. Inflamm bowel dis. 2015; 21: 2016-2025.
- Che L, Liu P, Yang Z, Che L, Hu L, Qin L, et al. Maternal high fat intake affects the development and transcriptional profile of fetal intestine in late gestation using pig model. Lipids Health Dis. 2016; 15: 90.
- Chu DM, Antony KM, Ma J, Prince AL, Showalter L, Moller M, et al. The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med. 2016; 8: 77.
- Galley JD, Bailey M, Dush CK, Schoppe-Sullivan S, Christian LM. Maternal obesity is associated with alterations in the gut microbiome in toddlers. Plos One. 2014; 9: e113026.
- Gohir W, Ratcliffe EM, Sloboda DM. Of the bugs that shape us: maternal obesity, the gut microbiome, and long-term disease risk. Pediatr Res. 2015; 77: 196-204.
- Gruber L, Kisling S, Lichti P, Martin FP, May S, Klingenspor M, et al. High fat diet accelerates pathogenesis of murine crohn's disease-like ileitis independently of obesity. Plos One. 2013; 8: e71661.
- Ding S, Chi MM, Scull BP, Rigby R, Schwerbrock NM, Magness S, et al. High-fat diet. bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS One. 2010; 5: e12191.
- Schulz MD, Atay C, Heringer J, Romrig FK, Schwitalla S, Aydin B, et al. High-fat-diet-mediated dysbiosis promotes intestinal carcinogenesis independently of obesity. Nature. 2014; 514: 508-512.
- Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen YY, et al. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009; 137: 1716-1724.
- Ma J, Prince AL, Bader D, Hu M, Ganu R, Baquero K, et al. High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nat Commun. 2014; 5: 3889.
- Reynolds RM, Allan KM, Raja EA, Bhattacharya S, McNeill G, Hannaford PC, et al. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: follow-up of 1 323 275 person years. Brit Med J. 2013; 347: f4539.
- Blackmore HL, Niu YG, Fernandez-Twinn DS, Tarry-Adkins JL, Giussani DA, Ozanne SE. Maternal diet-induced obesity programs cardiovascular dysfunction in adult male mouse offspring independent of current body weight. Endocrinology. 2014; 155: 3970-3980.
- Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EHJM, et al. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance - A novel murine model of developmental programming. Hypertension. 2008; 51: 383-392.
- Liang C, Oest ME, Prater MR. Intrauterine exposure to high saturated fat diet elevates risk of adult-onset chronic diseases in C57BL/6 mice. Birth Defects Res B Dev Reprod Toxicol. 2009; 86: 377-384.
- Wen X, Triche EW, Hogan JW, Shenassa ED, Buka SL. Prenatal factors for childhood blood pressure mediated by intrauterine and/or childhood growth? Pediatrics. 2011; 127: e713.
- Blackmore HL, Ozanne SE. Maternal diet-induced obesity and offspring cardiovascular health. J Dev Orig Health Dis. 2013; 4: 338-347.
- Wang JY, Ma H, Tong C, Zhang HY, Lawlis GB, Li YD, et al. Overnutrition and maternal obesity in sheep pregnancy alter the JNK-IRS-1 signaling cascades and cardiac function in the fetal heart. FASEB J. 2010; 24: 2066-2076.
- Jarvie E, Hauguel-de-Mouzon S, Nelson SM, Sattar N, Catalano PM, Freeman DJ. Lipotoxicity in obese pregnancy and its potential role in adverse pregnancy outcome and obesity in the offspring. Clin Sci. 2010; 119: 123-129.
- McCurdy CE, Bishop JM, Williams SM, Grayson BE, Smith MS, Friedman JE, et al. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest. 2009; 119: 323-335.
- Gluckman PD, Hanson MA, Buklijas T, Low FM, Beedle AS. Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nat Rev Endocrinol. 2009; 5: 401-408.
- Mouralidarane A, Soeda J, Visconti-Pugmire C, Samuelsson AM, Pombo J, Maragkoudaki X, et al. Maternal obesity programs offspring nonalcoholic fatty liver disease by innate immune dysfunction in mice. Hepatology. 2013; 58: 128-138.
- Oben JA, Mouralidarane A, Samuelsson AM, Matthews PJ, Morgan ML, McKee C, et al. Maternal obesity during pregnancy and lactation programs the development of offspring non-alcoholic fatty liver disease in mice. J Hepatol. 2010; 52: 913-920.
- Oben JA, Roskams T, Yang S, Lin H, Sinelli N, Torbenson M, et al. Hepatic fibrogenesis requires sympathetic neurotransmitters. Gut. 2004; 53: 438-445.
- Chang GQ, Gaysinskaya V, Karatayev O, Leibowitz SF. Maternal high-fat diet and fetal programming: Increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. J Neurosci. 2008; 28: 12107-12119.
- Tozuka Y, Wada E, Wada K. Diet-induced obesity in female mice leads to peroxidized lipid accumulations and impairment of hippocampal neurogenesis during the early life of their offspring. FASEB J. 2009; 23: 1920-1934.
- Walker CD, Naef L, d'Asti E, Long H, Xu ZF, Moreau A, et al. Perinatal maternal fat intake affects metabolism and hippocampal function in the offspring a potential role for leptin. Ann NY Acad Sci. 2010; 1144: 189-202.
- Niculescu MD, Lupu DS. High fat diet-induced maternal obesity alters fetal hippocampal development. Int J Dev Neurosci. 2009; 27: 627-633.
- Yu Y, Wang Q, Huang XF. Energy-restricted pair-feeding normalizes low levels of brain-derived neurotrophic factor/tyrosine kinase b mrna expression in the hippocampus, but not ventromedial hypothalamic nucleus, in diet-induced obese mice. Neuroscience. 2009; 160: 295-306.
- Molteni R, Barnard RJ, Ying Z, Roberts CK, Gomez-Pinilla F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 2002; 112: 803-814.
- Kanoski SE, Meisel RL, Mullins AJ, Davidson TL. The effects of energy-rich diets on discrimination reversal learning and on BDNF in the hippocampus and prefrontal cortex of the rat. Behav Brain Res. 2007; 182: 57-66.
- Louis GW, Myers MG. The role of leptin in the regulation of neuroendocrine function and CNS development. Rev Endocr Metab Dis. 2007; 8: 85-94.
- Oomura Y, Hori N, Shiraishi T, Fukunaga K, Takeda H, Tsuji M, et al. Leptin facilitates learning and memory performance and enhances hippocampal CA1 long-term potentiation and CaMK II phosphorylation in rats. Peptides. 2006; 27: 2738-2749.
- Liu ZJ, Bian J, Liu J, Endoh A. Obesity reduced the gene expressions of leptin receptors in hypothalamus and liver. Home Metab Res. 2007; 39: 489-494.
- Gumbs AA, Bessler M, Milone L, Schrope B, Chabot J. Contribution of obesity to pancreatic carcinogenesis. Surg Obes Relat Dis. 2008; 4: 186-193.
- Yogev Y, Catalano PM. Pregnancy and obesity. Obstet Gyn Clin N Am. 2009; 36: 285-300.
- Oben JA, Patel T, Mouralidarane A, Samuelsson AM, Matthews P, Pombo J, et al. Maternal obesity programmes offspring development of non-alcoholic fatty pancreas disease. Biochem Biophys Res Commun. 2010; 394: 24-28.
- Montales MTE, Melnyk SB, Simmen FA, Simmen RCM: Maternal metabolic perturbations elicited by high-fat diet promote Wnt-1-induced mammary tumor risk in adult female offspring via long-term effects on mammary and systemic phenotypes. Carcinogenesis. 2014; 35: 2102-2112.
- Hilakivi-Clarke L. Mechanisms by which high maternal fat intake during pregnancy increases breast cancer risk in female rodent offspring. Breast Cancer Res Tr. 1997; 46: 199-214.
- Zambrano E, Martinez-Samayoa PM, Rodriguez-Gonzalez GL, Nathanielsz PW. Dietary intervention prior to pregnancy reverses metabolic programming in male offspring of obese rats. J Physiol-London. 2010; 588: 1791-1799.
- Stoffers DA, Desai BM, DeLeon DD, Simmons RA. Neonatal exendin-4 prevents the development of diabetes in the intrauterine growth retarded rat. Diabetes. 2003; 52: 734-740.
- Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr. 2005; 135: 1382-1386.
- Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, et al. Neonatal leptin treatment reverses developmental programming. Endocrinology. 2005; 146: 4211-4216.
- Miles JL, Huber K, Thompson NM, Davison M, Breier BH. Moderate daily exercise activates metabolic flexibility to prevent prenatally induced obesity. Endocrinology. 2009; 150: 179-186.
- Miles JL, Landon J, Davison M, Krageloh CU, Thompson NM, Triggs CM, et al. Prenatally undernourished rats show increased preference for wheel running v. lever pressing for food in a choice task. Br J Nutr. 2009; 101: 902-908.
- Smith J, Cianflone K, Biron S, Hould FS, Lebel S, Marceau S, et al. Effects of maternal surgical weight loss in mothers on intergenerational transmission of obesity. J Clin Endocrinol Metab. 2009; 94: 4275-4283.