Abstract
Changes of interleukin levels have been associated with advanced stages of several cancer types. Increased levels of cytokines induce tumorigenesis, being a poor prognostic marker for malignant disease. Chronic inflammation is a pathological feature of cancer and interleukin-17 (IL-17A) is an inflammatory cytokine with diverse functions in host defense. This study aimed to clarify the direct role of IL-17 on tumor cells. Several human tumors cells from glioma, bladder and esophagus were first analyzed for IL-17 receptor (IL-17RA) expression through qRT-PCR. The cells were then treated with different concentrations of IL-17, ranging from 10 pg/mL to 100 ng/mL, and evaluated for IL-17 ability to modify tumor cell proliferation and viability through cell counting and MTT assay. Cell migration capacity and intracellular signaling pathways activated by IL-17 were assessed by wound healing assay and flow cytometry, respectively. IL-17RA mRNA expression was detected on the cell lines tested, but the incubation of IL-17 did not alter tumor cell viability or proliferation. However, the incubation of IL-17 was able to promote tumor cell migration and AKT, ERK and P38 phosphorylation in vitro. Our data show, for the first time, the ability of IL-17RA/IL-17 to induce migration of human tumor cell lines in vitro, possibly via activation of MAP kinases and AKT.
Keywords: IL-17A; IL-17RA; Glioma; Bladder tumor; Esophageal tumor; Cell migration
Introduction
Interleukin-17 (IL-17A) is classified as a pro-inflammatory cytokine and it is shown to be elevated in several types of cancer [1]. Data regarding the role of IL-17 on tumor development are not consensual. It is important to highlight that exogenously delivered IL-17 might display different effects in comparison to endogenous IL- 17 [2]. Recently, researches on this field point out the idea that IL-17 promotes tumor growth, especially through the activation of IL-6- STAT3 cascade, an oncogenic transcription factor that up regulates pro-survival and pro-angiogenic genes [1-4]. In contrast, other studies suggest that IL-17 may protect against tumors, by promoting immune system-mediated tumor rejection [1,5,6].
After binding to its receptor (IL-17/R), intracellular IL-17 signaling includes transcription factor NF-κB [7,8], which induces the coordinated expression of several inflammatory genes causing the perpetuation of the inflammatory response [8]; PI3K, MAPKs, JNK, ERK and p38 are clearly involved in IL-17-induced responses [7,8].
Studies about the role of IL-17 in cancer are still very few regarding to different types of cancer such as bladder, brain and esophageal cancer. In relation to gliomas, Hu et al. 2011, described that IL-17 expression may play an important role in tumorigenesis and progression, since they observed high mRNA-positive ratios of IL-17 in glioma tissues, but not in tissues subjected to trauma [3]. For bladder cancer, the tumor was reduced in IL-17-/- mice, indicating a role of this cytokine in promoting tumor growth through IL-6/ STAT3 pathway [4]. Another study suggests that IL-17 may play an important role in the recruitment and infiltration of antitumor immune cells in early stages of bladder cancer [5]. In esophageal squamous cell carcinoma (ESCC), IL-17 led to the production of inflammatory chemokines (CXCL9, CXCL10 and CCL2, CCL20), which are associated with the migration of T cells, NK cells, and DCs, respectively. In addition, IL-17 enhanced the cytotoxic effects of NK cells against tumor cells [6]. The present study aimed to clarify the direct action of IL-17 in a series of solid tumor human cells, from glioma, esophageal carcinoma and bladder cancer.
Materials and Methods
Cell lines and cell culture
GL261 mouse glioma cells, M059J and U138 human glioma cells and T24 human bladder transitional cell carcinoma cells were obtained from ATCC (Rockville, Maryland, USA). OE-21 human esophageal squamous cell carcinoma was a gift from INCA (Rio de Janeiro, Brazil). M059J and U138 were cultured in DMEM/10% FBS and OE-21 and T24 were cultured in RPMI/10% FBS at a temperature of 37°C, a minimum relative humidity of 95%, and an atmosphere of 5% CO2 in air.
Analysis of IL-17RA mRNA expression
Cells (2 x 105) were collected and processed as previously described [9]. Briefly, total RNA was isolated using Trizol LS reagent (Invitrogen) and cDNA species synthesized with ImProm- II™ Reverse Transcription System (Promega). Quantitative PCR, using SYBR Green I as intercalating dye, was performed for IL-17RA (F: 5’-GCCCTGGACAGGTTCCGGGACTG-3’; R: 5’-CCCCTCCTCTGCGGCGAGCAC-3’) and for 18S and β-actin as reference genes. Relative mRNA levels were determined using 2-ΔΔCT method including individual efficiency calculated per sample using LinReg 11.0 Software
Cell viability and proliferation
For measuring cell viability and proliferation, we performed MTT assay and cell counting, respectively, as previously described [9]. Cells were treated with IL-17A (10, 20, 50, 100 pg/mL and 10, 20, 100 ng/mL) for 24 h.
Wound-healing migration assay
Cells were seeded in medium containing 10% FBS at 3x105 cells per well in 24-well plates. In order to minimize cell proliferation, cell cultures were grown to 80-90% confluence and deprived of serum medium for 18 h. The objective was to obtain a reproducible measurement of the migration of the wound edge towards the wound space with minimum cell proliferation, but without loss of cell viability over the experimental period. Wounds were made by sterile pipette tips and remaining cells were washed twice with CMF to eliminate detached cells and it was added DMEM/RPMI deprived serum medium. Cells were treated with IL-17A (10 ng/ml) and then incubated at 37°C. After 48 h, migrating cells at the wound front were photographed by Olympus inverted microscope IX71 (Tokyo, Japan) with a magnification×100 and compared. The cell-free area was correlated with tumor cell ability to migrate into the scratch. TScratch software was used to calculate the cell-free area. Three independent experiments were performed.
Intracellular signaling pathways activated by IL-17
In order to analyze IL-17 intracellular signaling activation, tumor cells were treated with IL-17A (10 ng/ml) for 1, 15 and 30 min and then processed according to the manufacturer’s instructions using anti-AKT, anti-p38 MAPK and anti-ERK 1/2 antibodies (BD Phosflow, BD Biosciences). Cells were analyzed on FACSCanto II Flow Cytometer (BD Biosciences) and the results were analyzed using FlowJo Software (Tree Star).
Statistical analysis
Data were analyzed by one-way analysis of variance (one-way ANOVA), followed by Tukey post-hoc test, using GraphPad Software version 5.0 (San Diego, CA, USA.). p values <0.05 were taken as statistically significant.
Results and Discussion
Human glioma, esophageal and bladder tumor cells express IL-17 receptor (IL-17RA) but their viability and proliferation are unaltered by exogenous IL-17A
In this study, we demonstrated, for the first time, that M059J and U138 human glioma cells, 0E-21 human esophageal cancer cells and T24 human bladder cancer cells express IL-17A receptor (IL- 17RA) (Figure 1A). A previous study has already shown the IL-17RA expression on GL261 cells [10]. When we tested the effects of different concentrations of IL-17 on these tumor cell lines, the exogenous IL- 17 had no direct effect on the tumor cells proliferation or viability in vitro (Figure 1B and Figure 1C). These results are in agreement with studies performed with GL261 mouse glioma cell line [10] and human hepatocarcinoma cell lines [11]. Another study showed that recombinant IL-17 protein or retroviral transduction of IL-17 gene into tumors did not affect in vitro proliferation, but in vivo–-IL-17- transfected cells grew more rapidly when compared with controls [12].
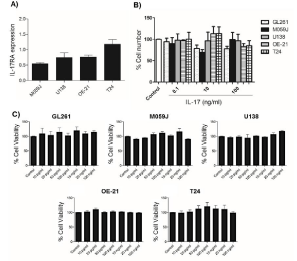
Figure 1: A: Relative gene expression profile of IL-17 on human tumor cells. Overall results from N=4 independent experiments using 18S and β-actin as reference
genes. B: Effect of IL-17 (100 pg/mL and 10, 100 ng/mL) on human tumor cells proliferation after 24 h. Control: non treated cells were considered 100% and used
to compare cell viability between the same cell line treated with IL-17. The experiments were carried out at least three times in triplicate. C: Effect of IL-17 (10, 20,
50, 100 pg/mL and 10, 20, 100 ng/mL) on tumor cells viability after 24 h. The experiments were carried out at least three times in triplicate. Each column represents
the mean ± SEM, as determined by ANOVA/Tukey test.
IL-17 prompts tumor cell migration in vitro
Although IL-17 is primarily associated with the induction of tissue inflammation, the other biological functions of IL-17, such as wound-healing functions, still remains to be thoroughly explored [13]. Here we showed that tumor cells stimulated with IL-17 (10 ng/ml) exhibited an increase in wound closure rates after 48 h when compared to non treated tumor cells (Figure 2A), suggesting that IL-17 is capable of promoting tumor cell migration in vitro. A study has showed that IL-17 increases the invasive capability of the JEG-3 human choriocarcinoma cell line, but the mechanisms of action involved in this effect remain unclear [14]. It is known that Matrix Metalloproteinases (MMPs) degrade ECM and facilitate cell migration. Regarding this mechanism, a study showed that IL-17 is able to induce invasion through MMPs (MMP-2, MMP- 9) transcription and expression [11] leading to cell migration [13]. Furthermore, the IL-17 capacity to stimulate IL-8 production can, in turn, promote granulocyte recruitment and stimulates MMPs expression, which causes the extracellular matrix remodeling and promotes cancer cell invasion [14].
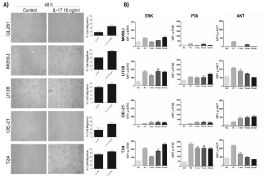
Figure 2: A: Effect of IL-17 (10 ng/ml) on cell migration after stimulation for 48 h (100×magnification). The experiment was carried out at least three times.
Percentage of open area were calculated using TScratch software [26]. Each column represents the mean ± SEM. *p < 0.05, **p < 0.01 and ***p < 0.001, as
determined by Student’s t-test. B: Effect of IL-17 (10 ng/ml) on phosphorylation of ERK, P38 and AKT after 1, 15 and 30 minutes of IL-17 (10 ng/ml) stimulation.
The experiments were carried out at least two times. Each column represents the mean ± SEM. *p < 0.05, **p < 0.01 and ***p < 0.001, as determined by ANOVA/
Tukey test. NC: negative control. PC: positive control.
Intracellular signaling activated by IL-17 on tumor cells
As shown in (Figure 2B), IL-17 (10 ng/ml) induces ERK1/2 phosphorylation in all human tumor cells tested, occurring continuously (from the first minute until 30 min after IL-17 stimulation) on the U138 and OE-21 cell lines and only after 15 min or 30 min on T24 and on M059J cell lines, respectively. IL-17-induced p38 phosphorylation was continuous on 0E-21 and T24 cells and occurred only after 15 or 30 min of stimulation on M059J and U138 cells, respectively. IL-17-induced AKT phosphorylation occurred faster (only on the first minute of stimulation) on U138 and T24 cells, decreasing this activation in the following min. On the OE-21 cell line, IL-17-induced AKT phosphorylation in a continuous way and on the M059J cells, IL-17 failed to induce AKT phosphorylation. It was shown that IL-17-induced MMPs expression occurs via p38 and MAPK activation, suggesting that IL-17 enhances cell migration by increasing MMP-1 expression through these signal transduction pathways [13]. AKT signaling may also play an important role in inducing pro-invasive factors and hence tumor progression, as the expression of IL-6, IL-8, MMP2, and VEGF increased in parallel with AKT activation in cells treated with IL-17 [11].
Conclusion
In conclusion, our results suggest that IL-17 directly promotes the migration of different human tumor cultured cells, possible through the activation of ERK, AKT and p38. This study provides additional understanding on the IL-17 direct action on tumors.
References
- Zeng Y, Zhang Q, Wang H, Lu M, Kong H, Zhang Y, et al. Prognostic significance of interleukin-17 in solid tumors: a meta-analysis. Int J Clin Exp Med. 2015; 8: 10515-10536.
- Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2010; 10: 248-256.
- Hu J, Mao Y, Li M, Lu Y. The profile of Th17 subset in glioma. Int Immuno pharmacol. 2011; 11: 1173-1179.
- Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009; 206: 1457-1464.
- Baharlou R, Khezri A, Razmkhah M, Habibagahi M, Hosseini A, Ghaderi A, et al. Increased interleukin-17 transcripts in peripheral blood mononuclear cells, a link between T-helper 17 and proinflammatory responses in bladder cancer. Iran Red Crescent Med J. 2015; 17.
- Lu L, Pan K, Zheng HX, Li JJ, Qiu HJ, Zhao JJ, et al. IL-17A promotes immune cell recruitment in human esophageal cancers and the infiltrating dendritic cells represent a positive prognostic marker for patient survival. J Immuno ther. 2013; 36: 451-458.
- Ivanov S, Linden A. Interleukin-17 as a drug target in human disease. Trends Pharmacol Sci. 2009; 30: 95-103.
- Kehlen A, Thiele K, Riemann D, Rainov N, Langner J. Interleukin-17 stimulates the expression of IkappaB alpha mRNA and the secretion of IL-6 and IL-8 in glioblastoma cell lines. J Neuro immunol. 1999; 101: 1-6.
- Gehring MP, Kipper F, Nicoletti NF, Sperotto ND, Zanin R, Tamajusuku AS, et al. P2X7 receptor as predictor gene for glioma radio sensitivity and median survival. Int J Bio chem Cell Biol. 2015; 68: 92-100.
- Cantini G, Pisati F, Mastropietro A, Frattini V, Iwakura Y, Finocchiaro G, et al. A critical role for regulatory T cells in driving cytokine profiles of Th17 cells and their modulation of glioma microenvironment. Cancer Immunol Immunother. 2011; 60: 1739-1750.
- Gu FM, Li QL, Gao Q, Jiang JH, Zhu K, Huang XY, et al. IL-17 induces AKT-dependent IL-6/JAK2/STAT3 activation and tumor progression in hepatocellular carcinoma. Mol Cancer. 2011; 10: 150.
- Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, et al. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003; 101: 2620-2627.
- Wu Y, et al. Interleukin-17A stimulates migration of periodontal ligament fibroblasts via p38 MAPK/NF-kappaB -dependent MMP-1 expression. J Cell Physiol. 2014; 229: 292-299.
- Zhu X, Mulcahy LA, Mohammed RA, Lee AH, Franks HA, Kilpatrick L, et al. IL-17 expression by breast-cancer-associated macrophages: IL-17 promotes invasiveness of breast cancer cell lines. Breast Cancer Res. 2008; 10.
