Abstract
This mini-review summarizes our recent published findings on the: i) emetic potential of cysteinyl leukotrienes LTC4, LTD4 and LTE4 in the least shrew (Cryptotis parva) model of emesis, ii) mechanism of action of their corresponding cysteinyl leukotrienes receptor 1 (CysLT1R) antagonist, pranlukast, against LTC4- induced vomiting, and iii) potential of pranlukast as a new class of antiemetic for the suppression of the acute- and delayed phases of vomiting caused by the cytotoxic cancer chemotherapeutic agent, cisplatin. Pranlukastis currently used in patients for the treatment of various respiratory disorders including asthma. Our findings demonstrate that unlike other leukotrienes (e.g. LTA4, LTB4 and LTF4), the above discussed leukotrienes are effective emetogens with the following potency order: LTC4=LTD4>LTE4. Prior treatment with pranlukast was shown to completely suppress LTC4-evoked emesis suggesting that CysLT1Rs are involved in vomiting. These and other findings indicate an important role for the emetic leukotrienes in the mediation of chemotherapy-induced nausea and vomiting (CINV). In fact we have demonstrated that blockade of the CysLT1R by pranlukast, not only can reduce cisplatin-evoked vomiting, but also intracellular markers of cisplatin-induced emetic signals. Moreover, pranlukast potentiated the antiemetic efficacy of serotonin 5-HT3 receptor antagonists, tropisetron and palonosetron, against CINV. If analogs of pranlukast such as montelukast and zafirlukast can also provide similar antiemetic potential, then clinical trials should be initiated since this class of drugs are relatively inexpensive than available effective antiemetic regimens against CINV.
Keywords: Cisplatin; Pranlukast; Least shrew; Palonosetron; Tropisetron; Emesis
Abbreviations
CINV: Chemotherapy-Induced Nausea and Vomiting; GIT: Gastrointestinal Tract; 5-HT3R: Serotonergic 5-HT3 Receptors; DVC: Dorsal Vagal Complex; NK1R: Neurokinin NK1 Receptors; SP: Substance P; NTS: Nucleus of the Solitary Tract; CysLT1: Cysteinyl Leukotriene 1; IP: Intraperitoneal; ERK1/2: Extracellular Signal- Regulated Protein Kinases 1 and 2; PKA: Protein Kinase A; PKCa/β II: Protein Kinase C Alpha/Beta II
Introduction
In this short review we briefly discuss: i) the progression of neurotransmitter hypothesis of chemotherapy-induced nausea and vomiting (CINV) and the current Status of clinically-relevant antiemetics for the prevention of CINV, ii) cost-effectiveness of antiemetics, and iii) introduction of pranlukast as an inexpensive new class of antiemetic for the prevention of CINV.
The neurotransmitter basis for chemotherapy-induced nausea and vomiting (CINV) and the current status of clinically-relevant antiemetics for the prevention of CINV
It is well recognized that cisplatin-like cytotoxic cancer chemotherapeutics evoke nausea and vomiting in cancer patients. The initial bout of vomiting occurs within a few hours of completion of intravenous administration of such chemotherapeutics to cancer patients and is referred to as the early- or acute-phase CINV [1]. This phase often subsides one day after the start of chemotherapy. Thereafter, a quiescent phase is observed where there can be little or no emesis as exemplified in the case of the least shrew animal model of emesis in the middle of Figure 1A and Figure 1B. Cancer patients will then experience additional bouts emesis, called the delayed-phase vomiting, which frequently starts from day three and can persist up to seven days post-treatment. Depending on the dose and route of administration, vomit-competent animals also exhibit both phases of CINV which can last from 2 (least shrews, see Figure 1) to 3 days (ferrets) post-cisplatin treatment [2-3]. Based upon the information obtained from animal models, the neurotransmitter basis of acuteand delayed-phases of CINV began in the late 1970s. Thus, initially it was proposed that the acute emesis is due to serotonin release from the enterochromaffin cells in the gastrointestinal tract (GIT), which will then stimulate the serotonergic 5-HT3 receptors (5-HT3R) located on the GIT vagal afferent neurons leading to afferent signaling to the brainstem [4]. Subsequently, the brainstem emetic nuclei in the dorsal vagal complex (DVC) are activated which evoke vomiting via vagal efferents. The delayed phase was thought to be due to activation of central neurokinin NK1 receptors (NK1R) subsequent to release of substance P (SP) in the medial nucleus of the solitary tract (NTS) in the DVC [4].
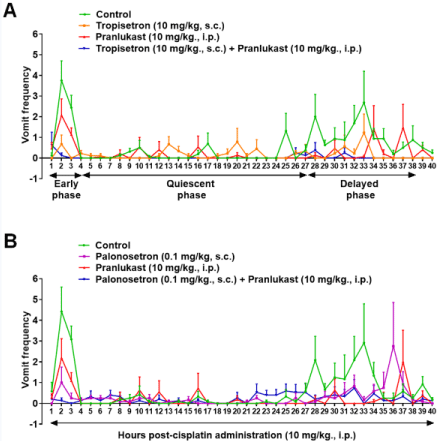
Figure 1: Antiemetic effects of the CysLT1R antagonist, pranlukast, either alone or in combination with a first generation 5-HT3R antagonist tropisetron, or a second
generation 5-HT3R antagonist palonosetron, on the mean frequency of vomiting per hour evoked by cisplatin. Different groups of least shrews were injected either
with their corresponding vehicles (control groups), pranlukast (10mg/kg, i.p.), tropisetron (10mg/kg, s.c.) or palonosetron (0.1mg/kg, s.c.), or a combination of the
said doses of pranlukast with tropisetron (Figure 1A), and pranlukast with palonosetron (Figure 1B) 30 min prior to cisplatin administration (10mg/kg, i.p.). Graphs
A and B show the mean frequency of vomits (means ± S.E.M.) per hour over the 40-hour observation period.
Nearly all cancer patients receiving large doses of cisplatin vomit. The above discussed emetic neurotransmitter dogma led to a renaissance in the development of new classes of prophylactic antiemetics (e.g. “the setrons”) which replaced the various classes of dopamine D2 receptor antagonists which were partially helpful in less than 30% of patients receiving chemotherapy [5]. Thus, introduction of the first generation 5-HT3R antagonists (e.g. ondansetron, granisetron and dolasetron) in the clinic began in early 1990s for the treatment of early-phase CINV. The setrons are often combined with a glucocorticoid such as dexamethasone for their anti-inflammatory/antiemetic properties and the combination has been effective in 47–61% of patients receiving moderately or highly emetogenic chemotherapeutics (MEC and HEC, respectively) [6-8]. A decade later, the NK1R antagonist aprepitant was introduced for reduction of delayed-phase vomiting [9]. The combination of the three discussed classes of antiemetics is often referred to as the “triple antiemetic regimen” and has revolutionized prophylactic antiemetic treatment in the clinic approaching 73-89% complete response rates [7,9]. Moreover, use of dexamethasone with longer-acting NK1R antagonists such as netupitant or rolapitant combined with a first or second generation 5-HT3R antagonist (e.g. palonosetron) can achieve a similar or greater clinical success against both phases of CINV [10- 11]. Latest antiemetic data obtained from the least shrew [12] and ferret [3] animal models of CINV support the efficacy of these new antiemetics in the above discussed clinical settings.
Furthermore, recent basic and clinical findings argue against the aforementioned well-established neurotransmitter CINV dogma in that serotonin released from the GIT evokes the acute phase CINV, while release of SP in the brainstem accounts for the delayed phase. Based up on our findings from the least shrew [2] and latest published clinical data [13] encompassing the full cycle of CINV, we proposed a revision of this elegant yet simple CINV neurotransmitter hypothesis to include other emetic mediators and concomitant involvement of both central and peripheral anatomical loci for the mediation of both early and delayed emesis. Indeed, early- and delayed-phases of CINV require not only serotonin, but also SP to be concomitantly released in the GIT and brainstem, as well as simultaneous release of other emetic neurotransmitters (such as dopamine) and mediators including prostaglandins and leukotrienes [5]. In fact as discussed earlier, palonosetron, netupitant and rolapitant do attenuate both phases of CINV in patients [14-15] and animal models of CINV, but not completely [3,12].
Cost-effectiveness
Even though improved efficacy by newer antiemetics can reduce healthcare resource utilization, it comes at a high cost [16-17]. In addition, with all of the recent advances in prophylactic antiemetic therapy, a significant number of cancer patients as well as animals still experience CINV following treatment with large doses of cisplatin-like drugs [3,7,12]. The latter findings suggest involvement of additional emetic mediator’s for induction of CINV. Thus, physicians should not only consider the choice of antiemetic regimen for cancer patients, but also the overall cost which can confound prophylactic antiemetic therapy since the price of newer drugs such as the palonosetron/netupitant oral preparation (Akynzeo), or rolapitant (Varubi), can approach $600 per capsule/tablet in the USA. Thus, many patients in the USA and most people in the developing countries may not afford such prices. In fact a recent meta-analysis [18] considers the high cost of antiemetic regimen a factor in clinical decision making. Although the exact mechanism of antiemetic action of the discussed glucocorticoid dexamethasone (a very affordable drug) still remains unknown, this class of drugs is inexpensive and helps to attenuate CINV especially when combined with older setrons and NK1R antagonists. It has been suggested that glucocorticoids’ antiemetic efficacy could be due to their anti-inflammatory effects [19] probably via a reduction in the synthesis of prostaglandins and leukotrienes [20]. Although not all, but several prostaglandins (e.g. PGE2 and PGF2a) and cysteinyl leukotrienes (e.g. LTC4 and LTD4), appear to be potent emetogens [21-24]. Regarding LTC4, the evoked vomiting was shown to be suppressed in a dose-dependent manner in the least shrew by the anti-asthmatic drug pranlukast [21]. Although not available in the USA, the cost of other members of this class of drugs (montelukast and Zafirlukast) that are sold in the USA is less than one dollar per pill. Based on pranlukast’s efficacy against LTC4- induced vomiting [21], we envisaged it may have potential utility against cisplatin-evoked emesis.
Antiemetic potential of pranlukast against cisplatinevoked acute and delayed vomiting
We recently introduced a new class of antiemetic, the antiasthmatic drug pranlukast, against cisplatin-evoked vomiting in the least shrew model of CINV [25]. Pranlukast along with montelukast and zafirlukast are cysteinyl leukotriene 1 (CysLT1) receptor antagonists, which are well-tolerated drugs and are used clinically for the treatment of respiratory diseases including chronic sinusitis, asthma and allergic rhinitis [26-27]. An intraperitoneal (i.p.) dose of 10mg/kg pranlukast by itself significantly reduced the mean frequency of vomits by 70% and fully protected 46% of least shrews during the delayed-phase of cisplatin (10mg/kg, i.p.) - evoked vomiting (Figure 1). Although pranlukast tended to substantially reduce both the mean frequency of vomits and the number of shrews vomiting during the early-phase, these reductions failed to attain significance. When pranlukast was combined with a first (tropisetron) - or a second (palonosetron)-generation 5-HT3R antagonist, it potentiated their antiemetic efficacy during both acute- and delayed-phases of cisplatin-evoked vomiting (Figure 1A and Figure 1B). In fact per hour efficacy antiemetic profile of pranlukast combined with palonosetron or tropisetron during both phases of CINV in the least shrew resembles those of: i) the NK1 receptor antagonist netupitant (5mg/kg) plus palonosetron (0.1mg/kg) in the same species [12]; ii) netupitant plus ondansetron in ferrets [3]; and iii) ondansetron plus aprepitant in combination with dexamethasone in ferrets [3]; and iv) palonosetron plus netupitant in combination with dexamethasone in ferrets [3].
For validation of intracellular emetic markers for CINV in the least shrew, we recently examined time-dependent activation/ phosphorylation of extracellular signal-regulated protein kinases 1 and 2 (ERK1/2), protein kinase A (PKA) and protein kinase C alpha/beta II (PKCa/β II) in their brainstem at many timepoints following cisplatin (10 mg/kg, i.p.) injection [12]. Cisplatin significantly elevated brainstem phospho-ERK1/2 levels at all timepoints following cisplatin administration except at 40h post-cisplatin injection. PKA phosphorylation tended to be elevated throughout the delayed-phase, but a significant increase only occurred at the 33 h peak delayed-phase. Brainstem phospho-PKCa/β II levels were enhanced during acute-phase with a significant elevation occurring at the 2h peak immediate-phase. In corresponding behavioral studies, a combination of palonosetron (0.1mg/kg) and the NK1 receptor antagonist netupitant (5mg/kg) suppressed cisplatin-evoked vomiting nearly completely during both acute- and delayed phases [12]. In the latter study, neither palonosetron, netupitant nor their combination had any significant effect on elevated phospho-ERK1/2 levels during peak acute emetic phase (i.e. 2h post-cisplatin treatment). This suggests additional mechanism is probably responsible for the evoked acute emesis. In our latest published study [25], administration of either palonosetron, tropisetron or pranlukast alone also failed to prevent cisplatin-evoked enhancement of ERK1/2 phosphorylation levels at the 2h peak acute-phase, but unlike the discussed case for netupitant/palonosetron combination [12], a regimen of pranlukast with either palonosetron or tropisetron did reverse ERK1/2 activation at the peak acute-phase [25]. Furthermore, neither palonosetron nor netupitant alone [12], nor tropisetron or palonosetron by themselves [25], could prevent cisplatin-evoked ERK1/2 activation during the 33h peak delayed-phase. Surprisingly, pranlukast pretreatment alone did prevent the evoked ERK1/2 activation during the 33h peak delayed-phase [25], and this suppression persisted even when pranlukast was combined with either palonosetron or tropisetron. On the other hand, netupitant could only prevent the latter effect when combined with palonosetron [12]. Thus, although both netupitant and pranlukast generally produce similar antiemetic profile against cisplatin-induced vomiting, their intracellular mechanism of action at the level of ERK1/2 phosphorylation differs.
Regarding cisplatin-evoked PKCa/β II phosphorylation observed at the 2h peak acute-phase, single pretreatment with either netupitant [12], palonosetron, tropisetron or pranlukast [25] failed to prevent the evoked activation. However, as was observed with netupitant plus palonosetron combination [12], pranlukast prevented the evoked increase in PKCa/β II phosphorylation when it was combined with either palonosetron or tropisetron [25]. Thus, in the latter phosphorylation event, both netupitant and pranlukast behave in a similar manner. In the case of cisplatin-induced PKA phosphorylation at the peak delayed emetic-phase, individual pretreatment with either netupitant [12], palonosetron or pranlukast [25] did prevent the evoked increase. Furthermore, a combination of netupitant plus palonosetron [12], or pranlukast combined with either tropisetron or palonosetron [25], did produce a similar inhibitory effect on the evoked PKA phosphorylation. Thus, the overall behavioral and intracellular signaling effects of pranlukast via blockade of CysLT1 receptors generally appear to be similar to the NK1R antagonist netupitant with some differences.
Conclusion
We have introduced a new class of antiemetic pranlukast [25] for the suppression of delayed-phase of cisplatin evoked vomiting. When it is combined with a first or second generation 5-HT3 receptor antagonist, it potentiates their antiemetic efficacy during both phases of CINV. Clinical potential of pranlukast and its analogs (montelukast, zafirlukast, etc) as a new class of antiemetic against CINV and other causes of vomiting requires initiation of clinical trials.
Acknowledgement
This manuscript was supported by the Western University of Health Sciences Research Funds. The authors do not have any conflicts of interests that would confound interpretation of the research data presented.
References
- Hesketh PJ, Van Belle S, Aapro M, Tattersall FD, Naylor RJ, Hargreaves R, et al. Differential involvement of neurotransmitters through the time course of cisplatin-induced emesis as revealed by therapy with specific receptor antagonists. Eur J Cancer. 2003; 39: 1074-1080.
- Darmani NA, Crim JL, Janoyan JJ, Abad J, Ramirez J. A re-evaluation of the neurotransmitter basis of chemotherapy-induced immediate and delayed vomiting: evidence from the least shrew. Brain Res. 2009; 1248: 40-58.
- Rudd JA, Ngan MP, Lu Z, Higgins GA, Giuliano C, Lovati E, et al. Profile of Antiemetic Activity of Netupitant Alone or in Combination with Palonosetron and Dexamethasone in Ferrets and Suncus murinus (House Musk Shrew). Front Pharmacol. 2016; 7: 263.
- Rudd JA, Andrews PLR. Mechanisms of acute, delayed and anticipatory vomiting in cancer and cancer treatment. In: Hesketh P (ed), Management of nausea and vomiting in Cancer and cancer treatment. Jones and Barlett Publishers Inc, New York. 2004; 15-66.
- Darmani NA, Ray AP. Evidence for a re-evaluation of the neurochemical and anatomical bases of chemotherapy-induced vomiting. Chem Rev. 2009; 109: 3158-3199.
- Hesketh PJ, Roman A, Hesketh AM, Perez EA, Edelman M, Gandara DR. Comparative review of 5-HT3 receptor antagonists in the treatment of acute chemotherapy-induced nausea and vomiting. Cancer Invest. 2000; 18: 163- 173.
- Rojas C, Raje M, Tsukamoto T, Slusher BS. Mechanisms and latest clinical studies of new NK1 receptor antagonists for chemotherapy-induced nausea and vomiting: Rolapitant and NEPA (netupitant/palonosetron). Eur J Pharmacol. 2015; 722: 26-37.
- Tricco AC, Blondal E, Veroniki AA, Soobiah C, Vafaei A, Ivory J, et al. Comparative safety and effectiveness of serotonin receptor antagonists in patients undergoing chemotherapy: a systematic review and network metaanalysis. BMC Med. 2016; 14: 216.
- Hesketh PJ, Grunberg SM, Gralla RJ, Warr DG, Roila F, de Wit R, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapyinduced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin--the Aprepitant Protocol 052 Study Group. J Clin Oncol. 2003; 21: 4112-4119.
- Hesketh PJ, Rossi G, Rizzi G, Palmas M, Alyasova A, Bondarenko I, et al. Efficacy and safety of NEPA, an oral combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy: a randomized dose-ranging pivotal study. 2014; Ann Oncol. 25: 1340-1346.
- Rapoport B, Schwartzberg L, Chasen M, Powers D, Arora S, Navari R, et al. Efficacy and safety of rolapitant for prevention of chemotherapy-induced nausea and vomiting over multiple cycles of moderately or highly emetogenic chemotherapy. Eur J Cancer. 2016; 57: 23-30.
- Darmani NA, Zhong W, Chebolu S, Mercadante F. Differential and additive suppressive effects of 5-HT3 (palonosetron)- and NK1 (netupitant)-receptor antagonists on cisplatin-induced vomiting and ERK1/2, PKA and PKC activation. Pharmacol Biochem Behav. 2015; 131: 104-111.
- Higa GM, Auber ML, Altaha R, Piktel D, Kurian S, Hobbs G, et al. 5-Hydroxyindoleacetic acid and substance P profiles in patients receiving emetogenic chemotherapy. J Oncol Pharm Pract. 2006; 12: 201-209.
- Hesketh PJ, Schnadig ID, Schwartzberg LS, Modiano MR, Jordan K, Arora S, et al. Efficacy of the neurokinin-1 receptor antagonist rolapitant in preventing nausea and vomiting in patients receiving carboplatin-based chemotherapy. Cancer. 2016; 122: 2418-2425.
- Aapro M, Rugo H, Rossi G, Rizzi G, Borroni ME, Bondarenko I, et al. A randomized phase III study evaluating the efficacy and safety of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy. Ann Oncol. 2014; 25: 1328-1333.
- Broder MS, Faria C, Powers A, Sunderji J, Cherepanov D. The Impact of 5-HT3RA Use on Cost and Utilization in Patients with Chemotherapy-Induced Nausea and Vomiting: Systematic Review of the Literature. Am Health Drug Benefits. 2014; 7: 171-182.
- Schwartzberg LS, Rugo HS, Aapro MS. New and emerging therapeutic options for the management of chemotherapy-induced nausea and vomiting. Clin Adv Hematol Oncol. 2015; 13: 3-13.
- Zhang Y, Yang Y, Zhang Z, Fang W, Kang S, Luo Y, et al. Neurokinin-1 Receptor Antagonist-Based Triple Regimens in Preventing Chemotherapy- Induced Nausea and Vomiting: A Network Meta-Analysis. J Natl Cancer Inst. 2017; 109.
- Sam TS, Chan SW, Rudd JA, Yeung JH. Action of glucocorticoids to antagonise cisplatin-induced acute and delayed emesis in the ferret. Eur J Pharmacol. 2001; 417: 231-237.
- Vane JR, Botting RM. Mechanism of action of anti-inflammatory drugs. Scand J Rheumatol Suppl. 1996; 102: 9-21.
- Chebolu S, Wang Y, Ray AP, Darmani NA. Pranlukast prevents cysteinyl leukotriene-induced emesis in the least shrew (Cryptotis parva). Eur J Pharmacol. 2010; 628: 195-201.
- Darmani NA, Chebolu S. The role of endocannabinoids and arachidonic acid metabolites in emesis. Murillo-Rodriguez E, Onaive ES, Darmani NA, Wagner E. editors. In: Endocannabinoids: Molecular, pharmacological, behavioral and clinical features. Bentham. e Books, Chapter 2. 2013; 25-59.
- Kan KK, Jones RL, Ngan MP, Rudd JA. Actions of prostanoids to induce emesis and defecation in the ferret. Eur J Pharmacol. 2002; 453: 299-308.
- Kan KK, Jones RL, Ngan MP, Rudd JA. Action of prostanoids on the emetic reflex of Suncus murinus (the house musk shrew). Eur J Pharmacol. 2003; 477: 247-251.
- Darmani NA, Chebolu S, Zhong W, Kim WD, Narlesky M, Adams J, et al. The anti-asthmatic drug pranlukast suppresses the delayed-phase vomiting and reverses intracellular indices of emesis evoked by cisplatin in the least shrew (Cryptotis parva). Eur J Pharmacol. 2017; 809: 20-31.
- Back M, Dahlén SE, Drazen JM, Evans JF, Serhan CN, Shimizu T, et al. International Union of Basic and Clinical Pharmacology. LXXXIV: leukotriene receptor nomenclature, distribution, and pathophysiological functions. Pharmacol Rev. 2011; 63: 539-584.
- Cingi C, Muluk NB, Ipci K, Sahin E. Antileukotrienes in upper airway inflammatory diseases. Curr Allergy Asthma Rep. 2015; 15: 64.
