Abstract
Vesicular medication delivery system, for example, Niosome is a novel medication delivery system, in which the solution is enclosed in vesicle which is made by Non-ionic surfactant. The niosomes provides several important advantages over conventional drug therapy. Structurally, niosomes are similar to liposomes, in that they are also made up of a bilayer. However, the bilayer in the case of niosomes is made up of non-ionic surface active agents rather than phospholipids as seen in case of liposomes. Niosomes tackled the issue of insolubility, instability, low bioavailability and fast debasement of medications. This paper overviews the method of preparation of Niosomes along with applications in pharmaceutical areas.
Keywords: Niosomes; Method of preparation; Evaluation study; Application of Niosomes.
Introduction
Niosomes are a novel drug delivery system, which entrapped the hydrophilic drug in the core cavity and hydrophobic drugs in the non-polar region present within the bilayer hence both hydrophilic and hydrophobic drugs can be incorporated into niosomes [1]. The niosomes are ampiphillic in nature, in which the medication is encapsulated in a vesicle which is made by non- ionic surfactant and hence the name niosomes. The niosomes size is a very small and microscopic [2]. The first niosome formulations were developed and patented by L’Oreal in 1975. In the presence of proper mixtures of surfactants and charge inducing agents from the thermodynamically stable vesicles. Niosomes are mostly studied as an alternative to liposomes because they alleviate the disadvantages associated with liposomes [3]. Niosomes overcome the disadvantages associated with liposomes such as chemical instability. Chemical instability of liposomes is due to their predisposition to oxidative degradation and variable purity of phospholipids. The main purpose of developing niosomal system is chemical stability, biodegradability, biocompatibility, chemical stability, low production cost, easy storage and handling and low toxicity [4,5]. Niosomes can be administrated through various routes such as oral, parenteral, topical. Niosomes are used as a carrier to deliver different types of drugs such as synthetic and herbal, antigens, hormones and other bioactive compounds [6,7,8]. This article presents some Salient features of niosomes along with an overview of the preparation techniques and the current applications of niosomes in encapsulation and delivery of bioactive compounds.
Salient features of niosomes [2,9,10]
- Niosomes can entrap solutes.
- Niosomes are osmotically active and stable.
- Niosomes have an infra-structure comprising of hydrophobic and hydrophilic for the most part together thus likewise oblige the medication atoms with an extensive variety of dissolvability.
- Niosome discharge the medication in a controlled way by means of its bilayer which give supported arrival of the encased medication, so niosomes fill in as medication warehouse in the body.
- Targeted medication conveyance can likewise be accomplished utilizing niosomes the medication is conveyed specifically to the body part where the remedial impact is required. There by lessening the measurement required to be managed to accomplish the coveted impact.
- They improve the solubility and oral bioavailability of poorly soluble drugs and also enhance the skin permeability of drugs when applied topically.
- Niosomes exhibits flexibility in their structural characteristics (composition, fluidity and size) and can be designed according to the desired situation.
- Niosomes can improve the performance of the drug molecules.
- Better availability to the particular site, just by protecting the drug from biological environment.
- Niosomes increase the stability of the entrapped drug.
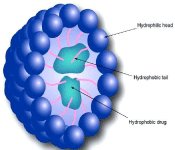
Figure 1: Structure of Niosome.
Advantages [6,11]
- Bioavailability Improvement: The term bioavailability alludes to the part of a dosage that is accessible at the site of activity in the body. Niosomes have unmistakable preferences over regular plans sin1ce the vesicles can go about as medication stores and shields sedate from acidic and enzymatic debasement in the gastro-intestinal tract which brings about bioavailability improvement and furthermore expanded the capacity to cross the anatomical hindrance of gastrointestinal tract.
- They enhance the restorative execution of the medication particles by postponed leeway from the dissemination, shielding the medication from natural condition and limiting impacts to target cells.
- Niosomal dispersion in an aqueous phase can be emulsified in a non aqueous phase to regulate the delivery.
- Rate of drug and administer normal vesicle in external non-aqueous phase.
- They are osmotically active and stable, as well as they increase the stability of entrapped drug.
- Handling and storage of surfactants requires no special conditions.
- They improve oral bioavailability of poorly absorbed drugs and enhance skin penetration of drugs.
- They can be made to reach the site of action by oral, parenteral as well as topical routes.
Comparison of liposomes and niosomes [11,12,13]
In spite of the fact that the liposomes and niosomes are practically same, both can be utilized as a part of focused and managed sedate conveyance framework, property of both relies on structure of the bilayer and strategies for their planning and both increment bioavailability and abatement the body leeway. Niosomes are prepared from uncharged single-chain surfactant and cholesterol whereas liposomes are prepared from double chain phospholipids, he major differences between liposomes and niosomes are described as follows, (Table 1, Figure 1)
Types of Niosomes [2,14]
Bola surfactant containing niosomes
The surfactant use in Bola surfactant containing niosomes are made of omega hexadecylbis-(1-aza-18 crown-6) (bola surfactant): span- 80/cholesterol in 2:3:1molar ratio.
Proniosomes
Proniosomes is made from the carrier and surfactant mixture. After the hydration of proniosomes, Niosomes are produced.
Aspasomes
Aspasomes is produced using the mix of acorbylpalmitate, cholesterol and exceptionally charged lipid diacetyl phosphate prompts the arrangement of vesicles. Aspasomes are first hydrated with water/fluid arrangement and afterward it is subjected to sonication to get the niosomes. Aspasomes can be utilized to build the transdermal saturation of medications. Aspasomes have likewise been utilized to diminish scatter caused by responsive oxygen species as it has innate cell reinforcement property.
Niosomes in carbopolgel
Niosomes were prepared from drug, spans and cholesterol then it is incorporated in carbopol-934 gel (1%w/w) base containing propylene glycol (10% w/w) and glycerol (30% w/w).
Vesicles in water and oil system (v/w/o)
In this strategy, the aqueous niosomes into an oil stage frame vesicle in water in oil emulsion (v/w/o). This can be set up by expansion of niosomes suspension figured from blend of sorbitol monostearate, cholesterol and solulan C24 (Poly-24-Oxyethylene cholesteryl ether) to oil stage at 60 0C.Thisresult in the formation of vesicle in water in oil (v/w/o)emulsion which by cooling to room temperature forms vesicle in water in oil gel (v/w/o gel). The v/w/o gel thus obtained can entrap proteins/ proteinous drugs and also protect it from enzymatic degradation after oral administration and controlled release.
Niosomes of hydroxyl propyl methyl cellulose
In this type, a base containing 10% glycerin of hydroxyl propyl methyl cellulose was first prepared and then niosomes were incorporated in it.
Deformable niosomes
The mixture of non-ionic surfactants, ethanol and water forms the deformable niosomes. These are smaller vesicles and easily pass through the pores of stratum corneum, which leads to increase penetration efficiency. It can be used in topical preparation [15,16]
The niosomes are also classified according to the number and size of bilayer which is as follows,
- i) Multi Lamellar Vesicles (MLV): Multilamellar vesicles are the most widely used niosomes. It consists of a number of bilayer. The approximate size of vesicles is 0.5-10 μm diameter .It is simple to make and are mechanically stable upon storage for long periods.
- ii) Large Unilamellar Vesicles (LUV): These are the large unilamellar vesicles which having a high aqueous/lipid compartment ratio, so that larger volumes of bio-active materials can be entrapped.
iii) Small Unilamellar Vesicles (SUV): These small unilamellar vesicles are mostly prepared from multilamellar vesicles by sonication method, French press and extrusion method.
Sr.no.
Liposomes
Niosomes
1
More expensive
Less expensive
2
Phospholipids are prone to oxidative Degradation.
But non-ionic surfactants are stable toward this.
3
Required special method for storage, handling and purification of phospholipids.
No special methods are required for such formulations Comparatively.
4
Phospholipids may be neutral charged.
Non-ionic surfactants are uncharged.
Table 1: Differences between Liposomes and Niosomes.

Figure 2: Probsonicator.
Components of Niosomes
The two major components utilized for the readiness of niosomes are, Cholesterol and Nonionic surfactants. Cholesterol is utilized to give unbending nature and appropriate shape, adaptation to the niosomes. The part surfactants assume a noteworthy part in the development of niosomes. The accompanying non-ionic surfactants are for the most part utilized for the arrangement of niosomes the spans (span 60,40,20,85,80), tweens (tween 20,40,60,80) and (brij 30,35,52,58,72,76).
Cholesterol [9]
Cholesterol is an amphiphilic molecule; it orients its OH group towards aqueous phase and aliphatic chain towards surfactant’s hydrocarbon chain. Cholesterol a waxy steroid metabolite is usually added to the non-ionic surfactants to provide rigidity Cholesterol is also known to prevent leakage by abolishing gel to liquid phase transition.
Non-ionic surfactants [9,17]
Niosomes are non-ionic surfactant unilamellar or multilamellar vesicles formed from synthetic non-ionic surfactants. Non-ionic surfactant possesses hydrophilic head group and hydrophobic tail. As HLB value increases therefore alkyl chain increases, the size of niosome increases. Hence HLB value 14-17 is not suitable for niosome formulation. HLB values 8 have highest entrapment efficiency. Non- ionic surfactants are as follows,
Ether linked surfactant: These are surfactants contain hydrophilic and hydrophobic moieties which are linked by ether, polyoxyethylene alkyl ethers with the general formula (CnEOm), where n; i.e. number of carbon atoms varies between 12 and 18 and m; i.e. number of oxyethylene unit varies between 3 and 7.
Di-alkyl chain surfactant: Surfactant was used as a principal component of niosomal preparation of stibogluconate and its potential in delivering sodium stibogluconate in experimental marine visceral leishmaniasis has been explored.
C16H33CH-O[-CH2-CH-O]7-H
| |
CH2 CH2OH
|
C12H25-O (mol. Wt. 972)
Ester linked: These surfactants have ester linkage between hydrophilic and hydrophobic groups; hence it is also called as Ester linked surfactants.
C15H31CO[O-CH2-CH-CH2]2-OH
|
OH (mol. Wt. 393)
This surfactant was also studied for its use in the preparation of stibogluconate bearing niosomes and in delivery of sodium stibogluconate to the experimental marine visceral leishmaniasis.
Sorbitan esters: These are the ester linked surfactants. The commercial sorbitan esters are mixtures of the partial esters of sorbital and its mono and di-anhydrides with oleic acid (Figure 2).
CH2
|
H-C-OH
|
RCOO- C-H
|
H-C-OH
|
H-C-OOC-R
|
CH2OOC-R
Where, R is H or an alkyl chain.
These have been used to entrap wide range of drugs viz doxorubicin.
Fatty acid and amino acid compounds: Long chain fatty acids and amino acid moieties have also been used in some niosomespreparation which form “Ufasomes” vesicles.
Charge inducers
There are two types of charged inducers such as Positive and Negative charge inducers. It increases the stability of the vesicles by induction of charge on the surface of the prepared vesicles. It act by preventing the fusion of vesicles due to repulsive forces of the same charge and provide higher values of zeta potential. The commonly used positive charge inducers are sterylamine and cetylpyridinium chloride and negative charge inducers are dicetyl phosphate, dihexadecyl phosphate and lipoamine acid [9].
Method of Preparation
Ether injection [18]
In this method, slow injection of surfactant: cholesterol (150micro.mol.) in 20ml ether through a 14 gauze needle (25ml/min.) in preheated 4ml aqueous phase maintained at 600c. The ether solution was evaporated using rotary evaporator, after evaporation of the organic solvent it forms single layered vesicles.
Sonication [19]
Niosomes using sonication method were prepared by Baillie et al 1986. In this method, surfactant: cholesterol (150micro.mol.) mixture was dispersed in 2ml aqueous phase in vial. The dispersion is subjected to probe sonication for 3 min. at 600c.This method involved the formation of MLVs which are subjected to ultrasonic vibration. Sonicator is two type Probe and Bath sonicator. Probe sonicator is use when sample volume is small and Bath sonicator is use when sample volume is large.
Hand shaking method [19]
In this method, surfactant: cholesterol (150micro.mol.) mixture was dissolved in 10ml diethylether in RBF. The ether is evaporated under vacuum at room temperature in rotary evaporated. Upon hydration the surfactant swells and is peeled off the support in to a film. Swollen amphiphiles eventually fold to form vesicles. The liquid volume entrapped in vesicles appears to be small which 5-10% is.
Extrusion method [19]
In this method, niosomes were prepared using C16G2, a chemically defined non -ionic surfactant by extrusion through a polycarbonate membrane. These studies not only demonstrate the effect of number of extrusion on vesicles size but also the effect of size on encapsulation of drug.
Reverse phase evaporation technique [18,19]
In this method, surfactant is dissolved in chloroform and added into the 0.25 volume phosphate saline buffer solution is emulsified to get w/o emulsion. The mixture is then solicited and subsequently chloroform is evaporated under reduce pressure. The lipid or surfactant forms a gel first and subsequently hydrates to form vesicles.
Bubble method [6,18]
It is novel technique for the one step preparation of liposomes and niosomes without the use of organic solvents. It consists of round-bottomed flask with three necks placed in water bath to control the temperature. Water-cooled reflux and thermometer is positioned in the first and second neck and nitrogen supply through the third neck. Cholesterol and surfactant are dispersed together in this buffer (pH 7.4) at 70°c. A continuous stream of nitrogen gas bubbles is generated and introduced through the dispersion and produce a niosomes.
Micro fluidization method [20]
Micro fluidization is a current strategy to plan unilamellar vesicles of characterized estimate circulation. Based on submerged jet principle, in this strategy two fluidized streams connect at ultrahigh speeds, in correctly characterized smaller scale channels inside the interaction chamber. The impingement of thin liquid sheet along a common front is arranged such that the energy supplied to the system remains within the area of niosomes formation. The outcome is a more prominent consistency, smaller size and better reproducibility of niosomes shaped.
Separation of unentrapped drug [21-27]
The removal of unentrapped solute from the vesicles can be done by various techniques, such as dialysis, gel filtration and centrifugation.
(i) Dialysis: Dialysis is one of most important technique used for removal of unentrapped drug from vesicles. In this technique, the aqueous niosomal dispersion is dialyzed in dialysis tubing against phosphate buffer or normal saline or glucose solution.
(ii) Gel Filtration: In this technique, the unentrapped drug is removed by gel filtration of niosomal dispersion through a Sephadex-G-50 column and elution with phosphate buffered saline or normal saline.
(iii) Centrifugation: The niosomal suspension is centrifuged and the supernatant is separated. The pellet is washed and then resuspended to obtain a niosomal suspension free from unentrapped drug [26,28].
Factors Affecting Physico-chemical Properties of Niosomes
Various factors that affect the physico-chemical properties of niosomes are discussed further.
Amount and type of surfactant [29]
The mean size of niosomes increases proportionally with increase in the HLB of surfactants like Span 85 (HLB 1.8) to Span 20 (HLB 8.6) because the surface free energy decreases with an increase in hydrophobicity of surfactant. The bilayers of the vesicles are either in the supposed fluid state or in gel state, contingent upon the temperature, the kind of lipid or surfactant and the nearness of different segments, for example, cholesterol. In the gel state, alkyl chains are available in an all-around requested structure, and in the fluid express, the structure of the bilayers is more confused. The surfactants and lipids are portrayed by the gel-fluid stage change temperature (TC). Phase transition temperature (TC) of surfactant also effects entrapment efficiency i.e. Span 60 having higher TC, provides better entrapment.
Nature of Surfactants [29]
A surfactant utilized for readiness of niosomes must have a hydrophilic head and hydrophobic tail. The hydrophobic tail may comprise of maybe a couple alkyl or perfluoroalkyl gatherings or now and again a solitary steroidal gathering. The hydrophobic tail of ether sort surfactants with single chain alkyl is more poisonous than comparing dialkylether chain. The ester type surfactants are chemically less stable than ether type surfactants and the former is less toxic than the latter due to ester-linked surfactant degraded by esterase’s to triglycerides and fatty acid. The surfactants with alkyl chain length from C12-C18 are suitable for preparation of niosomes. Surfactants such as C16EO5 (poly-oxyethylenecetyl ether) or C18EO5 (polyoxyethylenesteryl ether) are used for preparation of polyhedral vesicles. Span series surfactants having HLB number of between 4 and 8 can form vesicles.
Nature of encapsulated drug [30]
The physico-synthetic properties of typified medicate impact charge and unbending nature of the niosome bilayer. The medication cooperates with surfactant head gatherings and builds up the charge that makes shared aversion between surfactant bilayers and subsequently expands vesicle estimate. The aggregation of vesicles is prevented due to the charge development on bilayer. In Polyoxyethylene Glycol (PEG) coated vesicles; some drug is entrapped in the long PEG chains, thus reducing the tendency to increase the size. The hydrophilic lipophilic balance of the drug affects degree of entrapment.
Structure of surfactants [31]
The geometry of vesicle to be shaped from surfactants is influenced by surfactant's structure, which can be characterized by basic pressing parameters. Geometry of vesicle to be shaped can be predicated on the premise of basic pressing parameters of surfactants. Critical packing parameters can be defined using following equation,
CPP (Critical Packing Parameters) =V/lc × a0
Where,
V = hydrophobic group volume,
lc = the critical hydrophobic group length,
a0= the area of hydrophilic head group
Critical packing parameter value type of miceller structure formed can be ascertained as given below,
If CPP < ½ formation of spherical micelles,
If ½ < CPP < 1 formation of bilayer micelles,
If CPP > 1 formation inverted micelles.
Temperature of hydration [30]
Hydration temperature influences the shape and size of the niosome, temperature change of niosomal system affects assembly of surfactants into vesicles by which induces vesicle shape transformation. Ideally the hydration temperature for noisome formation should be above the gel to liquid phase transition temperature of system.
Resistance to osmotic stress [8]
Addition of a hypertonic salt solution to a suspension of niosomes brings about reduction in diameter. In hypotonic salt solution, there is initial slow release with slight swelling of vesicles probably due to inhibition of eluting fluid from vesicles, followed by faster release, which may be due to mechanical loosening of vesicles structure under osmotic stress.
Evaluation [2,8,29,30]
Entrapment efficiency
After preparing niosomal dispersion, unentrapped drug is separated by dialysis centrifugation and gel filtration. The drug remains entrapped in niosomes s determined by complete vesicle disruption using 50% n-propanol or 0.1% Triton X-100 and analyzed resultant solution by appropriate assay method using following equation.
Entrapment efficiency = (Amount entrapped / total amount) x 100
Bilayer Formation
Assembly of non-ionic surfactants to form a bilayer vesicle is characterized by an X-cross formation under light polarization microscopy.
Size
Shape of niosomal vesicles is assumed to be spherical, and their mean diameter can be determined by using laser light scattering method. Also, diameter of these vesicles can be determined by using electron microscopy, molecular sieve chromatography, ultracentrifugation, photon correlation microscopy, optical microscopy and freeze fracture electron microscopy.
Numbers of lamellae
This is determined by using Nuclear Magnetic Resonance (NMR) spectroscopy, small angle X-ray scattering and electron microscopy.
Membrane rigidity
Membrane rigidity can be measured by means of mobility of fluorescence probe as a function of temperature.
In-vitro release
A method of in-vitro release rate study includes the use of dialysis tubing. A dialysis sac is washed and soaked in distilled water. The vesicle suspension is pipetted into a bag made up of the tubing and sealed. The bag containing the vesicles is placed in 200ml of buffer solution in a 250ml beaker with constant shaking at 25°C or 37°C. At various time intervals, the buffer is analyzed for the drug content by an appropriate assay method.
Microscopic evaluation
Transmission electron microscopy was used for microscopic evaluation of niosomal dispersions. TEM used for determination of size and used for identified whether it is spherical or not.
Application of Niosomes
Niosome as a carrier for hemoglobin
Niosomal suspension shows a visible spectrum super imposable onto that of free hemoglobin so can be used as a carrier for hemoglobin. Vesicles are also permeable to oxygen and hemoglobin dissociation curve can be modified similarly to non-encapsulated hemoglobin [1].
Niosomes as drug carriers
Niosomes have likewise been utilized as transporters for iobitridol, a symptomatic operator utilized for X-ray imaging. Topical niosomes may fill in as solubilization grid, as a neighborhood station for maintained arrival of dermally dynamic mixes, as entrance enhancers, or as rate-restricting layer obstruction for the tweak of foundational ingestion of medications [2].
Ophthalmic drug delivery
It is difficult to achieve excellent bioavailability of drug from ocular dosage form like ophthalmic solution, suspension and ointment due to tear production, impermeability of corneal epithelium, non-productive absorption and transient residence time. But to achieve good bioavailability of drug niosomal vesicular systems have been proposed [29]. Carter et al. reported that multiple dosing with sodium stibogluconate loaded niosomes was found to be effective against parasites in the liver, spleen and bone marrow as compared to simple solution of sodium stibogluconate [17,29].
Delivery of peptide drugs
Yoshida et al investigated the stability of peptide increased by niosomes. In Yoshida et al for oral delivery of 9-desglycinamide, 8-arginine vasopressin entrapped in niosomes in an in-vitro intestinal loop model and reported that the stability of peptide increased by niosomes [30].
Transdermal delivery of drugs by niosomes
In transdermal route of delivery, when drug is incorporated in niosomes penetration of drug through skin is enhanced [30].
Neoplasia
The anthracyclic antibiotic such as Doxorubicin which shows broad spectrum anti tumour activity, produces a dose depend antirreversible cardio toxic effect. This drug increased the lifespan and decreased the rate of proliferation of sarcoma when administered by niosomal delivery into mice bearing S-180 tumor [30].
Use in studying immune response [32]
Because of their immunological selectivity, low danger and more noteworthy solidness; niosomes are being utilized to ponder the idea of the insusceptible reaction incited by antigens. Nonionic surfactant vesicles have plainly exhibited their capacity to work as adjuvant after parenteral organization with various distinctive antigens and peptides.
Anti-inflammatory agents [31]
Niosomal formulation of Diclofenac sodium with70% cholesterol exhibits greater anti-inflammator activity as compare to free drug. Niosomal formulation of Nimesulide and Flurbiprofen shows greater anti-inflammatory activity as compared to free drug. Sharma et al (2009) was developed span-60 niosomal oral suspension of fluconazole in the treatment of fungal infection. It is effective as compare to capsule and tablets [33].
Leishmaniasis [12,34,35]
Niosomes can be utilized for focusing of medication in the treatment of maladies in which the contaminating life form lives in the organ of reticulo-endothelial framework. Leishmaniasis is such an infection in which parasite attacks cells of liver and spleen.
Immunological application [12]
Niosomes have been used for studying the nature of the immune response provoked by antigens. Brewer and Alexander have reported niosomes as potent adjuvant in terms of immunological selectivity, low toxicity and stability.
Niosomes in gene delivery [20]
Novel niosome detailing in light of the 2,3-di (tetradecyloxy) propan-1-amine cationic lipid, joining with squalene and polysorbate 80 to assess the transfection productivity in rodent retinas. Lipoplexes at 15/1 proportion were 200 nm in measure, 25mV in zeta potential and displayed circular morphology. At this proportion, it was seen that niosomes consolidated and secured the DNA from enzymatic processing.
Tetanus toxoid (TT) [20,36]
Yoshika et al defined Span/CHOL/DCP niosomes containing lockjaw toxoid which was a vesicle-in water-in oil framework. Cottonseed oil was utilized and gave better immunological properties when contrasted with free antigen. Katare et al (2006) developed the polysaccharide-capped niosomes for oral immunization of tetanus toxoid and he was concluded that the niosomes were good approaches for oral immunization of tetanus toxoid.
Dignostic imaging with niosomes Niosomal framework can be utilized as demonstrative operators. Conjugated niosomal with N-palmitoylglucosamine (NPG), PEG 4400 and both PEG and NPG display essentially enhanced tumor focusing of an evaluated paramagnetic specialist evaluated with MR imaging [37].
Wagh et al (2012), was developed itraconazole niosomes drug delivery system and study its antimycotic against Candida albicans. He was enhanced the skin permeability of itraconazole by niosomal drug delivery [34].
Srivastav et al (2014) was developed the niosomes of ofloxacin and study its antimicrobial activity. He was prepared ofloxacin niosomes by using ether injection method [38].
Mishra et al (2014) was developed the formulation of Niosomes of Aceclofenac used as NSAID [39].
Bayindir et al (2010) was developed niosomes prepared with various nonionic surfactants for paclitaxel oral delivery. Paciltaxel is an antineoplastic agent. Pacitaxel niosomal formulation was prepared by various surfactants such as Tween 20, 60, Span 20, 40, 60, Brij 76, 78, 72 by film hydration method [40].
Patel et al developed Lopinavir niosomal gel using span 40 and cholesterol. Lopinavir is a highly potent protase inhibitor used in a treatment of AIDS. Lopinavir has low systemic bioavailability due to its high presystemic metabolism hence it is coadministered with ritonavir. In a study reported by patel et al, a niosomal gel enhanced the systemic bioavailability of lopinavir via transdermal route also enhanced its skin permeability [41].
Yasam et al (2014) developed the nifedipine proniosome for transdermal delivery. Nifedipine is a calcium channel blocker used in a treatment of hypertension. It undergoes rapidly first pass metabolism hence it has low bioavailability. In a study reported by Yasam et al, peformed the ex- vivo permeation studies in rat skin and concluded that the niosomes as good permeation enhancers [42].
Shahiwala et al () was developed niosomally entrapped nimesulide for topical application. Nimesulide is a non- steroidal anti- inflammatory drug having low skin permeability. In a study reported by Shahiwala, concluded that niosomes as good permeation enhancers for nimesulide [43].
Many drugs have poor aqueous solubility that result in variable bioavailabilities. This problem can be overcome by entrapping the drug into niosomes [44].
Jadon et al (2009) developed niosomes preparation of griseofulvin using different methods of preparation by varying nonionic surfactants, their concentration and cholesterol concentration. Griseofulvin has a poor and variable oral bioavailability use as antifungal agent. In a study reported by Jadon, concluded that span 60 provided highest entrapment efficiency and sustained release so could be one of the promising delivery system for griseofulvin [45].
Abdelbary et al (2011) developed parental administration of nystatin. Nystatin is an antifungal agent. In a study reported by Abdelbary, concluded that niosomal encapsulation for parental administration of nystatin, reducing its toxicity and making it a more active antifungal agent [46].
Ning et al (2005) developed niosomal gel of clotrimazole. Clotrimazole is widely used for the treatment of mycotic infections of the genitourinary tract. An alternative formulation was developed for the vaginal administration of clotrimazole to provide sustained and controlled release for local vaginal therapy by formulation in niosomes. In a study reported by Ning et al, the prepared vesicle gel system was evaluated by antifungal activity and tolerability on tissue level in rat and result it’s showed sustained and controlled release of the drug, clotrimazole [47].
Gupta et al (2011) developed Fluconazole-loaded niosomes using Span 40, Span 60, and Brij 72 surfactant.In a study reported by Gupta, concluded that the prepared formulation accumulated and formed localized drug depots in the skin, thereby releasing the contents in a sustained manner and is subjected to greatly enhanced cutaneous retention of the drug [48].
Barakat et al (2009) developed niosomal gel of naftifine hydrochloride as an antifungal drug. In a study reported that, the non-alcoholic niosomal formulation has not been detrimental to skin after repeated exposure [49].
Tamizharasi et al (2009) developed niosomal formulation of Gliclazide. Gliclazide is an oral antidiabetic drug which have bioavailability problem. Tamizharasi et al improved the oral bioavailability of Gliclazide by entrapment in non-ionic surfactant vesicles [50].
Hardia et al (2017) developed dexamethasone loaded niosomal gel. Dexamethasone is a corticosteroids used in treatment of inflammatory condition, cerebral edema, in alcohol withdrawal syndrome, and congenital adrenal hyperplasia, cerebral malaria, especially associated with a high dose of anticancer agents, Respiratory disorders, opportunistic mycobacterial infections, skin disorders and rheumatism. He developed niosomal Suspension of Dexamethasone prepared by Hand Shaking Method using Span 60, Tween 20 and Tween 80 with addition of Charge Inducer. Niosomal Gel Prepared by Carbopol 940 [51].
Conclusion
Drug consolidation in the niosomes to focus around the niosomes to the specific site is a promising drug delivery model. They shows a structure like liposome and consequently they can speak to elective vesicular frameworks as for liposomes, due to the niosome capacity to exemplify distinctive sort of medications inside their multi environmental structure and furthermore because of different elements like cost, stability and so on. These advantages over the liposomes make it a better targeting agent. Ophthalmic, topical, parenteral and various other routes are used for targeting the drug to the site of action for better efficacy.
References
- Shakya V. Niosomes: A Novel Trend in Drug Delivery. Ijrdp. 2014; 3: 1036-1041.
- Makeshwar K, Wasankar S. Niosomes: a novel drug delivery system. Asian J. Pharm. Res. 2013; 3: 16-20.
- Punithavalli G, Vignesh M. Formulation of Niosomal Suspension with enhanced oral bioavailability of Diclofenac sodium. Journal of Global Trends in Pharmaceutical Sciences. 2012; 3: 656-671.
- Katare R, Gupta P, Mahor S, Rawat A, Khatri K, Katare Y, Panda A, Vyas S. Development of polysaccharide-capped niosomes for oral immunization of tetanus toxoid. Journal Drug Del. Sci. Tech. 2006; 16: 167-172.
- Shahiwala A, Misra A. Studies in topical application of niosomally entrapped nimesulide. Journal of Pharm. Pharmaceut. Sci. 2002; 5: 220-225.
- Bagheri A, Chu B, Yaakob H. Niosomal Drug Delivery Systems: Formulation, Preparation and Applications. World Applied Sciences Journal. 2014; 32: 1671-1685.
- Sudheer P, Kaushik K. Review on Niosomes- A Novel Approach For Drug Targeting, Journal of Pharmaceutical Research. 2015; 1-14.
- Sunilkumar M. Niosomes As novel drug delivery system. International Research Journal of Pharmaceutical and Applied Science. 2015; 5: 1-7.
- Sankhyan A, Pawar P. Recent Trends in Niosome as Vesicular Drug Delivery System. Journal of Applied Pharmaceutical Science. 2012; 2: 20-32.
- Gurjar P. Niosome: A Promising Pharmaceutical Drug Delivery. Int. J. Pharm Anal. 2014; 2: 425-431.
- Madhav N, Saini A. Niosomes: A novel drug delivery system. International journal of research in pharmacy and chemistry. 2011; 1: 498-511.
- Gandhi A, Sen S, Paul A. Current Trends in Niosomes As vesicular drug delivery system. Asian Journal of Pharmacy and Life Science. 2012; 2: 339-353.
- Chauhan S, Luorence MJ. The preparation of polyoxyethylene containing non-ionic surfactant vesicles. J. Pharm. Pharmacol. 1986; 4: 6.
- Verma A. A vital role of niosomes on Controlled and Novel Drug delivery. Indian Journal of Novel Drug Delivery. 2011; 3: 238-246.
- Arul J, Shanmuganathan S, Nagalakshmi. An Overview on Niosome as Carrier in Dermal Drug Delivery. Journal of pharmaceutical sciences and research. 2015; 7: 923-927.
- Moghassemi S, Hadjizadeh A. Nano-niosomes as Nanoscale Drug Delivery Systems: An illustrated review. Journal of Controlled Release. 2014; 2: 22-36.
- Tangriet P. Niosomes: Formulation and Evaluation. International Journal of Biopharmaceutics. 2011; 2: 47-53.
- Arul J. An Overview on Niosome as Carrier in Dermal Drug Delivery. J. Pharm. Sci. & Research. 2015; 7: 923-929.
- Vyas S, Khar R. Targeted and Controlled Drug Delivery, Novel Carrier System. CBS publication. 2007; 1: 249-279.
- Goswami S, Pathak D. Niosomes- A review of current status and application, World Journal of Pharmacy and Pharmaceutical Sciences. 2017; 6: 594-615.
- Gayatri D, Venkatesh P, Udupa N. Niosomal sumatriptan succinate for nasal administration. Int. J. Pharm. Sci. 2000; 62: 479-481.
- Hu C, Rhodes D. Proniosomes: A novel drug carrier preparation. Int J. Pharm. 1999; 185: 23-35.
- Silver BL. The physical chemistry of membranes. New York: Alan/Unwin and Solomon Press. 1985; 209-230.
- Khandare J, Madhavi G, Tamhankar B. Niosomes novel drug delivery system. The East Pharmacist. 1994; 37: 61-64.
- Maver L, Bally M, Hope M, Cullis P. Biochem. Biophys. Acta. 1985; 816: 294-302.
- Blazek-Walsh A, Rhodes D. SEM imaging predicts quality of niosomes from maltodextrin-based proniosomes. Pharm. Res. 2001; 18: 656-661.
- Baillie A, Florence A, Hume L, Muirhead G, Rogerson A. The preparation and properties of niosomes non-ionic surfactant vesicles. J. Pharm. Pharmacol. 1985; 37: 863-868.
- Debnath A, Kumar A. Structural and Functional significance of Niosome and Proniosome in Drug Delivery System. International Journal of Pharmacy and Engineering. 2015; 3: 621-637.
- Jindal K. Niosomes as a Potntial Carrier System: A Review. IJPCBS. 2015; 5: 947-959.
- Kaur H, Dhiman S, Arora S. Niosomes: A novel drug delivery system. Int. J. Pharm. Sci. Rev. Res. 2012; 15: 113-120.
- Navya M. Niosomes As novel vesicular drug delivery system- A review. Asian Journal of Research in Biological and Pharmaceutical Sciences. 2014; 2: 62-68.
- Verma N. Niosomes and Its Application -A Review. IJRPLS. 2014; 2: 182-184.
- Sharma S. Span-60 Niosomal Oral Suspension of Flucanazole: Formulation and in vitro evaluation. Asian journal of pharmaceutical research and health care. 2009; 1: 142-156.
- Suzuki K, Sokan K. The Application of Liposomes to Cosmetics. Cosmetic and Toiletries. 1990; 105: 65-78.
- Tabbakhian M, Tavakoli N, Jaafari M, Daneshamouz S. Enhancement of follicular delivery of finasteride by liposomes and niosomes. Int. J. Pharm. 2006; 323: 1-10.
- Namdeo A, Jain N. Niosomes as drug carriers. Indian Journal of Pharm. Sci. 1996; 58: 41-46.
- Rai A. Niosomes: An approach to current drug delivery-A Review. International Journal of Advances in Pharmaceutics. 2017; 06: 41-48.
- Srivastav A, Das P. To Study the Formulation of Niosome of Ofloxacin and Its Evaluation for Efficacy of Anti-Microbial. International Journal of Innovative Research in Science, Engineering and Technology. 2014; 3: 17958-17965.
- Mishra N, Srivastava V, Kaushik A, Chauhan V, Srivastava G. Formulation and in vitro- evaluation of Niosomes of Aceclofenac. JSIR. 2014; 3: 337-341.
- Bayindir Z, Yuksel N. Characterization of niosomes prepared with various nonionic surfactants for paclitaxel oral delivery. Journal of Pharmaceutical Sciences. 2010; 99: 2049-2060.
- Yasam V, Jakki S, Natarajan J. Novel vesicular transdermal delivery of nifedipine-preparation, characterization and in vitro in-vivo evaluation. Drug Deliv. 2014; 9: 1-12.
- Malhotra M, Jain N. Niosomes as drug carriers. Indian Drugs. 1994; 31: 81-86.
- Baillie A, Coombs G, Dolan T. Non-ionic surfactant vesicles, niosomes, as delivery system for the anti-leishmanial drug, sodium stribogluconate. Journal of Pharm. Pharmacol. 1986; 38: 502-505.
- Rogerson A, Cummings J, Willmott N, Florence A. The distribution of doxorubicin in mice following administration in niosomes. J. Pharm. Pharmacol. 1988; 40: 337-342.
- Jadon P, Gajbhiye V, Jadon R, Gajbhiye K, Ganesh N. Enhanced Oral Bioavailability of Griseofulvin via Niosomes. AAPS PharmSciTech. 2009; 10: 1186-1192.
- Abdelbary A, Essam T, Abd El-Salam R, AlyKassem A. Niosomes as a potential drug delivery system for increasing the efficacy and safety of nystatin (antifungal). Drug Dev Ind Pharm. 2011; 37: 149-508.
- Ning M, Guo Y, Pan H, Chen X, Gu Z. Preparation, in vitro and in vivo Evaluation of Liposomal/Niosomal Gel Delivery Systems for Clotrimazole. Drug Dev. Ind. Pharm. 2005; 31: 375-383.
- Gupta M, Vaidya B, Mishra N, Vyas SP. Effect of surfactants on the characteristics of fluconazole niosomes for enhanced cutaneous delivery. Artif. Cells Blood Substit. Immobil. Biotechnol. 2011; 39: 376-384.
- Barakat H, Darwish I, El-Khordagui L, Khalafallah N. Development of naftifine hydrochloride alcohol-free niosome gel. Drug Dev. Ind. Pharm. 2009; 35: 631-637.
- Tamizharasi S, Dubey A, Rathi V, Rathi J. Development and characterization of niosomal drug delivery of gliclazide. Journal Young Pharm. 2009; 1: 205-209.
- Hardia A, Jamindar D, Mahajan A, Hardia A. Formulation and in vitro skin permeability evaluation of dexamethasone loaded niosomal gel. Asian journal of pharmaceutical research and development. 2017; 5: 1-9.
- Okore V, Attma A. Formulation and Evaluation of Niosomes, Indian Journal of Pharmaceutical Sciences. 2011; 73: 323-328.
- Stan C, Tataringa G. Preparation and Characterization of Niosomes containing Metronidazole. FARMACIA. 2013; 61: 1178-1185.
- Mujoriya R, Dhamandeb K. Niosomal Drug Delivery System-A Review. International journal of applied pharmaceutical. 2011; 3: 7-10.
- Kumar A. Review on Niosomes as novel drug delivery system. IRJP. 2011; 2: 61-65.
- Pardakhty A, Moazeni E. Nano-niosomes in drug, vaccine and gene delivery: a rapid overview. Nano med Journal. 2013; 1: 1-12.
- Wagh V, Deshmukh O. Itraconazole niosomes drug delivery system and its antimycotic activity against Candida albicans. International scholarly research network pharmaceutics. 2012; 1-7.
- Patel K, Kumar P, Thakkar H. Formulation of niosomal gel for enhanced transdermal lopinavir delivery and its comparative evaluation with ethosomal gel. AAPS Pharm Sci Tech. 2012; 13: 1502-1510.
- Yoshioka T, Stermberg B, Florence AT. Preparation and properties of vesicles (niosomes) of sobitan monoesters (Span 20, 40, 60, and 80) and a sorbitan triester (Span 85). Int J Pharm. 1994; 105: 1-6.
- Solanki A, Parikh J and Parikh R. Preparation, Characterization, Optimization and Stability Studies of Aceclofenac Proniosomes. International Journal of Pharmaceutical Research. 2008; 7: 237-246.
