Abstract
Depression is a common and serious mental disorder characterized by persistent feeling of sadness and loss of interest in activities. Despite conventional treatments can improve depression symptoms and prevent a recurrence, complementary and alternative therapies are necessary to be involved because of the detrimental effects of the current therapy. Traditional Chinese Medicine (TCM) formulas are considered to be a potential complementary method with special advantages for treating the multiple pathogenesis of depression in a holistic way. However, the underlying pharmacological mechanism of TCM formula on depression therapy remained elusive. Therefore, in current study, a systems pharmacology approach integrating Absorption, Distribution, Metabolism, and Excretion (ADME) filtering, target fishing, gene enrichment analysis, network pharmacology, and pathway analysis was developed to comprehensively explore the multiple therapeutic mechanisms of Danzhi Xiaoyao San (DZXYS) for the treatment of depression. Finally, 111 active components and 112 potential targets of DZXYS were identified through ADME filtering and target fishing. Gene enrichment analysis found that DZXYS played a significant role in treating depression through the regulation of multiple pathogenesis including neurotransmitter systems imbalance, brain derived neurotrophic factor, hypothalamic-pituitary-adrenal axis dysregulation, inflammation, and immune. Moreover, the compound-target and target-pathway networks were constructed to elucidate the therapeutic mechanism of DZXYS from the system perspective. This study proposes a promising way for facilitating in-depth understanding of the complicated therapeutic mechanism of TCM on depression.
Keywords: Danzhi xiaoyao san; Systems pharmacology; Active compounds; Depression; Mechanisms
Introduction
Depression is a multifactorial psychiatric disorder, characterized by specific symptoms including listlessness, interest drops, extreme of inferiority, hopelessness or guilt and a reduced ability to enjoy life [1]. According to the report released by World Health Organization (WHO), more than 350 million people worldwide are affected by depression, leading to a tremendous public health burden [2]. Evidence showed that depression is a systemic disease rather than a mental disorder, which always involves different pathophysiology mechanisms including neurotransmitter systems imbalance, decreased Brain Derived Neurotrophic Factor (BDNF), abnormal gene expression, Hypothalamic-Pituitary-Adrenal (HPA) axis dysregulation, impaired inflammatory response and immune regulation.
Antidepressant drugs are commonly used to help alleviate the symptoms of depression and prevent relapse of the illness. The current available clinical antidepressant medications mainly act on monoamine transmitters including serotonin and norepinephrine [3]. However, these drugs often cause detrimental effects, such as lagging, low compliance and failed treatment, which will lead to the intolerant or refractory responses of many patients [4,5]. Therefore, it is urgent to develop antidepressant drugs based on multiple pathogenesis against depression. Traditional Chinese medicine (TCM) as one of the major complementary and alternative medicine therapies featured as multi-components and multiple targets have been shown to possess special advantages for the treatment of complex pathogenesis of depression [6,7].
Danzhi Xiaoyao San (DZXYS), recognized as a classical prescription from Abstract for Chinese Internal Medicine (Nei Ke Zhai Yao) has been widely used to treat depression for many years. This herbal formula is composed of Radix Bupleuri (Chai- Hu), Paeoniae Radix Alba (Bai-Shao), Angelica Sinensis Radix (Dang-Gui), Fu-Ling, Atractylodes macrocephala Koidz. (Bai-Zhu), Licorice (Gan-Cao), Moutan Cortex (Mu-Dan-Pi), and Gardeniae Fructus (Zhi-Zi). A previous clinical study suggested that DZXYS played a significant role in lowering the Hamilton depression scale (HAMD), reducing the scores of anxiety/somatization, reversing cognitive impairment and feeling of despair in depressive patients [8]. Although DZXYS exhibit multiple therapeutic effects for relieving the symptoms of depression, its active compounds, related targets, pathways and underlying pharmacological mechanism remain elusive.
Systems pharmacology has been suggested as a promising approach to shift away from the traditional “one-drug, one-target, one-disease” towards the “multi-component, multi-target” strategy, which will shed light on a comprehensive investigation to systemically dissect the relationship between drugs, targets, pathways and diseases, as well as the potential pharmacological mechanisms of herbal formulae on disease therapy. Growing evidence revealed that systems pharmacology method is capable of uncovering the complicated pharmacological mechanisms of TCM herbs and formulas on the treatment of various diseases. For example, the combinatorial rule and the pharmacological mechanisms of Qing-Luo-Yin and Liu-Wei-Di-Huang Pill for the treatment of rheumatoid arthritis and various diseases were elucidated from a network/systemic perspective [9,10]. In our previous work, systems pharmacology has been successfully used to explore the potential mechanisms of the single herb and herb pairs for various diseases therapy [11,12]. In addition, a series of systems pharmacology frameworks have been developed to disclose the underlying mechanisms of TCM formulas for stroke, asthma and obesity [13-15].
Therefore, in current study, an integrative systems pharmacology strategy, which combines ADME (absorption, distribution, metabolism, and excretion) filtering, target fishing, gene enrichment analysis, network pharmacology and pathway analysis is proposed to reveal the multiple mechanisms of DZXYS in treating depression based on a holistic and systemic way. The detailed work flowchart is shown in Figure 1. The systems pharmacology framework constructed in our study not only sheds light on providing a probe to explore the complex therapeutic mechanism of DZXYS for depression at the system level, but also promotes the modernization and internationalization of TCM formulas.
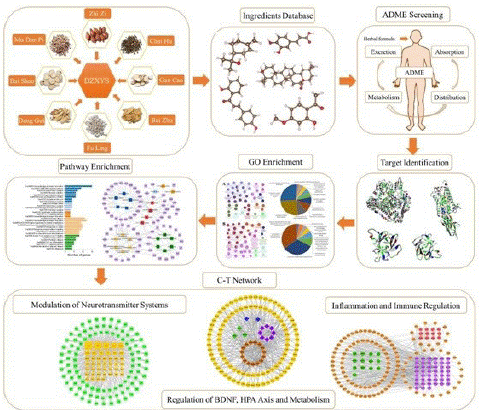
Figure 1: Overall survival, autologous stem cell transplant (ASCT) versus no ASCT (p=0.12).
Materials and Methods
Building the Compound Database of DZXYS
The chemical constituents of DZXYS were extracted from the TCMSP (Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform, https://tcmspw.com/tcmsp.php) database, and then manually supplemented from abundant literatures of CNKI and PubMed databases. All structures of these constituents were downloaded from TCMSP and NCBI PubChem databases, and then subjected to full geometry optimization using GUASS package with the same parameters as our previous work [16,17]. Considering that compounds with glycosyls cloud undergo glycosidase hydrolysis reaction before being absorbed, in this section, their corresponding aglycones were also added into the compound database of DZXYS for further research.
Active Compounds Screening
Predicting ADME properties of drug candidate molecules plays an important role in drug development, because of the fact that the failure of 60% of drug molecules is related to poor ADME properties [18]. Therefore, early ADME prediction of a candidate compound can significantly reduce the cost of drug research. For herbal medicine, it usually contains hundreds of constituents, but only a few bioactive compounds provide physiological benefits for human health. Identifying the active ingredients with favorable ADME properties is a fundamental step to uncover the therapeutic effects of DZXYS. In the present work, three in silico models, i.e., Drug-Likeness (DL), Oral Bioavailability (OB), and Blood-Brain Barrier (BBB) were employed to screen the potential active compounds of DZXYS.
Drug-likeness calculation: Drug-likeness is a qualitative profile used in drug design to optimize pharmacokinetic and pharmaceutical properties, such as solubility, and chemical stability. Therefore, for deciphering the molecular mechanism of functional food, it is first necessary to apply drug-likeness filter to remove non-drug-likeness compounds from the molecule database, and then just focus on the drug-likeness molecules [19]. In the present work, a robust in silico model was employed to screen the molecules with drug-likeness features from compound database of DZXYS. In this model, Tanimoto coefficient (as shown in Equation (1)) was used to calculate the DL index of each compound of DZXYS.
In which, A is the molecular descriptors of constituents of DZXYS, and B is the average molecular properties of 6511 small drug-like and drug molecules in DrugBank database (http://www.drugbank.ca). The DL threshold is ultimately set to 0.18, which is based on the average DL value in the DrugBank database.
Oral bioavailability evaluation: Being an important ADME parameter, oral bioavailability indicates the fractional extent of the oral bioactive molecule dosage which finally reaches its target site to exhibit the pharmacological activity [20]. In present work, OB value was also employed to screen the active constituents from DZXYS, and the values were computed through a reliable in silico model OBioavail 1.1 [21]. This model was built using metabolism (P450 3A4) and transport (P-glycoprotein) information of 805 drug and drug-like molecules. The linear (i.e., Partial Least Square (PLS), Multiple Linear Regression (MLR)), and nonlinear (support vector machine (SVR)) methods were used to construct this model, ending up with an optimal model holding a determination coefficient (R²) of 0.80 and a Standard Error of Estimate (SEE) of 0.31 for test sets. Finally, herbal constituents with proper OB values (OB ≥30%) were screened as the candidate compounds for further research.
BBB penetration: Blood-brain barrier is a highly selective semipermeable membrane that regulates the passage of small and large molecules into the Central Nervous System (CNS) [22]. Therefore, being the chief obstacle to the delivery of drugs to the CNS, BBB permeability was selected to identify the candidate ingredients that targeted the patient’s. CNS involved in the damp depression. Here, a reliable in silico BBB model constructed in our previous work [23] was employed to predict the BBB permeabilities of all compounds in DZXYS databases. Finally, the compounds with BBB values greater than -0.3 were identified as favorable BBB permeabilities, since molecules with less than -0.3 is not permeable.
Target Fishing and Gene Ontology Enrichment Analysis
Identifying compound-target interaction is a crucial task in drug discovery and elucidating the mechanism of drug action [24]. Here, a combinatorial method enriched with chemogenomic, similarity ensemble and data-mining approaches was employed to identify the compound-target interaction. First, the filtered active compounds were sent to the TTD (Therapeutic Targets Database http://bidd.nus.edu.sg/group/ttd/) and DrugBank databases to mine the compound-target interactions supported by abundant literatures. Second, two robust in silico models for identifying compound-target interaction, i.e., omics-based Ligand-Target Chemogenomic model (LTC) [25] and Similarity Ensemble Approach (SEA, http://sea.bkslab.org/) [26], were implemented to obtain the target proteins of filtered active compounds. Subsequently, all target information from the above two steps was sent to the database of Uniprot database (https://www.uniprot.org/) to eliminate the overlaps and noise. Finally, we mapped all target genes of DZXYS to the databases of Drug Bank, TTD, OMIM and DisGeNET to build the connections with depression.
Gene Ontology (GO) analysis was employed to reveal how active compounds of DZXYS acted on the multiple pathological mechanisms of depression. All depression- related target proteins of DZXYS were inputted into ClueGO plugin of Cytoscape 3.8.2 using p-value≤0.05 as the restriction, and GO enriched terms for cellular components, biological process and molecular functions were identified.
Network Construction and Analysis
Abundant experimental and clinical evidence has demonstrated that the pathological mechanism of depression mainly implicated in disorder of neurotransmitter, dysfunction of nervous system (BDNF, brain-derived neurotrophic factor), disorder of signal transduction (HPA (Hypothalamic Pituitary Adrenal) axis), metabolism imbalance, as well as dysregulation of inflammatory and immune responses. All target proteins of DZXYS were mapped onto these different pathological mechanisms of depression. Subsequently, Compound-Target network (C-T network) implicated in six individual pathological mechanisms were constructed to further discover the underlying mechanism of DZXYS in the treatment of depression. Pathway enrichment was employed to obtain the representative pathways of six pathological mechanism involved in depression based on Metascape databases [27]. Afterwards, these pathways and their linked target genes of DZXYS were used to construct T-P network. All visualized network graphs were constructed by Cytoscape 3.8.2 [28].
Results and Discussion
Filtering Active Components from DZXYS
A total of 1078 DZXYS candidate molecules were finally extracted from abundant literatures and TCMSP database, including 1036 ingredients from these 8 herbal medicines and 42 aglycones (Supplementary Table S1). Among them, Chai-Hu owned the largest number of constituents (347 molecules), as followed by Gan-Cao with 280 components and Dang-Gui with 126 chemicals. Other herbal medicines, Zhi-Zi, Bai- Shao, Mu-Dan-Pi, Bai-Zhu, and Fu-Ling contained 97, 84, 57, 55 and 33 compounds, respectively.
In general, an oral administrated medicine must overcome several in vivo obstacles like bioavailability, chemical and physical stabilities, before exhibition of the pharmacological effect at the binding site. Therefore, to identify the active molecules from DZXYS, we employed three classical in silico ADME parameters (including DL, OB and BBB). As a result, 106 active molecules from these eight herbal medicines in the DZXYS were found meeting the screening thresholds with satisfactory ADME profiles. For example, liquiritigenin, a flavonoid compound in Gan-Cao, displayed the favorable pharmacokinetic profiles (OB=32.76%, DL=0.18 and BBB=-0.29), and this compound was proved alleviating depressive-like behavior via inhibiting p-phosphatidylinositol 3-kinase (PI3K)/Akt/p-Mammalian Target of Rapamycin (mTOR) pathway and improving BDNF/p-tropomyosin-related kinase B (TrkB) pathway in mice model of depression [29]. A phytosterol compound beta-sitosterol (OB=36.91%, DL=0.87 and BBB=0.75) which is shared by Bai-Shao, Dang-Gui, Gan-Cao, Mu-Dan-Pi and Zhi-Zi also displayed anti-depressant-like activity in mice model. Betulinic acid (OB=55.38%, DL=0.78 and BBB=0.22), a pentacyclic triterpenoid shared by Bai-Shao, Gan-Cao and Mu-Dan-Pi, also reduced depressant-like behavior in tail suspension test [30]. A stigmastane-type phytosterol in Chai-Hu, a-spinasterol (OB=42.98%, DL=0.76 and BBB=0.79), which is a multitarget transient receptor potential cation channel subfamily V member 1 (TRPV1) antagonist and cyclooxygenase (PTGS1 (prostaglandin G/H synthase 1) and PTGS2 (prostaglandin G/H synthase 2)) inhibitor, exhibited anti-depressant-like effect in mice model [31,32]. Hederagenin (OB=36.91%, DL=0.75 and BBB=0.96), a triterpenoid saponin in Chai-Hu, also exhibited the anti-depression activity via regulating the expression of monoamine neurotransmitters and 5-hydroxytryptamine (serotonin) 1A receptor mRNA in rat model of depression [33]. a-Amyrin (OB=39.51%, DL=0.76 and BBB=1.28) was a pentacyclic triterpenoid from Bai-Zhu, and also exhibited the potent anti-depression effect through increasing the activities of amine oxidase [flavin-containing] A (MAOA) and Gamma Aminobutyric acid (GABA) in mice hippocampus [34]. Isoimperatorin (OB=45.46%, DL=0.23 and BBB=0.66), an active furocoumarin in Zhi-Zi, had a dual PTGS2 selective/5-lipoxygenase inhibitory property, making it possible for a novel anti-inflammatory drug. All these results convincingly proved the reliability and feasibility of our filter model.
As we know, different from the allopathy of western medicine, herbal formula featured as a holistic treatment regulates multiple therapeutic pathways to maintain the balance of human body. Factually, the pathological factors implicated in depression not only focus on the central nervous system, but also correlate with the cytokine hypothesis, oxidative stress and NO pathway [35]. Therefore, some major ingredients which possessed pharmacokinetic and pharmacological properties, but low BBB indexes, were also added into the active compound database, including paeoniflorin (OB=53.87%, DL=0.79, BBB=-1.86) and pachymic acid (OB=33.63%, DL=0.81, BBB=-0.57). For instance, paeoniflorin, the major active constituent found in Bai-Shao and Mu-Dan-Pi, had been reported to modulate HPA axis, via up-regulating serotonergic (serotonin and 5-hydroxyindoleacetic acid) and noradrenergic (noradrenaline) systems, ameliorating neuroinflammation and depressive-like behaviors, which contributed to a candidate molecule for the treatment of depression [36,37]. The other compound pachymic acid, a predominant triterpenoid in Fu-Ling, also had multiple pharmacological properties, such as anti-inflammation, anti-cancer and anti-vomiting [38].
To avoid the missed active constituents, a few active molecules were also manually added into the active molecules database of DZXYS based on text-mining in literature databases. For example, geniposide (OB=14.64%, DL=0.44, BBB=-2.61), as one of the main active molecules (73.44±2.62mg/g) in Zhi-Zi with both medicinal and nutritional effects [39], produced the antidepressant-like property via regulating HPA axis in chronic unpredictable mild stress-induced depressive rats [40], and this compound also ameliorated the depression-like behavior through up-regulating BDNF expression in streptozotocin-evoked diabetic mice [41]. In addition, albiflorin (OB=12.09%, DL=0.77, BBB=-2.19) had a high concentration (12.61±3.09 mg/g) in Bai-
Shao [42], exhibited obvious anti-depressant-like effect via up-regulating the expression levels of hippocampal serotonin (5-HT)/Norepinephrine (NE) and BDNF in mice model of depression [43]. Ferulic acid (OB=39.56%, DL=0.06, BBB=-0.03) in Dang-Gui is a marker for evaluating the quality of this herb, because of relative high concentration (0.21~1.75mg/g dry weight) and abundant pharmacological effects, like antidepressant-like, anti-inflammatory, anti-microbial, and anti-hyperlipidemic properties [44]. Therefore, these molecules were manually added into the active molecule database of DXP, although they obtained the low DL values or OB indexes.
By combining the results of filter model and text-mining, finally, 120 molecules were screened as the candidate active ingredients of DZXYS, and their ADME parameters were displayed in Table 1. Among them, Gan-Cao has the largest number of active ingredients (72 molecules), as followed by Chai-Hu and Mu-Dan-Pi both with
MOL ID
Molecule Name
CAS
DL
OB(%)
BBB
Herbs
CH257
(+)-Anomalin
73069-28-0
0.66
46.06
0.00
Chai-Hu
BZ016
(24S)-24-Propylcholesta-5-ene-3beta-ol
64997-52-0
0.78
36.23
1.09
Bai-Zhu
GC200
(2R)-7-Hydroxy-2-(4-hydroxyphenyl)
chroman-4-one41680-09-5
0.18
71.12
-0.25
Gan-Cao
GC173
1,3-Dihydroxy-9-methoxy-6- benzofurano[3,2-c]chromenone
-
0.43
48.14
-0.19
Gan-Cao
GC218
1-Methoxyphaseollidin
65428-13-9
0.64
69.98
0.48
Gan-Cao
CH205
3',4',5',3,5,6,7-Heptamethoxyflavone
17245-30-6
0.59
31.97
0.08
Chai-Hu
ZZ053
3-Epioleanolic acid
25499-90-5
0.76
32.03
0.39
Zhi-Zi
GC225
3'-Hydroxy-4'-O-Methylglabridin
-
0.57
43.71
0.73
Gan-Cao
GC233
3'-Methoxyglabridin
74046-05-2
0.57
46.16
0.47
Gan-Cao
BZ032
3β-Acetoxyatractylone
61206-10-8
0.22
54.07
1.08
Bai-Zhu
GC237
4'-Methoxyglabridin
-
0.52
36.21
0.61
Gan-Cao
MDP040
4-O-methylpaeoniflorin_qt
-
0.43
67.24
-0.15
Mu-Dan-Pi
GC247
6-Prenylated eriodictyol
-
0.41
39.22
-0.29
Gan-Cao
GC249
7-Acetoxy-2-methylisoflavone
3211-63-0
0.26
38.92
0.16
Gan-Cao
GC057
7-Methoxy-2-methyl isoflavone
19725-44-1
0.20
42.56
0.56
Gan-Cao
BZ055
8β-Ethoxy atractylenolide ?
113269-35-5
0.21
35.95
1.12
Bai-Zhu
ZZ062
Ammidin
482-44-0
0.22
34.55
0.92
Zhi-Zi
CH216
Areapillin
83162-82-7
0.41
48.96
-0.29
Chai-Hu
BS007
Beta-sitosterol
83-46-5
0.75
36.91
0.87
Bai-Shao
DG021
Beta-sitosterol
83-46-5
0.75
36.91
0.87
Dang-Gui
GC011
Beta-sitosterol
83-46-5
0.75
36.91
0.87
Gan-Cao
MDP010
Beta-sitosterol
83-46-5
0.75
36.91
0.87
Mu-Dan-Pi
ZZ014
Beta-sitosterol
83-46-5
0.75
36.91
0.87
Zhi-Zi
BS002
Betulinic acid
472-15-1
0.78
55.38
0.22
Bai-Shao
GC007
Betulinic acid
472-15-1
0.78
55.38
0.22
Gan-Cao
MDP005
Betulinic acid
472-15-1
0.78
55.38
0.22
Mu-Dan-Pi
ZZ069
Corymbosin
18103-41-8
0.41
51.96
-0.21
Zhi-Zi
FL028
Dehydroeburicoic acid
6879-05-6
0.83
44.17
-0.16
Fu-Ling
GC278
Dehydroglyasperins C
199331-35-6
0.37
53.82
-0.12
Gan-Cao
FL015
Eburicoic acid
560-66-7
0.81
38.70
-0.04
Fu-Ling
FL010
Ergosta-7,22e-dien-3beta-ol
1105-11-9
0.72
43.51
0.91
Fu-Ling
FL011
Ergosterol peroxide
2061-64-5
0.81
40.36
0.34
Fu-Ling
ZZ068
Ethyl oleate (NF)
111-62-6
0.19
32.40
1.10
Zhi-Zi
GC068
Euchrenone
-
0.57
30.29
0.39
Gan-Cao
GC175
Eurycarpin A
166547-20-2
0.37
43.28
-0.06
Gan-Cao
GC013
Formononetin
485-72-3
0.21
69.67
0.02
Gan-Cao
GC254
Gadelaidic acid
506-31-0
0.20
30.70
0.94
Gan-Cao
GC116
Gancaonin A
27762-99-8
0.40
51.08
0.13
Gan-Cao
GC117
Gancaonin B
124596-86-7
0.45
48.79
-0.10
Gan-Cao
GC258
Gancaonin G
126716-34-5
0.39
60.44
0.23
Gan-Cao
GC259
Gancaonin H
126716-35-6
0.78
50.10
-0.14
Gan-Cao
GC123
Gancaonin L
129145-50-2
0.41
66.37
-0.13
Gan-Cao
GC124
Gancaonin M
129145-51-3
0.41
30.49
0.21
Gan-Cao
GC126
Gancaonin O
129145-53-5
0.41
44.15
-0.28
Gan-Cao
GC170
Glabranin
41983-91-9
0.31
52.90
0.31
Gan-Cao
GC171
Glabrene
60008-03-9
0.44
46.27
0.04
Gan-Cao
GC168
Glabridin
59870-68-7
0.47
53.25
0.36
Gan-Cao
GC172
Glabrone
60008-02-8
0.50
52.51
-0.11
Gan-Cao
GC089
Glepidotin A
42193-83-9
0.35
44.72
0.06
Gan-Cao
GC090
Glepidotin B
87440-56-0
0.34
64.46
-0.09
Gan-Cao
GC070
Glyasperin B
142488-54-8
0.44
65.22
-0.09
Gan-Cao
GC073
Glyasperin C
142474-53-1
0.40
45.56
0.07
Gan-Cao
GC072
Glyasperin F
145382-61-2
0.54
75.84
-0.15
Gan-Cao
GC265
Glyasperins M
-
0.59
72.67
-0.04
Gan-Cao
GC139
Glycyrin
66056-18-6
0.47
52.61
-0.13
Gan-Cao
GC045
Glycyrol
23013-84-5
0.67
90.78
-0.20
Gan-Cao
GC096
Glypallichalcone
146763-58-8
0.19
61.60
0.23
Gan-Cao
GC167
Glyzaglabrin
65242-64-0
0.35
61.07
-0.20
Gan-Cao
FL024
Hederagenin
465-99-6
0.75
36.91
0.96
Fu-Ling
GC243
Icos-5-enoic acid
7329-42-2
0.20
30.70
1.09
Gan-Cao
GC034
Inermine
2035-15-6
0.54
75.18
0.40
Gan-Cao
GC239
Inflacoumarin A
158446-33-4
0.33
39.71
-0.24
Gan-Cao
GC204
Isobavachin
31524-62-6
0.32
36.57
-0.04
Gan-Cao
Table 1: The active ingredients and ADME parameters of DZXYS.
GC216
Isoformononetin
486-63-5
0.21
38.37
0.25
Gan-Cao
GC207
Isoglycyrol
23013-86-7
0.84
44.70
0.05
Gan-Cao
ZZ063
Isoimperatorin
482-45-1
0.23
45.46
0.66
Zhi-Zi
GC076
Isotrifoliol
329319-08-6
0.42
31.94
-0.25
Gan-Cao
GC008
Jaranol
3301-49-3
0.29
50.83
-0.22
Gan-Cao
GC077
Kanzonol B
-
0.35
39.62
-0.12
Gan-Cao
GC246
Kanzonol F
-
0.89
32.47
0.56
Gan-Cao
GC099
Kanzonol U
178330-48-8
0.38
58.44
0.34
Gan-Cao
GC082
Kanzonol W
184584-82-5
0.52
50.48
0.04
Gan-Cao
GC261
Licoagrocarpin
202815-29-0
0.58
58.81
0.61
Gan-Cao
GC270
Licoagroisoflavone
-
0.49
57.28
0.09
Gan-Cao
GC110
Licoarylcoumarin
125709-31-1
0.43
59.62
-0.23
Gan-Cao
GC022
Licochalcone a
58749-22-7
0.29
40.79
-0.21
Gan-Cao
GC109
Licochalcone G
1261240-29-
20.32
49.25
-0.04
Gan-Cao
GC142
Licocoumarone
118524-14-4
0.36
33.21
0.06
Gan-Cao
GC145
Licoisoflavanone
66067-26-3
0.54
52.47
-0.22
Gan-Cao
GC143
Licoisoflavone
66056-19-7
0.42
41.61
-0.27
Gan-Cao
GC144
Licoisoflavone B
66056-30-2
0.55
38.93
-0.18
Gan-Cao
GC115
Licoricone
51847-92-8
0.47
63.58
-0.14
Gan-Cao
CH122
Linoleyl acetate
5999-95-1
0.20
42.10
1.08
Chai-Hu
GC040
Liquiritigenin
578-86-9
0.18
32.76
-0.29
Gan-Cao
CH230
Longikaurin A
75207-67-9
0.53
47.72
0.09
Chai-Hu
GC054
Lupiwighteone
104691-86-3
0.37
51.64
-0.23
Gan-Cao
ZZ044
Mandenol
544-35-4
0.19
42.00
1.14
Zhi-Zi
GC047
Medicarpin
607363-34-8
0.34
49.22
0.53
Gan-Cao
CH234
Octalupine
6809-89-8
0.28
47.82
0.30
Chai-Hu
GC274
Odoratin
53948-00-8
0.30
49.95
-0.24
Gan-Cao
GC275
Phaseol
88478-02-8
0.58
78.77
-0.06
Gan-Cao
GC094
Phaseolinisoflavan
40323-57-7
0.45
32.01
0.46
Gan-Cao
GC050
Pinocembrin
480-39-7
0.18
64.72
0.12
Gan-Cao
CH249
Sainfuran
90664-32-7
0.23
79.91
0.23
Chai-Hu
GC067
Shinflavanone
157414-03-4
0.72
31.79
0.25
Gan-Cao
GC151
Shinpterocarpin
157414-04-5
0.73
80.30
0.68
Gan-Cao
CH051
Stigmasterol
83-48-7
0.76
43.83
1.00
Chai-Hu
DG026
Stigmasterol
83-48-7
0.76
43.83
1.00
Dang-Gui
ZZ020
Stigmasterol
83-48-7
0.76
43.83
1.00
Zhi-Zi
ZZ083
Sudan III
85-86-9
0.59
84.07
0.10
Zhi-Zi
ZZ046
Supraene
111-02-4
0.42
33.55
1.73
Zhi-Zi
FL004
Trametenolic acid
24160-36-9
0.80
38.71
-0.14
Fu-Ling
GC023
Vestitol
20879-05-4
0.21
74.66
0.30
Gan-Cao
GC276
Xambioona
82345-36-6
0.87
54.85
0.52
Gan-Cao
BZ011
a-Amyrin
638-95-9
0.76
39.51
1.28
Bai-Zhu
CH318
a-Spinasterol
481-18-5
0.76
42.98
0.79
Chai-Hu
BZ026
Atractylenolide I
73069-13-3
0.15
37.37
1.29
Bai-Zhu
BZ029
Atractylone
6989-21-5
0.13
41.10
1.85
Bai-Zhu
FL017
Pachymic acid
29070-92-6
0.81
33.63
-0.57
Fu-Ling
DG022
Ferulic acid
537-98-4
0.06
39.56
-0.03
Dang-Gui
MDP017
Paeonol
552-41-0
0.04
28.79
0.84
Mu-Dan-Pi
BS068
Paeoniflorin
23180-57-6
0.79
53.87
-1.86
Bai-Shao
MDP022
Paeoniflorin
23180-57-6
0.79
53.87
-1.86
Mu-Dan-Pi
BS071
Albiflorin
39011-90-0
0.77
12.09
-2.19
Bai-Shao
DG056
(Z)-Ligustilide
4431-01-0
0.07
51.30
1.24
Dang-Gui
ZZ080
Geniposide
24512-63-8
0.44
14.64
-2.61
Zhi-Zi
CH240
Saikosaponin a
20736-09-8
0.09
32.39
-2.93
Chai-Hu
CH242
Saikosaponin D
20874-52-6
0.09
34.39
-2.89
Chai-Hu
GC066
Enoxolone
471-53-4
0.74
22.05
-0.53
Gan-Cao
GC136
Glycyrrhizic acid
1405-86-3
0.11
19.62
-2.86
Gan-Cao
Table 1 off 1:
11 active molecules. In addition, Bai-Shao, Bai-Zhu, Mu-Dan-Pi, Dang-Gui and Bai-Shao contained 7, 6, 5, 4 and 4 active ingredients, respectively.
Target Identification and Gene Ontology Enrichment Analysis
To further decode the therapeutic targets of DZXYS, a comprehensive range of target identification was provided. As a result, 431 targets were collected for 119 active ingredients, while only one compound (octalupine, CH234) had no related target protein. In order to focus on depression-related genes, 850 depression-related targets were collected based on the search engine results from four gene-disease databases. And then 431 targets and 119 active compounds of DZXYS were mapped to these 850 depression-related targets to build the connections with depression. Finally, 111 active compounds of DZXYS were identified interacting with 112 depression-related proteins (as displayed in Table S2).
As shown in Table S2, the overwhelming majority of those active ingredients (105 out of 111 compounds) were connected with more than one target proteins, displaying their multiple mechanisms of action. For example, ferulic acid, the main active compound of Dang-Gui, not only inhibited MAOA selectively [45], but also prevented the activity of ornithine decarboxylase (ODC1) by 46% [46]. Glycyrrhizic acid, which is the major sweet-tasting ingredient of Gan-Cao, not only suppressed the activity of caspase-3 (CASP3) [47], but also inhibited corticosteroid 11-beta-dehydrogenase isozyme 1 (HSD11B1) activity in rat [48]. Paeoniflorin, the major active compound of Bai-Shao and Mu-Dan-Pi, exerted a neuroprotective effect through activating adenosine receptor A1 (ADORA1) in rat [49], and also inhibited TNF (tumor necrosis factor), IL6 (Interleukin-6) to improve the cardiac function in rat model with ventricular remodeling [50]. Enoxolone, also called as glycyrrhetinic acid, was a pentacyclic triterpenoid derivative of Gan-Cao, and inhibited the expression of cytochrome P450 2E1 (CYP2E1) to attenuate the acetaminophen-induced liver injury in mice [51]. Additionally, as the major active ingredient of Zhi-Zi, geniposide upregulated HMOX1 (heme oxygenase 1) expression to improve the capacity of anti-oxidant effect in primary hippocampal neurons [52]. All these results documented that the bioactivity compounds in herbal medicines exhibited the promiscuous actions.
In order to explore the underlying molecular mechanism of these 112 targets DZXYS, GO enrichment analysis, including biological process, molecular function, and cellular component were carried out. As shown in Figure 2A, the top 5 enriched GO molecular processes included G protein-coupled amine receptor activity, regulation of blood vessel diameter, cellular response to catecholamine stimulus, chemical synaptic transmission, postsynaptic, and neurotransmitter biosynthetic process, which have been suggested to implicate in the pathological mechanism of depression. For example, G protein-coupled amine receptor, which is the largest human membrane protein family, is involved in numerous physiological functions such as parasympathetic and
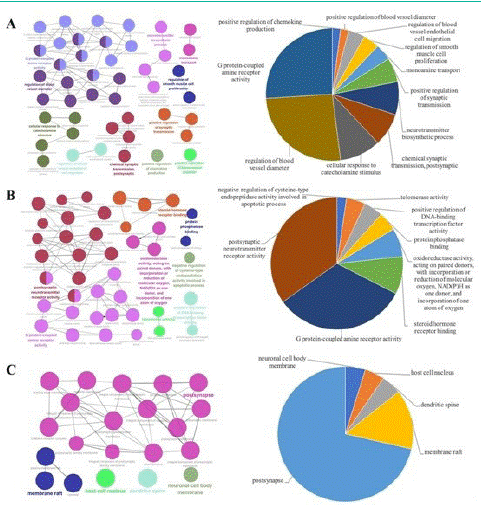
Figure 2: GO enrichment analysis for the biological process (A), molecular function (B) and cellular component (C) of 112 depression-related targets of DZXYS. The nodes show the representative- enriched GO terms.
sympathetic nervous functions, immune regulation and metabolism, and has been found to be major therapeutic targets in various human diseases like psychiatric diseases and neurodegeneration [53].
In term of GO molecular function (Figure 2B), the candidate chemicals were mainly enriched in postsynaptic neurotransmitter receptor activity, G protein-coupled amine receptor activity, and steroid hormone receptor binding, etc. These molecular functions are linked with the pathological pathways of depression. For example, anti-depression drugs, such as Selective Serotonin Reuptake Inhibitors (SSRIs), Tricyclic Antidepressants (TCA), and Serotonin–Norepinephrine Reuptake Inhibitors (SNRIs), primarily inhibited the reuptake of monoamine neurotransmitters (like dopamine, noradrenaline, and serotonin) in an attempt to enhance the persistence of these neurotransmitters in the synaptic space to trigger postsynaptic neurotransmission receptors [54,55]. For GO cellular component (Figure 2C), the major enriched GO ontologies were post-synapse, membrane raft, dendritic spine, and so on, which are closely related with depressive disease. For example, the postsynaptic release of endocannabinoids played an important role in long-term depression in striatum [56].
Network Analysis to Explore the Therapeutic Mechanisms of DZXYS
Compound-Target network analysis: Modulation of Neurotransmitter Systems Plenty of experimental and clinical studies documented that neurotransmitter systems play the fundamental roles in the pathological mechanism of depression [57,58]. Actually, the petroleum ether soluble fraction and water-EtOH soluble fraction of DZXYS displayed anti-depressive effects through regulating the monoamine neurotransmitters (like 5-HT, dopamine and noradrenaline) and amino acid neurotransmitters in the hippocampus of rat models with Chronic
Unpredictable Mild Stress (CUMS) [59]. Therefore, the C-T network of DZXYS implicated in neurotransmitter systems was constructed as displayed in Figure 3.
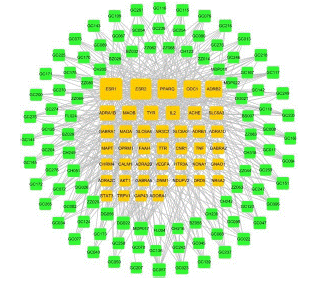
Figure 3: The C-T network of DZXYS involved in depression through modulating neurotransmitter systems. The green nodes represent the active ingredients of DZXYS, and the yellow nodes are the target proteins.
Figure 3 showed that 43 target proteins are implicated in disorder of neurotransmitter secretion. Among them, ESR1 (estrogen receptor), ESR2 (estrogen receptor beta), PPARG (peroxisome proliferator activated receptor gamma), ODC1, ADRB2 (beta-2 adrenergic receptor), ADRA1B (alpha-1B adrenergic receptor), MAOB (amine oxidase [flavin-containing] B), and TYR (tyrosinase), which linked with 69, 61, 51, 40, 36, 25, 21 and 21 active ingredients of DZXYS, respectively, were the hub targets of DZXYS for regulating neurotransmitter systems. For example, ESR1 played a certain role in etiology of postpartum depression probably via regulating the signaling of neurotransmitters [60]. Additionally, the researches of electrophysiology and microdialysis proved that the activation of PPARG weakened the ability of drug abuse to mediate dopaminergic neurons of the ventral tegmental area, and then decreased the level of dopamine (one type of neurotransmitter) released in the nucleus accumbens [61,62].
As displayed in Figure 3, five ingredients, including MDP017 (paeonol), DG022 (ferulic acid), GC057 (7-methoxy-2-methyl isoflavone), DG056 ((Z)-ligustilide), and GC023 (vestitol), were the main active ingredients of this formula to regulate neurotransmitter system, because of their hub roles in C-T network and/or high contents in the herbal medicine of DZXYS. For instance, paeonol was the main ingredient isolated from Mu-Dan-Pi, and interacted with 15 target proteins in C-T network (Figure 3), such as MAOA, MAOB, TNF (tumor necrosis factor), and ADRB1 (beta-1 adrenergic receptor), et al. In fact, this phenol compound was documented not only inhibiting MAOA and MAOB in a dose-dependent manner with IC50 values of 54.6 and 42.5μM [63], respectively, but also dose-dependently suppressing TNF formation [64].
Regulation of BDNF, HPA axis and metabolism: Based on the recent metabolomics studies, the proposed hypotheses for the pathological and pharmacological mechanism of depression included BDNF, hyperactivity of HPA axis, and metabolism. In fact, the clinical studies demonstrated that DZXYS was effective in modifying symptoms of depression patients by increasing the serum level of BDNF [8]. An experimental research documented that DZXYS acted on the arginine vasopressin to modulate HPA axis, and then displayed the anti-
depressant effect in the CUMS rat model [59]. Zhu XL et al. proved that DZXYS enhanced excitability and displayed anti-depressant properties via improving the metabolism of porphyrin, arachidonic acid, phenylalanine, D-arginine and D-ornithine, as well as biosynthesis of steroid hormone, unsaturated fatty acid and steroid in rat model of DZXYS [65]. Therefore, the anti-depressant effect of DZXYS via these three pathological mechanisms were also analyzed, and the corresponding C-T network was constructed as displayed in Figure 4.
An increasing number of evidence demonstrated that BDNF played a pivotal role in the pathogenesis of major depressive disorder and therapeutic efficacy of anti-depressant treatment [66]. As showed in Figure 4, we found that 16 targets, such as Androgen Receptor (AR), ESR2 and glycogen synthase kinase-3 beta (GSK3B), were
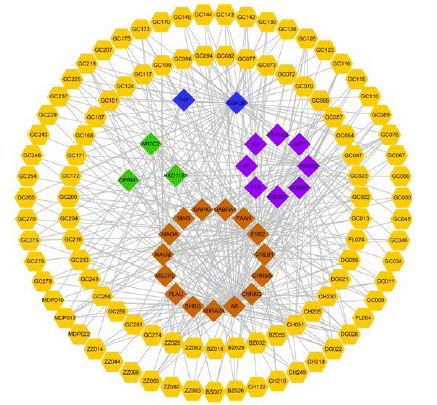
Figure 4: The C-T network of the active ingredients of DZXYS (yellow nodes) involved in depression through regulating the target proteins of BDNF (brown nodes), HPA axis (green nodes) and metabolism (purple nodes). The blue nodes show the shared target proteins of BDNF and metabolism, as well as HPA axis and metabolism.
implicated in the regulation of BDNF to display the anti-depression effect. For instance, the hub target AR, targeted by 64 active ingredients of DZXYS, was proved to modify the BDNF expression through modulating the expression of miR-204-5p, and then ameliorated the depressive-like behavior in the Chronic Mild Stress (CMS) mice model of depression [67]. Besides AR, several other targets, including ESR2, GSK3B and MAOB, which displayed a high number of interactions with the active compounds, were considered as the hub proteins to regulate BDNF to exhibit anti-depressive effects. For example, the weakened ESR2 signaling gave rise to down-regulated transcription of BDNF gene, which resulted in the low levels of BDNF in the hippocampal region [68]. All the evidence suggested that DZXYS may regulate multiple BDNF-related targets (like, AR, ESR2, GSK3B and MAOB) to exhibit the therapeutic effects on depression.
Dysregulation of HPA axis activity in patients with major depressive disorder is among the unanimous and powerful biological discoveries in psychiatry, which has been found to restore with efficacious treatment, although inconsistencies exist [69,70]. Factually, several researches had been proved that DZXYS could suppress the abnormal hyperactivity of HPA axis via acting on the arginine vasopressin to display anti-depressant property [59,71]. Some active ingredients of DZXYS in Figure 4, like paeoniflorin (MDP022, one of the major monoterpene glycosides in Bai-Shao) and glycyrrhizic acid (GC136, one of the major bioactive triterpene glycosides in Gan-Cao), also had been proved to exhibit anti-depressant effect through reducing HPA activity [72,73]. Taking paeoniflorin as an example, this compound ameliorated the depression-like behavior in male offspring rats, at least partially through restoring the HPA axis hyperfunction [73]. All these results indicated that several active ingredients of DZXYS(like paeoniflorin and glycyrrhizic acid) exhibited synergic anti-depressant effect through reducing the HPA axis dysfunction.
With the development of analytical technologies, several key metabolites, such as malonate, formate, glutamate, glucose, fructose, m-hydroxyphenylacetate, alanine, lipid/protein complexes, and amino acids were closely related to depressive disorder [74-76], and these metabolites were found mainly involved in energy metabolism, gut microbial metabolism, lipid metabolism and amino acids metabolism [57]. As displayed in Figure 4, 9 target proteins, like GSK3B, ADRB2, ABCB1 and ADRB1, have significant relationships with the pathological mechanism of depression via metabolomics. For example, the hub protein GSK3B, a serine threonine kinase, targeted by 48 active ingredients, was regarded as one of central regulators in the regulation of glucose metabolism [77]. Moreover, this protein was suggested to be involved in the pathogenetic mechanism of major depressive disorder, and to be a therapeutic target as an improver of anti-depressant action [78]. Therefore, all these findings proposed that DZXYS could regulate several metabolites to exhibit anti-depressant effects.
Inflammation and immune regulation: There is growing evidence that depression and inflammation have strong bidirectional links, fueling one another [79]. Specifically, all cardinal features of an inflammatory response were discovered in patients with major depression, such as enhanced expression of both pro-inflammatory cytokines and their receptors [80]. Therefore, the target proteins of DZXYS implicated in inflammatory response were also discussed in details. The C-T network (Figure 5) showed that 58 targets such as PTGS2, NOS2, PPARG, SCN5A and DPP4 related to inflammatory response. For example, PTGS2 was targeted by the 83 active ingredients of DZXYS, such as paeonol, glabrone, (Z)-ligustilide, ferulic acid, and kanzonol B. In rat models of depression, PTGS2 inhibition ameliorated the depression-like behaviors through suppressing neuroinflammation, neural apoptosis, and oxidative stress [81]. Besides, 15 active ingredients were experimentally validated to exhibit anti-inflammatory properties through inhibiting PTGS2 at mRNA and/or protein levels, such as (z)-ligustilide [82], ferulic acid [83], stigmasterol [84], hederagenin [85], formononetin [86], licochalcone A [87], liquiritigenin [88], glycyrol [89], pinocembrin [90], isotrifoliol [91], glabridin [92], paeonol [64], imperatorin [93], isoimperatorin [94], and a-spinasterol [95]. Another hub target, NOS2 linked with 56 target proteins were involved in the pathological mechanism of major depressive disorder. Recent studies showed that NOS2 inhibition was used as an adjuvant therapy to improve the therapeutic efficacy of serotonergic antidepressants [96], and the inhibition of NOS2 induced antidepressant-like properties in rodents [97,98]. In addition, several compounds like glycyrol [89], formononetin [99], licochalcone A [100], isotrifoliol [91], and glabridin [101], were experimentally confirmed to down-regulate the expression of NOS2, resulting in anti-inflammatory effects.
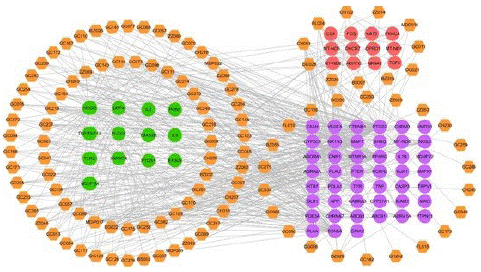
Figure 5: The C-T network of the active ingredients of DZXYS (orange nodes) involved in depression through regulating inflammation (purple nodes) and immune (red nodes) systems. The green nodes show the shared target proteins of inflammation and immune system.
Several animal models of depression revealed that a cell-mediated immune response induced depression-like behavior, blocking of which reversed the development of depression-like behavior [102-104]. Therefore, the target proteins of DZXYS implicated in the immune system were also discussed in Figure 5. It is worth noting that the expression of NOS2 was induced in several cell types by inflammatory and immunological stimuli, therefore, the hub target NOS2 implicated in the pathophysiology of depression not only through inflammatory system (as mentioned above), but also by regulating immune system. Additionally, IL-2 (interleukin-2), a central regulator of immune responses, had been displayed to directly modulate dopaminergic activity and neuronal excitability in mesolimbic pathway, which is implicated in the pathological outcomes of depression [105]. As displayed in Figure 5, IL-2 was targeted by 18 active ingredients of DZXYS, and was identified as the
hub target for the anti-depression property of DZXYS. All these results indicated that DZXYS exhibited the anti-depression effects via regulating multiple immune-related target proteins.
Pathway Analysis of DZXYS
To further decipher the underlying mechanism of DZXYS in the treatment of depression, we enriched the targets of DZXYS with KEGG signaling pathway based on Metascape tool. The top 3 to 8 clusters (one pathway per cluster) distributed in the modules of neurotransmitter, BDNF, HPA axis, metabolomics, inflammatory and
Immune systems were shown in Figure 6A. These pathways and linked genes were used to build T-P network as displayed in Figure 6B. As displayed in Figure 6A and 6B, the pathway of neuroactive ligand-receptor interaction had the highest number of linked targets, including 15 neurotransmitter-related genes, 12 inflammation-related targets, 4 BDNF-related proteins, and 3 targets implicated in HPA axis and metabolomics.
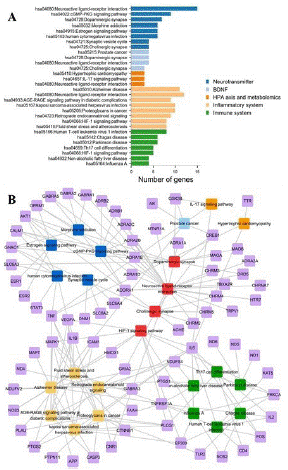
Figure 6: (A) Pathway enrichment of the different module-related genes. The y-axis is the characteristic pathways of the top 3 to 8 clusters, which are respectively enriched by the corresponding module-related targets. (B) T-P network of DZXYS. The purple nodes are the target proteins of DZXYS. And the blue, cornflower blue, coral, feldspar, green and red nodes are neurotransmitter, BDNF, HPA axis and metabolomics, inflammation, immune system, as well as the shared biological pathways.
Subsequently, HIF-1 signaling pathway showed the higher number of connections with 8 inflammation-related and 5-immune-related genes, followed by the pathway involved in Alzheimer disease with 11 inflammation-related genes.
All these pathways were closely related to the pathological mechanism of depression. For example, in the pathway of neuroactive ligand-receptor interaction, several neuroactive receptors were implicated in the therapeutic mechanism of depression, such as epinephrine and norepinephrine receptors (ADRA1D, ADRA2A,
ADRA1B, ADRA2B, ADRA2C, ADRB1 and ADRB2), GAGB receptors (GABRA1, GABRA2 and GABRA5), and dopamine receptors (DRD1 and DRD5), et al. In fact, the a2-adrenergic receptors (ADRA2A, ADRA2B and ADRA2C), which regulated the release of norepinephrine, serotonin and other neurotransmitters, have been proved to be the promising targets for the treatment of depression [106]. Additionally, Hypoxia Inducible Factor-1 (HIF-1), which driven transcriptional response in hypoxia, might have a favorable effect on depression. The target genes of HIF-1, like vascular endothelial growth factor and erythropoietin, had been documented to exhibit antidepressant properties in several models [107]. All these findings made HIF-1 signaling pathway a potential target of anti-depressive agents. The pathways involved in Alzheimer disease also showed the higher topological property in the T-P network of DZXYS, becoming the major therapeutic pathway in treatment of depression. In fact, depression is one of the most common neuropsychiatric disturbances in Alzheimer disease, with estimated prevalence ranging from 25% to 74.9% [108,109].
Conclusion
Depression is a multifactorial psychiatric disorder which involved various pathological mechanisms. DZXYS as a TCM formula plays a significant role in treating multiple pathogenesis against depression through multi-compounds, multi-target, and multi-pathway method. However, it remains unclear about the active compounds, potential targets, related pathways and complex therapeutic mechanisms of DZXYS in the treatment of depression. Therefore, a systems pharmacology method is developed to explore the pharmacological mechanisms of DZXYS on depression therapy.
In current study, 111 active compounds and 112 related protein targets were obtained by ADME evaluation system and target fishing. Then GO enrichment analysis was used to reveal the relationships between potential targets and multiple pathogenesis to further explore the therapeutic mechanism of DZXYS for the treatment of depression. Finally, C-T and T-P networks were established to exhibit the therapeutic effects of DZXYS against depression via regulating the neurotransmitter systems imbalance, BDNF, HPA, inflammation, and immune response. The present work will not only accelerate the in-depth understanding of the complicated therapeutic mechanism of DZXYS on depression therapy from a systematic perspective, but also will provide a way to promote TCM modernization and internationalization.
Author Statements
Acknowledgment
The research is supported by the Key Science and Technology Program of Henan Province (No. 192102310439), Joint Youth Fund for Basic and Applied Basic Research of Guangdong Province (No. 2019A1515111095), International Cooperative Research Project of Shenzhen Collaborative Innovation Program (No. GJHZ20190821173801659), and the Key Scientific Research Projects of Higher Education Institutions in Henan Province (No. 20B180006).
Conflict of Interest
The authors declare no conflict of interest.
References
- Tang Y-m, Zhou L-j, Liu Y. Changes of serum CRP, CER, Hcy and anti-CCP concentrations in patients with depression. Pract Prev Med. 2012; 9.
- Dong XZ, Li ZL, Zheng XL, Mu LH, Zhang GQ, et al. A representative prescription for emotional disease, Ding-Zhi-Xiao-Wan restores 5-HT system deficit through interfering the synthesis and transshipment in chronic mild stress-induced depressive rats. J Ethnopharmacol. 2013; 150: 1053-61.
- Zhou X, Wang J, Lu Y, Chen C, Hu Y, et al. Anti-depressive effects of Kai-Xin-San on lipid metabolism in depressed patients and CUMS rats using metabolomic analysis. J Ethnopharmacol. 2020; 252: 112615.
- Trivedi MH, Fava M, Wisniewski SR, Thase ME, Quitkin F, et al. Medication augmentation after the failure of SSRIs for depres sion. N Engl J Med. 2006; 354: 1243-52.
- Khawam EA, Laurencic G, Malone DA. Side effects of antidepressants: an overview. Cleve Clin J Med. 2006; 73: 351-3.
- Lee G, Bae H. Therapeutic effects of phytochemicals and medicinal herbs on depression. BioMed Res Int. 2017; 2017: 6596241.
- Yeung WF, Chung KF, Ng KY, Yu YM, Zhang SP, et al. Prescription of Chinese herbal medicine in pattern-based traditional Chinese medicine treatment for depression: a systematic review. Evid Based Complement Alternat Med. 2015; 2015: 160189.
- Li YJ, Luo HC, Qian RQ. Effect of Danzhi Xiaoyao powder on neuro-immuno-endocrine system in patients with depression. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2007; 27: 197-200.
- Liang X, Li H, Li S. A novel network pharmacology approach to analyse traditional herbal formulae: the Liu-Wei-Di-Huang pill as a case study. Mol Biosyst. 2014; 10: 1014-22.
- Zhang B, Wang X, Li S. An integrative platform of TCM network pharmacology and its application on a herbal formula, Qing-Luo-Yin. Evid Based Complement Alternat Med. 2013; 2013: 456747.
- Zhou W, Wang J, Wu Z, Huang C, Lu A, et al. Systems pharmacology exploration of botanic drug pairs reveals the mechanism for treating different diseases. Sci Rep. 2016; 6: 36985.
- Zhang J, Chen Z, Zhang L, Zhao X, Liu Z, et al. A systems-based analysis to explore the multiple mechanisms of Shan Zha for treating human diseases. Food Funct. 2021; 12: 1176-91.
- Zhou W, Chen Z, Li W, Wang Y, Li X, et al. Systems pharmacology uncovers the mechanisms of anti-asthma herbal medicine intervention (ASHMI) for the prevention of asthma. J Funct Foods. 2019; 52: 611-9.
- Zhou W, Chen Z, Wang Y, Li X, Lu A, et al. Systems pharmacology-based method to assess the mechanism of action of weight-loss herbal intervention therapy for obesity. Front Pharmacol. 2019; 10: 1165.
- Zhang J, Li Y, Chen X, Pan Y, Zhang S, et al. Systems pharmacology dissection of multi-scale mechanisms of action for herbal medicines in stroke treatment and prevention. PLOS ONE. 2014; 9: e102506.
- He X, Wu C, Qian Y, Li Y, Zhang L, et al. Highly sensitive and selective light-up fluorescent probe for monitoring gallium and chromium ions in vitro and in vivo. Analyst. 2019; 144: 3807-16.
- He X, Xiong W, Lilei Zhang C, Xu C, Fan J, et al. ESIPT-based ratiometric fluorescent probe for highly selective and sensitive sensing and bioimaging of group IIIA ions in living cancer cells and zebrafish. Dyes Pigments. 2020; 174: 108059.
- Mandlik V, Bejugam PR, Singh S. Application of artificial neural networks in modern drug discovery, Artificial Neural Network for Drug Design, Delivery and Disposition. Elsevier. 2016; 123-139.
- Shen M, Tian S, Li Y, Li Q, Xu X, et al. Drug-likeness analysis of traditional Chinese medicines: 1. property distributions of drug-like compounds, non-drug-like compounds and natural compounds from traditional Chinese medicines. J Cheminform. 2012; 4: 31.
- Kim MT, Sedykh A, Chakravarti SK, Saiakhov RD, Zhu H. Critical evaluation of human oral bioavailability for pharmaceutical drugs by using various cheminformatics approaches. Pharm Res. 2014; 31: 1002-14.
- Li X, Xu X, Wang J, Yu H, Wang X, et al. A system-level investigation into the mechanisms of Chinese Traditional Medicine: compound Danshen Formula for cardiovascular disease treatment. PLOS ONE. 2012; 7: e43918.
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood–brain barrier. Neurobiol Dis. 2010; 37: 13-25.
- Li L-t, Li Y, Wang Y-h, Zhang S-w. Prediction of BBB permeation based on molecular indices. Chin J Med Chem. 2007; 17: 221.
- Schenone M, Dancík V, Wagner BK, Clemons PA. Target identification and mechanism of action in chemical biology and drug discovery. Nat Chem Biol. 2013; 9: 232-40.
- Yu H, Chen J, Xu X, Li Y, Zhao H, et al. A systematic prediction of multiple drug-target interactions from chemical, genomic, and pharmacological data. PLOS ONE. 2012; 7: e37608.
- Keiser MJ, Roth BL, Armbruster BN, Ernsberger P, Irwin JJ, et al. Relating protein pharmacology by ligand chemistry. Nat Biotechnol. 2007; 25: 197-206.
- Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019; 10: 1523.
- Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003; 13: 2498-504.
- Tao W, Dong Y, Su Q, Wang H, Chen Y, et al. Liquiritigenin reverses depression-like behavior in unpredictable chronic mild stress-induced mice by regulating PI3K/Akt/mTOR mediated BDNF/TrkB pathway. Behav Brain Res. 2016; 308: 177-86.
- Machado DG, Cunha MP, Neis VB, Balen GO, Colla A, et al. Antidepressant-like effects of fractions, essential oil, carnosol and betulinic acid isolated from Rosmarinus officinalis L. Food Chem. 2013; 136: 999-1005.
- Socala K, Nieoczym D, Pieróg M, Wlaz P. a-spinasterol, a TRPV1 receptor antagonist, elevates the seizure threshold in three acute seizure tests in mice. J Neural Transm (Vienna). 2015; 122: 1239-47.
- Socala K, Wlaz P. Evaluation of the antidepressant- and anxiolytic-like activity of a-spinasterol, a plant derivative with TRPV1 antagonistic effects, in mice. Behav Brain Res. 2016; 303: 19-25.
- Liang BF, Huang F, Wang HT, Wang GH, Yuan X, et al. Involvement of norepinephrine and serotonin system in antidepressant-like effects of hederagenin in the rat model of unpredictable chronic mild stress-induced depression. Pharm Biol. 2015; 53: 368-77.
- Kun X, Zuhua G. Amyrin exerts potent anxiolytic and antidepressant effects via mechanisms involving monoamine oxidase and γ-aminobutyric acid in mouse hippocampus. Trop J Pharm Res. 2019; 18.
- Chopra K, Kumar B, Kuhad A. Pathobiological targets of depression. Expert Opin Ther Targets. 2011; 15: 379-400.
- Li J, Huang S, Huang W, Wang W, Wen G, et al. Paeoniflorin ameliorates interferon-alpha-induced neuroinflammation and depressive-like behaviors in mice. Oncotarget. 2017; 8: 8264-82.
- Qiu FM, Zhong XM, Mao QQ, Huang Z. Antidepressant-like effects of paeoniflorin on the behavioural, biochemical, and neurochemical patterns of rats exposed to chronic unpredictable stress. Neurosci Lett. 2013; 541: 209-13.
- Shah VK, Choi JJ, Han JY, Lee MK, Hong JT, et al. Pachymic acid enhances pentobarbital-induced sleeping behaviors via GABAA-ergic systems in mice. Biomol Ther (Seoul). 2014; 22: 314-20.
- Tsai TR, Tseng TY, Chen CF, Tsai TH. Identification and determination of geniposide contained in Gardenia Jasminoides and in two preparations of mixed traditional Chinese medicines. J Chromatogr A. 2002; 961: 83-8.
- Cai L, Li R, Tang WJ, Meng G, Hu XY, et al. Antidepressant-like effect of geniposide on chronic unpredictable mild stress-induced depressive rats by regulating the hypothalamus–pituitary–adrenal axis. Eur Neuropsychopharmacol. 2015; 25: 1332-41.
- Wang J, Duan P, Cui Y, Li Q, Shi Y. Geniposide alleviates depression-like behavior via enhancing BDNF expression in hippocampus of streptozotocin-evoked mice. Metab Brain Dis. 2016; 31: 1113-22.
- Tan YQ, Chen HW, Li J, Wu QJ. Efficacy, chemical constituents, and pharmacological actions of Radix Paeoniae rubra and Radix Paeoniae Alba. Front Pharmacol. 2020; 11: 1054.
- Wang YL, Wang JX, Hu XX, Chen L, Qiu ZK, et al. Antidepressant-like effects of albiflorin extracted from Radix paeoniae Alba. J Ethnopharmacol. 2016; 179: 9-15.
- Chao WW, Lin BF. Bioactivities of major constituents isolated from Angelica sinensis (Danggui). Chin Med. 2011; 6: 29.
- Badavath VN, Baysal I, Uçar G, Mondal SK, Sinha BN, et al. Monoamine oxidase inhibitory activity of ferulic acid amides: curcumin-based design and synthesis. Arch Pharmazie. 2016; 349: 9-19.
- Conney AH, Lysz T, Ferraro T, Abidi TF, Manchand PS, et al. Inhibitory effect of curcumin and some related dietary compounds on tumor promotion and arachidonic acid metabolism in mouse skin. Adv Enzyme Regul. 1991; 31: 385-96.
- Ramana KV, Singhal SS, Reddy AB. Therapeutic potential of natural pharmacological agents in the treatment of human diseases. Biomed Res Int. 2014; 2014: 573452.
- Yin YY, Ton SH, Kadir KBA. Effects of glycyrrhizic acid on 11 β-hydroxysteroid dehydrogenase (11 βHSD1 and 2) activities and HOMA-IR in rats at different treatment periods. Exp Clin Endocrinol Diabetes. 2010; 118: 617-24.
- Kong Y, Peng Q, Lv N, Yuan J, Deng Z, et al. Paeoniflorin exerts neuroprotective effects in a transgenic mouse model of Alzheimer’s disease via activation of adenosine A1 receptor. Neurosci Lett. 2020; 730: 135016.
- Chen H, Dong Y, He X, Li J, Wang J. Paeoniflorin improves cardiac function and decreases adverse postinfarction left ventricular remodeling in a rat model of acute myocardial infarction. Drug Des Dev Ther. 2018; 12: 823-36.
- Yang G, Zhang L, Ma L, Jiang R, Kuang G, et al. Glycyrrhetinic acid prevents acetaminophen-induced acute liver injury via the inhibition of CYP2E1 expression and HMGB1-TLR4 signal activation in mice. Int Immunopharmacol. 2017; 50: 186-93.
- Yin F, Liu J, Zheng X, Guo L, Xiao H. Geniposide induces the expression of heme oxygenase-1 via PI3K/Nrf2-signaling to enhance the antioxidant capacity in primary hippocampal neurons. Biol Pharm Bull. 2010; 33: 1841-6.
- Huang Y, Thathiah A. Regulation of neuronal communication by G protein-coupled receptors. FEBS Lett. 2015; 589: 1607-19.
- Hillhouse TM, Porter JH. A brief history of the development of antidepressant drugs: from monoamines to glutamate. Exp Clin Psychopharmacol. 2015; 23: 1-21.
- Andrade C, Rao NSK. How antidepressant drugs act: a primer on neuroplasticity as the eventual mediator of antidepressant efficacy. Indian J Psychiatry. 2010; 52: 378-86.
- Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002; 5: 446-51.
- Peng GJ, Tian JS, Gao XX, Zhou YZ, Qin XM. Research on the pathological mechanism and drug treatment mechanism of depression. Curr Neuropharmacol. 2015; 13: 514-23.
- Nemeroff CB. Recent advances in the neurobiology of depression. Psychopharmacol Bull. 2002; 36: 6-23.
- Wu LL, Liu Y, Yan C, Pan Y, Su JF, et al. Antidepressant-like effects of fractions prepared from Danzhi-Xiaoyao-San decoction in rats with chronic unpredictable mild stress: effects on hypothalamic-pituitary-adrenal axis, arginine vasopressin, and neurotransmitters. Evid Based Complement Alternat Med. 2016; 2016: 6784689.
- Pinsonneault JK, Sullivan D, Sadee W, Soares CN, Hampson E, et al. Association study of the estrogen receptor gene ESR1 with postpartum depression—a pilot study. Arch Womens Ment Health. 2013; 16: 499-509.
- De Guglielmo G, Melis M, De Luca MA, Kallupi M, Li HW, Niswender K et al. PPAR γ activation attenuates opioid consumption and modulates mesolimbic dopamine transmission. Neuropsychopharmacology. 2015; 40: 927-37.
- Ciccocioppo R, Ubaldi M. Nuclear peroxisome proliferator activated receptor-gamma (PPARγ) as a therapeutic target to treat neurodegeneration and dependence elicited by drugs of abuse. Neural Regen Res. 2021; 16: 984-5.
- Kong LD, Cheng CH, Tan RX. Inhibition of MAO A and B by some plant-derived alkaloids, phenols and anthraquinones. J Ethnopharmacol. 2004; 91: 351-5.
- Chou TC. Anti-inflammatory and analgesic effects of paeonol in carrageenan-evoked thermal hyperalgesia. Br J Pharmacol. 2003; 139: 1146-52.
- Zhu YL, Li SL, Zhu CY, Wang W, Zuo WF, et al. Metabolomics analysis of the antidepressant prescription Danzhi Xiaoyao Powder in a rat model of Chronic Unpredictable Mild Stress (CUMS). J Ethnopharmacol. 2020; 260: 112832.
- Jiang H, Chen S, Li C, Lu N, Yue Y, et al. The serum protein levels of the tPA–BDNF pathway are implicated in depression and antidepressant treatment, Translational psychiatry. Transl Psychiatry. 2017; 7: e1079.
- Hung YY, Huang YL, Chang C, Kang HY. Deficiency in androgen receptor aggravates the depressive-like behaviors in chronic mild stress model of depression. Cells. 2019; 8: 1021.
- Chhibber A, Woody SK, Karim Rumi MA, Soares MJ, Zhao L. Estrogen receptor β deficiency impairs BDNF–5-HT2A signaling in the hippocampus of female brain: a possible mechanism for menopausal depression. Psychoneuroendocrinology. 2017; 82: 107-16.
- McKay MS, Zakzanis KK. The impact of treatment on HPA axis activity in unipolar major depression. J Psychiatr Res. 2010; 44: 183-92.
- Guerry JD, Hastings PD. In search of HPA axis dysregulation in child and adolescent depression. Clin Child Fam Psychol Rev. 2011; 14: 135-60.
- Liu R, Xu L, Zhang S, Wang Y, Zhao P. Danzhi Xiaoyao Powder reduced the scratching behavior exaggerated by chronic psychological stress in mice; 2020.
- Yang G, Li J, Cai Y, Yang Z, Li R, et al. Glycyrrhizic acid alleviates 6-hydroxydopamine and corticosterone-induced neurotoxicity in SH-SY5Y cells through modulating autophagy. Neurochem Res. 2018; 43: 1914-26.
- Li Yc, Zheng Xx, Xia Sz, Li Y, Deng Hh, et al. Paeoniflorin ameliorates depressive-like behavior in prenatally stressed offspring by restoring the HPA axis-and glucocorticoid receptor-associated dysfunction. J Affect Disord. 2020; 274: 471-81.
- Zheng P, Wang Y, Chen L, Yang D, Meng H, et al. Identification and validation of urinary metabolite biomarkers for major depressive disorder. Mol Cell Proteomics. 2013; 12: 207-14.
- Zheng P, Gao HC, Li Q, Shao WH, Zhang ML, et al. Plasma metabonomics as a novel diagnostic approach for major depressive disorder. J Proteome Res. 2012; 11: 1741-8.
- Gao X, Guo B, Yang L, Liu J, Zhang X, et al. Selection and dynamic metabolic response of rat biomarkers by metabonomics and multivariate statistical analysis combined with GC–MS. Pharmacol Biochem Behav. 2014; 117: 85-91.
- Benzler J, Ganjam GK, Krüger M, Pinkenburg O, Kutschke M, et al. Hypothalamic glycogen synthase kinase 3β has a central role in the regulation of food intake and glucose metabolism. Biochem J. 2012; 447: 175-84.
- Duda P, Hajka D, Wójcicka O, Rakus D, Gizak A. GSK3β: a master player in depressive disorder pathogenesis and treatment responsiveness. Cells. 2020; 9: 727.
- Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. 2015; 172: 1075-91.
- Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016; 16: 22-34.
- Song Q, Feng YB, Wang L, Shen J, Li Y, et al. COX-2 inhibition rescues depression-like behaviors via suppressing glial activation, oxidative stress and neuronal apoptosis in rats. Neuropharmacology. 2019; 160: 107779.
- Chung JW, Choi RJ, Seo EK, Nam JW, Dong MS, et al. Anti-inflammatory effects of (Z)-ligustilide through suppression of mitogen-activated protein kinases and nuclear factor-κB activation pathways. Arch Pharm Res. 2012; 35: 723-32.
- Sadar SS, Vyawahare NS, Bodhankar SL. Ferulic acid ameliorates TNBS-induced ulcerative colitis through modulation of cytokines, oxidative stress, iNOs, COX-2, and apoptosis in laboratory rats. Excli J. 2016; 15: 482-99.
- Ahmad Khan MA, Sarwar AHMG, Rahat R, Ahmed RS, Umar S. Stigmasterol protects rats from collagen induced arthritis by inhibiting proinflammatory cytokines. Int Immunopharmacol. 2020; 85: 106642.
- Lee CW, Park SM, Zhao R, Lee C, Chun W, et al. Hederagenin, a major component of Clematis mandshurica Ruprecht root, attenuates inflammatory responses in RAW 264.7 cells and in mice. Int Immunopharmacol. 2015; 29: 528-37.
- Wang H, Zhang D, Ge M, Li Z, Jiang J, et al. Formononetin inhibits enterovirus 71 replication by regulating COX-2/PGE 2 expression. Virol J. 2015; 12: 35.
- Guo W, Liu B, Yin Y, Kan X, Gong Q, et al. Licochalcone A protects the blood–milk barrier integrity and relieves the inflammatory response in LPS-induced mastitis. Front Immunol. 2019; 10: 287.
- Tu C, Ma Y, Song M, Yan J, Xiao Y, et al. Liquiritigenin inhibits IL-1β-induced inflammation and cartilage matrix degradation in rat chondrocytes. Eur J Pharmacol. 2019; 858: 172445.
- Shin EM, Zhou HY, Guo LY, Kim JA, Lee SH, et al. Anti-inflammatory effects of glycyrol isolated from Glycyrrhiza uralensis in LPS-stimulated RAW264. 7 macrophages. Int Immunopharmacol. 2008; 8: 1524-32.
- Zhou LT, Wang KJ, Li L, Li H, Geng M. Pinocembrin inhibits lipopolysaccharide-induced inflammatory mediators production in BV2 microglial cells through suppression of PI3K/Akt/NF-κB pathway. Eur J Pharmacol. 2015; 761: 211-6.
- Li H, Yoon JH, Won HJ, Ji HS, Yuk HJ, et al. Isotrifoliol inhibits pro-inflammatory mediators by suppression of TLR/NF-κB and TLR/MAPK signaling in LPS- induced RAW264. 7 cells. Int Immunopharmacol. 2017; 45: 110-9.
- Wu Y, Geng J, Lei X, Wu Q, Chen T, Zhong L. Glabridin downregulates lipopolysaccharide- induced oxidative stress and neuroinflammation in BV-2 microglial cells via suppression of nuclear factor-κB signaling pathway. Pharmacogn Mag. 2020; 16: 675.
- Singh G, Kaur J, Kaur M, Singh P, Bhatti R. Anti-nociceptive and anti-inflammatory effect of imperatorin: evidences for involvement of COX-2, iNOS, NFκB and inflammatory cytokines. Int J Neurosci. 2020; 130: 176-85.
- Moon TC, Jin M, Son JK, Chang HW. The effects of isoimperatorin isolated from Agelicae dahuricae on cyclooxygenase-2 and 5-lipoxygenase in mouse bone marrow-derived mast cells. Arch Pharm Res. 2008; 31: 210-5.
- Brusco I, Camponogara C, Carvalho FB, Schetinger MRC, Oliveira MS, et al. a-spinasterol: a COX inhibitor and a transient receptor potential vanilloid 1 antagonist presents an antinociceptive effect in clinically relevant models of pain in mice. Br J Pharmacol. 2017; 174: 4247-62.
- Harkin A, Connor TJ, Burns MP, Kelly JP. Nitric oxide synthase inhibitors augment the effects of serotonin re-uptake inhibitors in the forced swimming test. Eur Neuropsychopharmacol. 2004; 14: 274-81.
- Inserra A, Mastronardi CA, Rogers G, Licinio J, Wong ML. Neuroimmunomodulation in major depressive disorder: focus on caspase 1, inducible nitric oxide synthase, and interferon-gamma. Mol Neurobiol. 2019; 56: 4288-305.
- Montezuma K, Biojone C, Lisboa SF, Cunha FQ, Guimarães FS, et al. Inhibition of iNOS induces antidepressant-like effects in mice: pharmacological and genetic evidence. Neuropharmacology. 2012; 62: 485-91.
- El-Bakoush A, Olajide OA. Formononetin inhibits neuroinflammation and increases estrogen receptor beta (ERβ) protein expression in BV2 microglia, International immunopharmacology. 2018; 835: 325-37.
- Kim JK, Shin EK, Park JH, Kim YH, Park JHY. Antitumor and antimetastatic effects of licochalcone A in mouse models. J Mol Med (Berl). 2010; 88: 829-38.
- Yehuda I, Madar Z, Leikin-Frenkel A, Tamir S. Glabridin, an isoflavan from licorice root, downregulates iNOS expression and activity under high-glucose stress and inflammation. Mol Nutr Food Res. 2015; 59: 1041-52.
- Maes M. Depression is an inflammatory disease, but cell-mediated immune activation is the key component of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011; 35: 664-75.
- Maes M, Yirmyia R, Noraberg J, Brene S, Hibbeln J, et al. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab Brain Dis. 2009; 24: 27-53.
- Sluzewska A, Rybakowski J, Bosmans E, Sobieska M, Berghmans R, et al. Indicators of immune activation in major depression. Psychiatry Res. 1996; 64: 161-7.
- Rankin JS, Zalcman SS, Zhu Y, Siegel A. Short- and long-term effects of interleukin-2 treatment on the sensitivity of periadolescent female mice to interleukin-2 and dopamine uptake inhibitor. PLOS ONE. 2013; 8: e64473.
- Schramm NL, McDonald MP, Limbird LE. The a2A-adrenergic receptor plays a protective role in mouse behavioral models of depression and anxiety. J Neurosci. 2001; 21: 4875-82.
- Li G, Zhao M, Cheng X, Zhao T, Feng Z, et al. FG-4592 improves depressive-like behaviors through HIF-1-mediated neurogenesis and synapse plasticity in rats. Neurotherapeutics. 2019; 17: 664-675.
- Lyketsos CG, DelCampo L, Steinberg M, Miles Q, Steele CD, et al. Treating depression in Alzheimer disease: efficacy and safety of sertraline therapy, and the benefits of depression reduction: the DIADS. Arch Gen Psychiatry. 2003; 60: 737-46.
- Benoit M, Dygai I, Migneco O, Robert PH, Bertogliati C, et al. Behavioral and psychological symptoms in Alzheimer’s disease. Relation between apathy and regional cerebral perfusion. Dem Geriatr Cogn Disord. 1999; 10: 511-7.
