Abstract
Diabetic Retinopathy (DR) is a condition caused by damage to the blood vessels in the retina, leading to abnormal blood vessel growth, swelling, and leakage. Salvia Miltiorrhiza (SM), a traditional Chinese herb used for medicinal purposes, has shown potential benefits for treating DR due to its antioxidant, anti-inflammatory, vasodilatory, and anti-angiogenic effects. This study employed network pharmacology and molecular docking techniques to identify and validate potential mechanisms of SM in the treatment of DR.
The Active constituents of SM used in this study were retrieved from the Traditional Chinese Medicine Systems Pharmacology (TCMSP), and DR-associated targets were collected from GeneCards and Therapeutic Target Database (TTD) databases. Overlapping targets between SM constituents and DR-associated targets were utilized to create the Salvia Miltiorrhiza constituents target-diabetic retinopathy network and protein-protein interaction network. Gene enrichment analysis identified potential therapeutic SM mechanisms in DR treatment, including biological processes, cellular components, molecular functions, and signaling pathways. Docking results confirmed stable interactions between SM constituents (tanshinone IIA and luteolin) and DR targets with the highest binding affinities of -10.3 and -8.9 kcal/mol.
The study offers reasonable molecular mechanisms for treating DR using SM constituents and suggests further wet lab experiments to corroborate the computational analysis findings. The findings provide a basis for additional research and the potential development of SM-based therapies for DR.
Keywords: Diabetic retinopathy; Salvia Miltiorrhiza; Network pharmacology; Molecular docking; Gene enrichment analysis; Luteolin; Tanshinones IIA.
Introduction
Diabetes-related microvascular retinopathy is a well-known sight-threatening condition. Globally, 93 million individuals are currently affected by Diabetic Retinopathy (DR) [1]. Chronic high blood glucose is the direct cause of diabetic retinopathy, which damages retinal capillaries and leads to capillary leakage and blockage. It may lead to loss of vision ultimately resulting in blindness. Between the ages of 20 and 65, diabetic retinopathy is the primary cause of visual loss in persons of working age. Approximately one in three persons with diabetes has DR, and one in ten will develop a vision-threatening condition [2]. Several therapies have been employed clinically, including anti-Vascular Endothelial Growth Factor (anti-VEGF) medication, long-acting steroids, and laser photocoagulation surgery [3,4]. Multiple studies have revealed that numerous variables, including oxidative stress, hyperglycemia, and inflammatory cytokines, contribute to DR's progression, despite its unknown pathophysiology [3,5,6. Hyperglycemia is recognized as the primary instigating component in advancing diabetic retinopathy [7], indicating that stringent glycemic management helps prolong the onset of DR. Moreover, researchers believe high blood glucose causes oxidative stress, which drives an inflammatory reaction and is vital in advancing diabetic retinopathy. Continuous hyperglycemia is a hallmark of the metabolic condition known as type 2 diabetes mellitus [8,9]. Long-term complications of hyperglycemia may induce retinal, renal, and cardiovascular disease, as well as decreased blood flow. Changes in lifestyle or the use of therapeutic medications can prevent or delay the onset of impaired glucose tolerance. Some of these medications are derived from plants or microorganisms, like the galegine extracted from Galega officinalis, which is very similar to the diabetes medication metformin. Other anti-diabetic products of natural origin include Salvia Miltiorrhiza, quinones, cactus plants, picnogenol, acarbose, miglitol, and voglibose from microbes [10-12]. Chronic hyperglycemia and insulin resistance may lead to chronic impairment and dysfunction of various tissues, especially the livers, eyes, kidneys, heart, and nerves, accounting for major morbidity and mortality causes [13]. Small molecules of natural substances are the lead research targets in treating metabolic complications; besides diabetes mellitus, they have been treating various health-threatening complications such as Cancer, Alzheimer, cardiovascular diseases, etc. Salvia Miltiorrhiza, also known as Danshen, is used in China and other countries or regions to treat microcardiovascular related diseases,including complications of diabetes. Active constituents in Salvia Miltiorrhiza extracts include water-soluble luteolin and lipid-soluble Tanshinone IIA, which have been used in this study based on their stable binding affinities with the DR targets. Studies proved that both ingredients possess anti-diabetic properties [14]. They also have other beneficial activities, such as reactive oxygen species scavenging, antioxidant effects [15], and anti-Endoplasmic Reticulum Stress. In addition, they have demonstrated anticancer effects [16], anti-inflammatory properties [17], and alleviation of DR [18], among other potential benefits. However, their molecular mechanisms are still unclear. Understanding the interaction of natural substances with disease targets is crucial for facilitating drug discovery. Hence, this study seeks to determine the molecular mechanism of SM constituents on DR using network pharmacology and molecular docking techniques.
Materials and Methods
Active Constituents of Salvia Miltiorrhiza (SM) and SM-related Target Screening
We retrieved the Constituents of SM using the keywords "Salvia Miltiorrhiza" and "Danshen" in the TCMSP-Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (tcmsp-e.com). OB: Oral bioavailability refers to the ability of a medication to enter the bloodstream. Drug likeness (DL) is a mean sure that considers a molecule's molecular, biological, and physical characteristics and determines its similarity to existing treatments. Drug candidates with good OB and DL indicate that it has desirable qualities that make them [4]. This study utilized OB=30% as a Constituent screening criterion, and a drug similarity DL=0.2. The study identified 59 candidate Candidates for SM after the screening. The study collected Miltiorrhiza-related active Constituent targets using the TCMSP platform. We utilized the UniProt database (https://www.uniprot.org, August 2022) to gather gene IDs and associated information regarding the Salvia Miltiorrhiza targets acquired from TCMSP.
Diabetic Retinopathy-Related Target Screening
The word "Diabetic Retinopathy" was entered to retrieve gene IDs of DR-associated targets from the GeneCards(https://www.genecards.org) and Therapeutic Target Database (TTD) (https://db.idrblab.org/ttd/, last update July 31, 2023). After filtering targets by importance score, = 3,500 targets were pin-pointed.
Generation of the Salvia Miltiorrhiza Constituents-Target-Diabetic Retinopathy Network
The Venny (2.1.0, https://bioinfogp.cnb.csic.es/tools/venny/) online tool was utilized to examine the overlap between the genes linked with DR and the SM constituents' targets. We plotted the Venn diagram and loaded the intersecting targets and constituents of SM into the network visualization platform Cytoscape version 3.9.1. Then, we used this information to generate the Salvia Miltiorrhiza-constituents-target-diabetic retinopathy network.
PPI Network Establishment
To establish the network, overlapping targets of SM and DR were imported into the STRING 11.5 platform, updated on August 12, 2021 (https://string-db.org/cgi/); we defined the protein type as "Homo sapiens" using the multiple protein options and saved in TSV-format file [19]. Using CytoScape version 3.9.1, we next analyzed the network of PPI.
Analyses of GO terms and KEGG Pathways for Enrichment
Using the online Metascape platform (http://metascape.org/gp/index.html), this study performed GO: Gene Ontology and KEGG: Kyoto Encyclopedia of Genes and Genomes pathway enrichment analysis to examine the biological activities and metabolic pathways of all overlapping genes between SM and DR.
Constituents-Targets Molecular Docking
We downloaded the Three-Dimensional (3D) structure of the ATK1 (PDB ID: 1UNQ), TP53 (PDB ID: 4XZV), VEGFA (PDB ID:4ZFF), and TNF (PDB ID: 5E1T) from the RCSB PDB database. Meanwhile, we retrieved the three-dimensional structures of the constituents Luteolin (CID_5280445) and Tanshinone IIA (CID_164676) from the PubChem online chemical database in SDF format. We converted them to PDB format using PyMOL. We utilized AutoDockTools-1.5.6 software to import molecules and correlated proteins to accomplish the docking process. We included polar hydrogen, assigned AD4 Type atom, and Gasteiger and Kolman charges within the environment. Besides, we employed the default value to set the grid of every protein at X, Y, and Z. We established the grid box size via AutoDock Vina version 1.5.6; then, this study visualized the docking results using the Discovery Studios software. TP53, ATK1, VEGFA, and TNF were receptors, while Luteolin and Tanshinone IIA were ligands. The ligand coordinate identified the target protein complex's potential molecular docking site [20].
Results
The study evaluates Salvia Miltiorrhiza and Diabetic Retinopathy via network Pharmacology and molecular docking analysis. The study aimed to identify the SM-DR's target genes, construct a network of SM-DR-associated genes, and analyze potential mechanisms involved with the SM-DR target interaction. Then, molecular docking was conducted to investigate the findings further, as stipulated in (Figure 1).
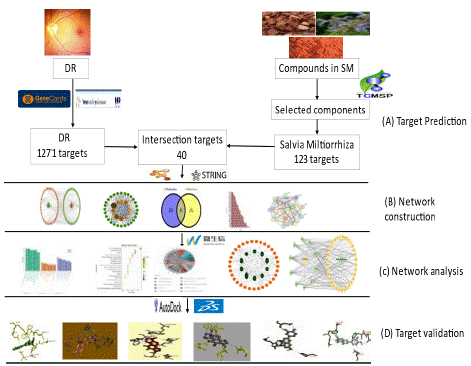
Figure 1: Salvia Miltiorrhiza and Diabetic Retinopathy network Pharmacology and molecular docking analysis flow chart. (A) Initially, the study aimed to identify the target for the study as the first step (B) We constructed a network in the second step (C) The analysis of potential mechanism in the third step; and (D) Finally, molecular docking was conducted to investigate the findings further.
Screening of Active Constituents and Shared targets
We acquired 59 constituents and 935 Salvia Miltiorrhiza-related targets that satisfy the OB=30% benchmark and a DL=0.20. After removing repeated gene targets, we collected 123 targets with SM target-specific symbols. In addition, the GeneCards and TTD databases provided us with 4334 and 102 DR-associated targets, respectively. We filtered the data to remove redundant gene IDs and recovered 4,342 DR-associated targets with the above databases.
We found 1271 Diabetic Retinopathy-associated targets after screening them using the relevant standard (please refer to the Supplementary data Table 1).
Ingredients
Ingredients ID
Degree Size
OB%
DL
Luteolin
MOL000006
23
36.16262934
0.24552
Tanshinone iia
MOL007154
16
49.88730004
0.39781
Dihydrotanshinlactone
MOL007100
8
38.6847683
0.32227
1-methyl-8,9-dihydro-7H-naphtho[5,6-g]benzofuran-6,10,11-trione
MOL007127
8
34.72082213
0.36634
2-isopropyl-8-methylphenanthrene-3,4-dione
MOL007041
7
40.86015408
0.22897
przewaquinone c
MOL007069
7
55.7416731
0.40408
4-methylenemiltirone
MOL007049
6
34.34867589
0.22726
2-(4-hydroxy-3-methoxyphenyl)-5-(3-hydroxypropyl)-7-methoxy-3-benzofurancarboxaldehyde
MOL007050
6
62.78414726
0.39628
danshenspiroketallactone
MOL007094
6
50.43128103
0.3067
IsoTanshinone II
MOL007111
6
49.91602574
0.39674
neocryptoTanshinone ii
MOL007124
6
39.46299114
0.23157
Tanshindiol B
MOL007151
6
42.66581049
0.45303
DehydroTanshinone II A
MOL002651
5
43.76228599
0.40019
3-beta-Hydroxymethyllenetanshiquinone
MOL007059
5
32.16103376
0.40894
Methylenetanshinquinone
MOL007061
5
37.07319368
0.36017
Przewaquinone B
MOL007068
5
62.24005962
0.41374
(6S,7R)-6,7-dihydroxy-1,6-dimethyl-8,9-dihydro-7H-naphtho[8,7-g]benzofuran-10,11-dione
MOL007070
5
41.31045706
0.453
cryptoTanshinone
MOL007088
5
52.34196226
0.39555
epidanshenspiroketallactone
MOL007105
5
68.27315929
0.30549
isocryptotanshi-none
MOL007108
5
54.98193246
0.39449
(2R)-3-(3,4-dihydroxyphenyl)-2-[(Z)-3-(3,4-dihydroxyphenyl)acryloyl]oxy-propionic acid
MOL007132
5
109.3805241
0.35119
salviolone
MOL007145
5
31.72415039
0.23568
(6S)-6-hydroxy-1-methyl-6-methylol-8,9-dihydro-7H-naphtho[8,7-g]benzofuran-10,11-quinone
MOL007150
5
75.38587847
0.4551
Przewaquinone E
MOL007152
5
42.85485204
0.45301
1,2,5,6-tetrahydroTanshinone
MOL001601
4
38.74538672
0.35791
3a-hydroxytanshinone?a
MOL007045
4
44.92933597
0.44272
formyltanshinone
MOL007058
4
73.444622
0.41736
tanshinaldehyde
MOL007079
4
52.4747043
0.45196
dan-shexinkum d
MOL007093
4
38.88302101
0.55453
deoxyneocryptotanshinone
MOL007098
4
49.40034705
0.28555
dihydrotanshinone?
MOL007101
4
45.04327919
0.36015
miltionone ?
MOL007119
4
49.68439433
0.32125
miltionone ?
MOL007120
4
71.02970321
0.43711
prolithospermic acid
MOL007130
4
64.37096207
0.31017
(6S)-6-(hydroxymethyl)-1,6-dimethyl-8,9-dihydro-7H-naphtho[8,7-g]benzofuran-10,11-dione
MOL007155
4
65.25893771
0.44871
tanshinone ?
MOL007156
4
45.63730602
0.29549
sugiol
MOL002222
3
36.11353486
0.27648
5,6-dihydroxy-7-isopropyl-1,1-dimethyl-2,3-dihydrophenanthren-4-one
MOL007036
3
33.76525236
0.28585
przewaquinone f
MOL007071
3
40.30788399
0.45925
Danshenol B
MOL007081
3
57.9508753
0.55764
Danshenol A
MOL007082
3
56.96524899
0.52172
C09092
MOL007107
3
36.06948986
0.2474
Miltirone
MOL007122
3
38.75698635
0.25418
neocryptoTanshinone
MOL007125
3
52.48799701
0.32306
salvilenone ?
MOL007143
3
32.43470856
0.22895
digallate
MOL000569
2
61.84861803
0.25635
(E)-3-[2-(3,4-dihydroxyphenyl)-7-hydroxy-benzofuran-4-yl]acrylic acid
MOL007048
2
48.24363244
0.31229
Salvilenone
MOL007085
2
30.38365387
0.37639
miltipolone
MOL007121
2
36.55611206
0.36803
Table 1: The 49 corresponding SM-ingredient to shared targets.
After intersecting the Diabetic Retinopathy-related targets of 1271 and Salvia Miltiorrhiza-related targets of 123 (Supplementary data Table 2) using the Venn diagram, we identified 40 shared targets (as indicated in Figure 2a.) that may serve as possible targets of SM in treating diabetic retinopathy. We then used these targets for succeeding network construction, pathway enrichment, and docking analysis.
Pathway ID
Pathway name
KEGG Target Gene
Gene Count
Degree Score
hsa04151
PI3K-Akt signaling pathway
AKT1, BCL2L1, CASP9, CDK2, EGFR, HRAS, IL2, IL4, IL6R, INSR, KDR, MCL1, PIK3CG, TP53, VEGFA
15
16
hsa05417
Lipid and atherosclerosis
AKT1, BCL2L1, CASP9, CD40LG, FOS, HRAS, CXCL8, JUN, MMP1, MMP9, SRC, TNF, TP53
13
14
hsa05161
Hepatitis B
AKT1, BIRC5, CASP9, CCNA2, CDK2, FOS, HRAS, CXCL8, JUN, MMP9, SRC, TNF, TP53
13
14
hsa05418
Fluid shear stress and atherosclerosis
AKT1, EDN1, FOS, GSTP1, JUN, KDR, MMP2, MMP9, SRC, TNF, TP53, VEGFA
12
13
hsa05207
Chemical carcinogenesis - receptor activation
AKT1, BIRC5, CYP1A2, CYP3A4, EGFR, ESR1, FOS, HRAS, JUN, PPARA, SRC, VEGFA
12
13
hsa04926
Relaxin signaling pathway
AKT1, EDN1, EGFR, FOS, HRAS, JUN, MMP1, MMP2, MMP9, SRC, VEGFA
11
12
hsa05205
Proteoglycans in cancer
AKT1, EGFR, ESR1, HRAS, KDR, MMP2, MMP9, SRC, TNF, TP53, VEGFA
11
12
hsa04010
MAPK signaling pathway
AKT1, EGFR, FOS, HRAS, HSPB1, INSR, JUN, KDR, TNF, TP53, VEGFA
11
12
hsa05167
Kaposi sarcoma-associated herpesvirus infection
AKT1, CASP9, FOS, HRAS, CXCL8, JUN, PIK3CG, SRC, TP53, VEGFA
10
11
hsa05166
Human T-cell leukemia virus 1 infection
AKT1, BCL2L1, CCNA2, CDK2, FOS, HRAS, IL2, JUN, TNF, TP53
10
11
Table 2: KEGG Top 10 Signaling Pathways by Gene Count and CytoScape-CytoNCA Plugin calculated Degree Value.
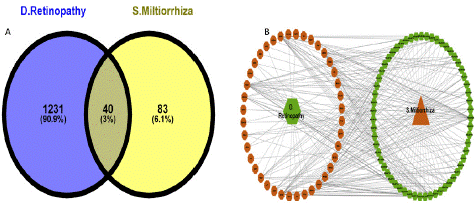
Figure 2: Targets Prediction and Network Construction. (A). Venn diagram of the 40 possible shared targets. (B) The 59 Salvia Miltiorrhiza-Diabetic Retinopathy-shared-targets network. Green Ellipse represents Salvia Miltiorrhiza ingredients. Yellow Ellipse represents the shared targets.
Salvia Miltiorrhiza-Diabetic Retinopathy PPI Network Analysis
This study performed the protein-protein Interaction (PPI) network to unveil the link between SM and DR targets, with a local clustering coefficient of 0.759 and PPI enrichment p-value: <1.0e-16. The study employed the establishment of 123 targets PPI network of 59 ingredients (supplementary data, figure 1a)., then imported the data from STRING to CytoScape to obtain visualization (Supplementary Data, Figure 1b). The result presented that the ingredients' target PPI network had 123 nodes and 910 edges. Additionally, we constructed the DR-associated targets network with PPI data (supplementary data Figure 2a) and developed a result generated from CytoScape (Supplementary Data Figure 2b).
We also smuggled the active constituents and shared targets of SM and DR into CytoScape 3.7.2 to build an active constituents-DR target network. The network (Figure 2b.) showed the 59 SM active constituents in a hexagonal green shape on the right, and the 40 shared targets were represented in an octagonal brown shape on the left of the network. The PPI data of the shared targets exported from STRING was sent to CytoScape to build the PPI network depending on shared targets. To enhance the clarity of the network in Cytoscape, we utilized the attribute circular layout algorithm for network visualization. The network consisted of 40 nodes and 365 edges, and other parameters were kept as mentioned above. The edge thickness signifies edge betweenness, and a thicker line designates a stronger target relationship [21].
Following the shared targets, we established that 49 of the 59 active SM constituents were tangled in DR treatment. Details on the 49 active constituents (compound name, MOL ID, OB, and DL) are itemized in Table 1. The SM and DR 40 shared targets corresponding to the 49 constituents of SM were named the 49-SM-Constituents-40-shared-targets-SM-DR network (Figure 3a). The network contains 180 nodes and 917 edges. Nodes with more edges perform higher degree values and larger node sizes in the network, illustrating high value. The network analysis disclosed that the threshold degree value of the 49 candidate constituents was 5.06. The first 12 compounds in Table 1 represent the top nodes with the highest degree values. All 12 constituents had degree values more elevated than the threshold degree value (5.06): (MOL000006, degree=23), (MOL007154, degree=16), (MOL007100 and MOL007127, degree=8), (MOL007041 and MOL007069, degree=7), (MOL007049, MOL007050, MOL007094, MOL007111, MOL007124 and MOL007151, degree=6) making them highly connected hub constituents in the network. Therefore, these compounds might serve as virtual nodes in the network and strongly treat diabetic retinopathy, illustrating that SM treats DR through numerous SM ingredients. Based on the confidence degree, the 40 shared targets were utilized to make a shared targets network(Figure 3b) and a bar chart (Figure 3d); the network showed brown color nodes situated in the middle of the shared targets network, representing the 12 hubs genes based on degree corresponding to the following shapes and deepness of their colors: octagon(degree=38), hexagon (degree=33), diamond (degree=32), triangle(degree=28), V triangle(degree=27) and ellipse(degree=26). The Threshold Degree Value (TDV) of the shared targets' PPI network values at 18 was calculated from the Cytoscape-Centiscape 2.2 Menu. Then, we produced hub targets for the PPI network, as displayed in Figure 3c. The selected 12 hub genes (AKT1, VEGFA, TP53, TNF, EGFRR, SRC, ESR1, JUN, HRAS, MMP9, CXCL8, and FOS) contained scores more significant than the TDV score of the shared targets. Several diabetic retinopathy-associated genes, like AKT1, VEGFA, SRC, HRAS, and TNF, were connected with SM's therapeutic outcomes on DR. These data suggested that our SM constituents perhaps affected DR primarily through the 12 hub genes targets.
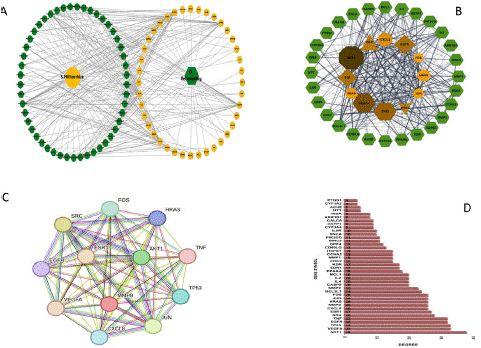
Figure 3: Active ingredient-DR target network. (A) The 49-SM-Constituents-40-shared-targets-SM-DR network. The 49 active ingredients are in a hexagonal green shape on the right, and the 40 shared targets are in an octagonal brown shape on the left. (B) The 40 shared targets of DR and SM with the 12 hub genes in the middle in brown color with varying shapes based on threshold degree value. (C) String database network of the 12 hub genes. (D) The bar column plot of the 40 shared targets plotted as genes symbol(X-axis) against the degree of confidence(Y-axis).
GO Terms Enrichment Analysis
This analysis provides possible target genes of SM in comprehending diabetic retinopathy therapy. The SM and DR 40 shared targets annotation was sub-sectioned into three groups: BP: Biological Processes, CC: Cellular Component, and MF: Molecular Functions. This study gathered 894 statistically significant GO terms (Supplementary Data Table 4), including 809 of BP, 24 of CC, and 61 of MF. The first 20 terms (BP, CC, and MF) bubble charts appear in Figure 4a-c. Figure 4d depicts the 10 most significant enrichment terms of BP, CC, and MF, with the highest count value in the column chart. Positive regulation of protein phosphorylation, regulation of kinase activity, positive regulation of phosphorylation, positive regulation of cell migration, positive regulation of cell motility, positive regulation of locomotion, regulation of protein kinase activity, cellular response to nitrogen compound, cellular response to organonitrogen compound, and cell population proliferation are primarily associated with the shared genes, as determined by BP analysis. Targets are primarily collected for CC analysis at the side of the membrane, the membrane raft, the membrane microdomain, the axon, the perinuclear region of the cytoplasm, the cell leading edge, the external side of the plasma membrane, the neuronal cell body, the cell body, and the transcription regulator complex. Key functions in MF analysis include kinase binding, kinase regulator activity, signaling receptor activator activity, receptor-ligand activity, protein domain specific binding, protein kinase binding, protein kinase regulator activity, enzyme activator activity, protein homodimerization activity, and cytokine activity.
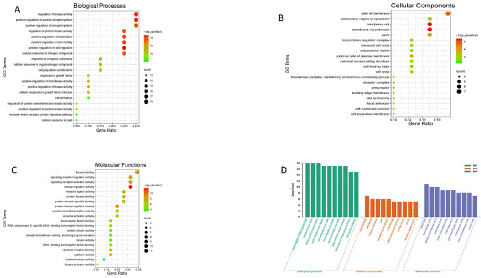
Figure 4: Overall survival, autologous stem cell transplant (ASCT) versus no ASCT (p=0.12).
KEGG Shared Gene Enrichment Analyses
The biological pathway was determined by conducting a KEGG analysis on the 40 shared targets for Salvia Miltiorrhiza treatment of DR. There were 134 statistically significant pathways (Supplementary Table 5), and we picked the best 20 pathways with the top count value for visualization (Figure 5a). The results proposed that SM-DR shared targets are mainly PI3K-Akt signaling, lipid and atherosclerosis, hepatitis B, and fluid shear stress and atherosclerosis. Then, we design a circle diagram to determine the 10 best KEGG pathways enrichment analysis among 12 hub genes, which reveals that the bulk of hub genes were enriched in PI3K-Akt signaling (Figure 5b). Additionally, we explore the connection between Salvia Miltiorrhiza constituents and diabetic retinopathy-shared targets using KEGG key pathways network analysis genes (Figure 5c). The network analysis of the 10 KEGG pathway key genes disclosed 33 genes among the shared genes; detailed information regarding the 10 key pathways is listed in Table 2. We also constructed the KEGG's best 10 pathways based on gene count and the CytoScape-CytoNCA plugin's calculated degree value, as indicated in Figure 5d. These networks asserted that SM components engaged various DR therapeutic targets.
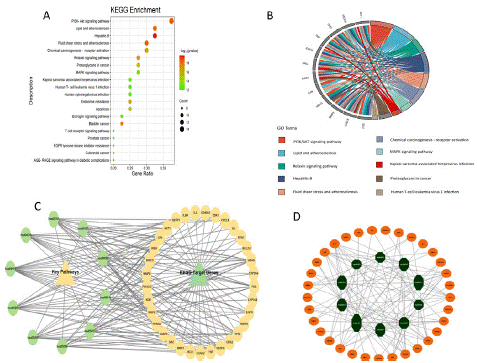
Figure 5: Potential Salvia miltiorrhiza therapy targets: KEGG analysis for enrichment. (A) The dot plot of KEGG top 20 pathways in the KEGG enrichment study of common targets. The changes from red to green indicate that the -log10 (p-value) varies from big to small. The bigger the surface area, the bigger the enrichment degree. (B) KEGG 10 key pathways Circle diagram of the 12 hub genes. The genes are connected to their pathways via different colored ribbons; the bigger ribbons, the more targets were enriched in the pathways. (C) The KEGG target genes and key pathways network, the green octagon shape represents the KEGG key pathways, and the brown ellipse represents the target genes in the pathway. (D) Key pathways network based on degree, the brown ellipse represents the KEGG pathways enriched genes, and the green octagon represents the 10 key pathways arranged by size according to degree values.
Selected Targets Docking Analysis
Depending on the KEGG analysis outcomes, we investigated numerous pathways that could be strongly associated with diabetic retinopathy, namely the PI3K-AKT signaling pathway, lipid and atherosclerosis, and the fluid shear stress and atherosclerosis pathways. PPI network highlighted that AKT1, VEGFA, TP53, and TNF are crucial target genes in the pathways above, and all of these PPI nodes were very significant. Therefore, they were identified as DR-related primary targets. The SM constituents-DR targets network analysis picked their exact corresponding ingredients with high threshold degree values to validate them via molecular docking. The related active constituents are Luteolin (MOL ID: MOL000006) and Tanshinone IIA (MOL ID: MOL007154). The crystal structures of the major target proteins AKT1 (1UNQ), VEGFA (4ZFF), TP53 (4XVV), and TNF were acquired from the PDB database (5E1T). Experts in TCM find it challenging to point out the specific binding sites on target genes for natural ingredient compounds. The active binding sites for DR-associated targets for SM constituents remain unknown [4,22]. To confirm the authenticity of the molecular docking model performance, we applied DeepSite to forecast the target proteins' potential docking locations [4,23]. The ligands and receptors docking analysis was conducted with AutoDock Vina. The binding regions were confined to the docking sites. Docking results confirmed stable interactions between SM constituents (tanshinone IIA and luteolin) and DR targets with the highest binding affinities of -10.3 and -8.9 kcal/mol. Figures 7 and 8 illustrate the docking designs and binding affinities. The docking outcomes are presented as molecular stick model representations for the interaction residues. The ligand is represented as a scaled ball and stick model in the middle of the residues at the binding site Figures 7a, b, c, d, e, and f. A list of the interaction residues can be seen in the supplementary data table 6. Interacting bonds between the receptor and ligand are represented by dashed lines. Figure 8 shows the binding affinities details. A significant likelihood of interaction between the ligand and receptor is indicated by negative binding energy [24-26]. The docking approach exhibited overall binding affinities greater than -5 kcal/mol, indicating a stable association between the DR-SM complex.
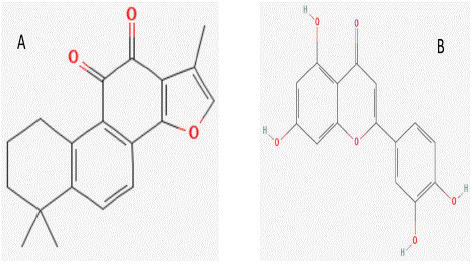
Figure 6: 2-Dimensional structure of ingredients. (A) 2- Dimensional structure of Tanshinone IIA. (B) 2- Dimensional structure of Luteolin.
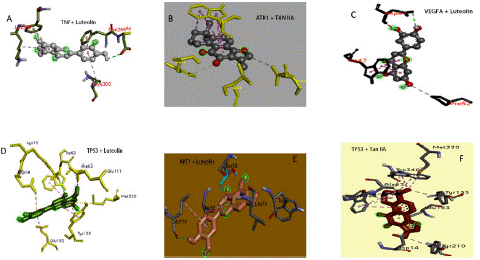
Figure 7: Molecular docking findings. Salvia miltiorrhiza active constituents (Luteolin and Tanshinone IIA) docking patterns of key targets (AKT1, VEGFA TP53, and TNF).
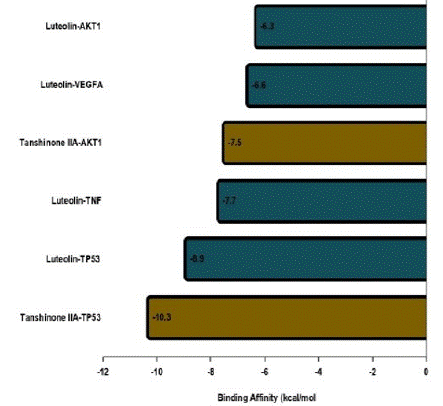
Figure 8: Binding affinities (kcal/mol) of key targets and their targeted active constituents of salvia miltiorrhiza. The constituents and target proteins(X-axis) plotted against the binding affinities (Y-axis).
Discussion
Diabetic retinopathy is an eye condition caused by damage to the blood vessels of the light-sensitive tissue at the back of the eye (retina) [27]. DR is clinically characterized by retinal microvascular pathologic changes, such as microaneurysms, hemorrhages, capillary occlusion, and neovascularization, finally leading to severe vision loss and irreversible blindness [28]. Salvia Miltiorrhiza constituents such as luteolin and tanshinone IIA, a Chinese Traditional Medicine (TCM) extracts, have been demonstrated to have in vivo and in vitro medicinal benefits on DR. Still, their mechanism is yet to be well defined. The mechanisms of action of TCM treatments are intricate, with numerous constituents and targets. When the pathophysiology of pathogens is yet to be explicated, it becomes more problematic to scrutinize the mechanical action of a TCMs treatment effects [22]. Network pharmacology and Molecular docking analysis enable us to organize the investigation of the targets, active constituents, and pathways of medications at the molecular level, thereby enhancing our understanding of the links between constituents-targets and pathways in treating disease.
Employing network pharmacology and molecular docking techniques, we discovered 59 active constituents and 123 targets of SM, and 1271 targets of DR; 40 targets overlapped with the therapeutic targets of DR. An investigation of the SM active constituent-target network revealed that luteolin and tanshinone IIA two of the 49 corresponding SM active components, may act strongly on many network targets. This finding suggests that these two constituents may be essential for the efficiency of the therapy of SM in DR. Luteolin prevails as the greatest potential target, accompanied by tanshinone IIA in the 49-constituents-40 shared targets network.
One of the key elements in the pathophysiology of metabolic syndrome and diabetes is inflammation. The primary cause of type 1 diabetes and a common sign of type 2 diabetes is chronic inflammation. TCM extracts have been reported to have pharmacological effects in disease treatment by mitigating factors or biomarkers associated with diseases. Reports have shown the antioxidant ability of luteolin. A study on active components SM, mainly Asiatic acid and Salvianolic acid j, reported that the SM components (Asiatic acid and Salvianolic acid j) might regulate cell proliferation and response to inflammation and hypoxia [29]. Previous research shows that Luteolin augments insulin sensitivity and raises insulin-mediated glucose uptake [30]. It has also been demonstrated to inhibit increased glucose-induced VEGF synthesis, lowering ROS manufacture and fat buildup [31,32]. In an animal model of diabetes-induced retinal neurodegeneration, Luteolin reduced inflammatory markers, including oxidative stress and cytokines levels [31,33,34]. Heme-Oxygenase-1 (HO-1) is an antioxidant and a cytoprotectant. Luteolin increases HO-1 expression and phosphorylation of AKT and prevents morphological destruction in diabetic-associated complication tissues.
Tanshinone IIA has also been reported to enhance the morphological functioning of the retina in diabetic mice and inhibit diabetic retinopathy through its anti-inflammatory, anti-angiogenic, and antioxidant properties [35-37]. Excessive production of VEGF can cause retinal neovascularization. Findings have shown that Tanshinone IIA significantly reduced VEGF expression in both in vivo and in vitro studies [35,38]. Tanshinone IIA has shown its anti-inflammatory ability in TNF (a pro-inflammatory cytokine that leads to increased vascular permeability, breakdown of the Blood-Retinal Barrier (BRB), and loss of capillary pericytes) by reducing the contents of TNF in the retina of diabetic mouse [35]. The inflammatory cytokines reduce beta cell function and induce insulin resistance. Luteolin and Tanshinone IIA attenuate inflammation-induced diabetes and diabetes-induced inflammation. The TCM extracts might exert their protective effects by ameliorating the hyperglycemia-induced increase in inflammatory markers. In conformity with the above reports, these constituents may serve as therapeutic effects on DR.
AKT1, VEGFA, TP53, TNF, EGFR, SRC, ESR1, JUN, HRAS, MMP9, CXCL8, and FOS were the top 12 targets acting on DR in the PPI network among the 40 shared targets in the SM-DR network, according to the degree of the node. The study selected the first four genes (AKT1, VEGFA, TP53, and TNF mainly connected to the core components of SM Luteolin and Tanshinone IIA base on the order of high-degree nodes for docking analysis. These genes are regarded as hub genes in the study's network and may play essential roles in the therapeutic efficacy of SM in DR. These target genes are implicated in oxidative stress, inflammation, vascular permeability, and immune system modulation [22,29], which are hallmark for DR progression.
For instance, the AKT1 protein targeted by this study as a hub gene is abundantly present in insulin-responsive tissues. It initiates a mechanism that increases glucose absorption by cells by activating glucose transport proteins, thereby improving glucose metabolism [39,40]. The PI3K/AKT pathway, which controls cell proliferation and survival, is among the major mechanisms involving AKT1 in DR. This system can be dysregulated in diabetes, resulting in increased inflammation, oxidative stress, and aberrant blood vessel formation in the eye, which are defining characteristics of DR. AKT1 controls these activities and targeting AKT1 or the PI3K/AKT pathway has been suggested as a promising diabetic retinopathy treatment [41]. Preclinical studies using animal models indicate that modulating AKT1 and VEGFA activities through various means, such as pharmacological inhibitors or gene silencing techniques, can reduce retinal inflammation, abnormal blood vessel growth, and oxidative stress leading to improved outcomes in diabetic retinopathy [42].
The hub target, VEGFA, is present in microvessels of arteries, veins, and lymphatic vessels and in the endothelial cells of larger vessels [43]. The VEGFA gene is also implicated in the aberrant development of retinal blood vessels. Hyperglycemia, as observed in diabetes, can destroy retinal blood vessels [44] , triggering the release of VEGF-A, a gene-encoded protein. VEGF-A stimulates neovascularization in the retina, which can disturb normal retinal function and potentially lead to vision loss. In DR, the overexpression of VEGF-A due to an increase in VEGFA gene activity contributes to the progress of aberrant blood vessels in the retina, which can leak fluid and blood, resulting in retinal edema, hemorrhages, and other severe complications. Therefore, VEGFA and its protein product VEGF-A are targeted in treating DR to inhibit abnormal blood vessel growth and reduce vision-threatening complications.
According to research, TP53, one of our 12 hub genes, is connected with cancer and may also play a role in the growth of DR by inducing inflammation, oxidative stress, retinal neovascularization, and vascular endothelial dysfunction. In a study conducted by Zafer et al., it was observed that there is a notable augmentation in the expression levels of the p53 protein in retinal pericytes subjected to elevated glucose concentrations. The investigation revealed a concomitant escalation in the O-linked Β-N-acetylglucosamine (O-GlcNAc) modification levels of p53 within retinal pericytes exposed to high glucose conditions. The outcomes of the study strongly propose that the post-translational O-GlcNAc modification of p53, coupled with its heightened expression, potentially plays a contributory role in the selective early demise of pericytes during diabetic conditions [45].
TNF is a pro-inflammatory cytokine that is vital to the pathophysiology of DR. Chronic hyperglycemia in diabetes releases TNF and other pro-inflammatory cytokines, which cause retinal blood vessel inflammation and damage. This injury causes the flow of fluid and blood into the retina and the formation of aberrant blood vessels, which can result in vision loss and blindness. In addition, TNF promotes blood-retinal barrier degradation, which is essential for maintaining the integrity of the retina. It also stimulates the creation of other inflammatory molecules and apoptosis in retinal cells. Our investigation revealed that the Fluid shear stress and atherosclerosis pathway contains a notable concentration of the four major targets AKT1, VEGFA, TP53, and TNF. Additionally, AKT1, TP53, and VEGFA were revealed to be considerably enriched in diabetes complication-associated PI3K-Akt signaling and Lipid and atherosclerosis pathways. The antioxidant, anti-inflammatory, and anti-neovascularization activities of SM extracts such as Luteolin and Tan-IIA have been reported by previous studies. Our study showed Luteolin and Tanshinone-IIA are mainly connected to the core genes associated with diabetic retinopathy and may serve as targeted components in DR treatment.
KEGG enrichment findings indicate numerous signaling pathways contributing to DR progression and initiation. We chose three of the top 20 pathways enriched with our four core genes. These pathways consist of the PI3K-Akt, Lipid and Atherosclerosis, and Fluid Shear Stress and Atherosclerosis pathways. Thus, it is believed that the constituents of SM may possess anti-diabetic potential by acting on these signaling pathways. PI3K-AKT, a pathway that plays a significant role in DR formation and growth [46], has exhibited a crucial role in forming new blood vessels in DR. When activated, PI3K/AKT can prolong the lifespan of endothelial cells and work in tandem with VEGF to facilitate cell survival and movement, culminating in the initiation of neovascularization [47,48].
Moreover, PI3K signaling may be triggered in diabetic conditions by hypoglycemia and hypoxia, and activation of AKT may facilitate vascular dilation, remodeling, and vascularization [49]. Once activated, AKT may perform diverse functions by acting upon various downstream target proteins. For example, it can promote cell growth and proliferation by phosphorylating mTOR or impede cell apoptosis by inhibiting the Low-Density Lipoprotein (LDL) cholesterol expression [50]. PI3K/AKT signaling pathway has been reported that its activation cause the pathogenesis of DR and stimulates the propagation and movement of retina endothelial cells [51].
Lipids, a form of fat, can potentially influence the progress of DR in atherosclerosis. Increasing Low-Density Lipoprotein (LDL) cholesterol, commonly regarded as "bad" cholesterol, can lead to atherosclerosis, a condition characterized by the accumulation of fatty deposits inside the arterial walls. The progression of DR may be aided by the restriction of blood flow to the retina caused by atherosclerosis, which narrows the blood vessels. In addition, the atherosclerosis pathway is associated with inflammation, which contributes to the development of diabetic retinopathy [52,53]. Atherosclerosis can also produce blood clots, which can restrict blood flow to the retina and contribute to improving DR. Therefore, targeting lipids and atherosclerosis by inhibiting the atherosclerosis pathway might serve as a mechanism for treating DR.
Fluid shear stress and atherosclerosis, which have demonstrated significant roles in the formation and progression of DR, are other essential pathways reported in this work. Blood flow exerts a physical force known as fluid shear stress on the inner walls of blood vessels, including the retina. Under certain conditions, fluid shear stress can protect blood arteries by stimulating the production of anti-inflammatory chemicals. However, it can also contribute to the advancement of atherosclerosis. For instance, aberrant blood flow patterns, such as those that occur at branching points or bends in blood vessels, can result in areas of lower shear stress [54], which can stimulate the release of pro-inflammatory genes and promote the formation of atherosclerotic plaques [52]. Furthermore, blood flow alterations and elevated glucose and lipid levels can create excessive shear stress on the retinal blood vessels in diabetic retinopathy. This can result in oxidative stress, inflammation, and endothelial dysfunction [55], which are crucial factors in the growth of DR. Luteolin and Tanshinone-IIA have exhibited anti-atherosclerosis efficacy in preclinical studies. A study conducted in a mouse model showed luteolin to remarkably attenuated atherosclerosis in high-fat diet-induced ApoE-/- mouse via alleviating inflammation [56]; Tan IIA, on the other hand, has been shown to ameliorate endothelial cell dysfunction and prevent oxidative stress and inflammatory damage to vascular endothelial cells [57]. Based on the mechanisms mentioned earlier, SM constituents are hypothesised to reduce DR's progression and maintain retinal function by inhibiting oxidative stress, inflammation, neovascularization and endothelial dysfunction through PI3K/Akt signaling and Lipid and Fluid shear stress-induced atherosclerosis pathways. The reported data propose that the Salvia Miltiorrhiza targets identified via network pharmacology may have a therapeutic role against DR.
To further identify the mechanism behind the therapeutic impact of SM in DR, GO analysis was done on the 40 shared targets of Salvia Miltiorrhiza. In this study, we chose the top 20 most significantly enriched BP, CC, and MF terms. The data indicate that Salvia Miltiorrhiza has intricate and multifaceted effects on DR. The GO analyses demonstrated that regulation of kinase activity and positive regulation of protein phosphorylation significantly impact the advancement of DR. Kinases are enzymes crucial in numerous biological activities, such as cell signaling and the expression gene regulation. Kinases have been connected to multiple features of diabetic retinopathy, including the onset of retinal neovascularization and inflammation [6,58]. In diabetic retinopathy, positive regulation of protein phosphorylation is an essential biological process that plays a significant role in the onset and progression of the disease. Protein phosphorylation is a process by which phosphate groups are added to specific amino acid residues of proteins. This modification is vital for regulating various cellular processes involving metabolism, cell signaling, and gene expression. High blood glucose levels in DR can cause oxidative stress and retinal inflammation. This can activate various signaling pathways that result in the abnormal phosphorylation of proteins involved in angiogenesis, inflammation, and apoptosis. For instance, the phosphorylation of VEGF receptor-2 can promote angiogenesis, forming new blood vessels that can be leaky and dysfunctional [59]. The investigation revealed that the molecular functions of our target genes were primarily associated with kinase binding and kinase regulation. For the examination of cellular components, it was also observed that the targets were abundant in the membrane side and membrane raft. Collectively, these results demonstrate the intricacy of the pathogenic mechanism underlying DR. The GO annotation and KEGG signaling pathways showed that the hub constituents of Salvia Miltiorrhiza, including Luteolin and Tanshinone-IIA, may play a role in inhibiting oxidative stress, inflammation, neovascularization and endothelial dysfunction by regulating the binding and recognition of intracellular (such as AKT1, TNF and TP53) and extracellular (such as VEGF) related proteins, via PI3K/Akt signaling and Lipid and Fluid shear stress-induced atherosclerosis pathways.
Docking technology efficiently determines how traditional medicine constituents bind with essential target proteins related to diseases. This strategy can assist researchers in selecting which herbal compounds to examine in the wet lab [60]. To authenticate the predictions derived from network pharmacology assessment, docking was done on four hub genes linked with DR. This study used Luteolin and Tanshinone IIA as ligands to dock the DR-associated hub genes. The results proved that Luteolin binds to all 4 hub genes, while Tanshinone IIA binds with AKT1 and TP53. The ingredients bind to their target genes with affinities scores more significant than -5kcal/mol. Docking results confirmed stable interactions between SM constituents (tanshinone IIA and luteolin) and DR targets with the highest binding affinities of -10.3 and -8.9 kcal/mol. These findings demonstrate a persistent connection between the receptors and ligands.
Extracts from Salvia Miltiorrhiza (SM), such as tanshinone IIA and Luteolin, have been utilized in clinical interventions for metabolic and other diseases [35,61,62]. However, comprehensive clinical studies are imperative to ascertain the efficacy and safety of these compounds in Diabetic Retinopathy (DR). Presently, pharmaceutical strategies for diabetic retina predominantly involve blood lipid regulation, blood sugar reduction, blood pressure management, and anti-VEGF drugs. Despite the prevalent use of anti-VEGF drugs, their limited half-life necessitates repeated administration, prompting the need for rigorous, long-term efficacy and safety validation [63]. This investigation, consistent with prior research, suggests that tanshinone IIA and Luteolin, acting as multi-targeted drugs, hold promise in impeding neovascularization, mitigating oxidative stress, and inhibiting inflammation.
These compounds can be employed individually or in synergy as an SM-hybrid or potentially in combination with other pharmaceutical agents for treating diabetic retinopathy. Their observed effects on shared targets such as TNF, ATK1, and VEGF, among others, signify potential biomarkers or therapeutic targets for diabetic retinopathy interventions. The demonstrated efficacy of tanshinone IIA and Luteolin in targeting neovascularization, oxidative stress, and inflammation proposes a more sustained and effective treatment avenue compared to current anti-VEGF drugs.
Understanding the intricate biological processes influenced by SM aids in elucidating the mechanisms underlying its reparative effects on diabetic retinopathy. These pathways emerge as potential targets for drug development or interventions aimed at modulating the progression of diabetic retinopathy retinopathy.
Conclusion
The investigation uncovered that potent pharmacological mechanisms are strictly linked to endothelial dysfunction, neovascularization, inflammation, and oxidative stress. Based on the results, luteolin and tanshinone-IIA may inhibit oxidative stress, inflammation, neovascularization, and endothelial dysfunction by controlling the binding and recognition of related extracellular (VEGF) and intracellular (AKT1, TNF, and TP53) proteins. The biological characteristics are mainly controlled via the PI3K/Akt signaling and Lipid and Fluid shear stress-induced atherosclerosis pathways. The outcomes of molecular docking suggested that the active constituents of SM bind tightly with essential targets, including AKT1, VEGFA, TP53, and TNF, in DR treatment. The identified targets, pathways, and constituents from SM provide a robust foundation for further research and the development of drugs or interventions for diabetic retinopathy. Additionally, this knowledge furnishes a roadmap for translational research, holding the potential to unveil novel therapeutic agents in the ongoing pursuit of effective treatments for diabetics. However, this study has limitations, like the possibility that some compounds or targets may not have been involved in the analysis and the absence of experimental authentication for the results obtained employing network pharmacology and docking. Therefore, we suggest that our group conduct further wet lab experimental studies to confirm these findings.
Author Statements
Data Availability Statement
The original data presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
Conceptualization KL; MY; Formal analysis, KL, SLG, and QZ; Investigation, KL; Resources, MY; Data curation, K. L, and MY; Writing-original draft preparation, KL, and SLG; Writing-review & editing, KL, ZL, and ML, Funding acquisition, ZL All authors have read and agreed to the published version of the manuscript.
Acknowledgements
This project was supported by Zhejiang Province "leading goose" research and development project (number: 2023C02017).
Conflicts of Interest
The authors have no conflicts of interest to declare relevant to this article's content.
References
- Tilahun M, et al. Prevalence of Diabetic Retinopathy and Its Associated Factors among Diabetic Patients at Debre Markos Referral Hospital, Northwest Ethiopia, 2019: Hospital-Based Cross-Sectional Study. Diabetes Metab Syndr Obes. 2020; 13: 2179-2187.
- Lee R, TY Wong, C Sabanayagam. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond). 2015; 2: 17.
- Chen WP, et al. Danhong Huayu Koufuye combined with metformin attenuated diabetic retinopathy in Zucker diabetic fatty rats. Int J Ophthalmol. 2015; 8: 1094-100.
- Zhang M, et al. Network pharmacology and molecular docking study on the active ingredients of qidengmingmu capsule for the treatment of diabetic retinopathy. Scientific Reports. 2021; 11: 7382.
- Zheng L, TS Kern. Role of nitric oxide, superoxide, peroxynitrite and PARP in diabetic retinopathy. Front Biosci (Landmark Ed). 2009; 14: 3974-87.
- Tang J, TS Kern. Inflammation in diabetic retinopathy. Prog Retin Eye Res. 2011; 30: 343-58.
- Zhang L, et al. Effect of Salvia miltiorrhiza on retinopathy. Asian Pac J Trop Med. 2013. 6: 145-9.
- Giri B, et al. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: An update on glucose toxicity. Biomedicine & Pharmacotherapy. 2018; 107: 306-328.
- Ormazabal V, et al. Association between insulin resistance and the development of cardiovascular disease. Cardiovascular Diabetology. 2018; 17: 122.
- Ríos JL, F Francini, GR Schinella. Natural Products for the Treatment of Type 2 Diabetes Mellitus. Planta Med. 2015; 81: 975-94.
- Guo Y, et al. Salvia miltiorrhiza improves type 2 diabetes: A protocol for systematic review and meta-analysis. Medicine (Baltimore). 2021; 100: e23843.
- Scott LJ, CM Spencer. Miglitol: A review of its therapeutic potential in type 2 diabetes mellitus. Drugs. 2000; 59: 521-49.
- Daryabor G, et al. The Effects of Type 2 Diabetes Mellitus on Organ Metabolism and the Immune System. Frontiers in Immunology. 2020; 11.
- Jia Q, et al. Salvia miltiorrhiza in diabetes: A review of its pharmacology, phytochemistry, and safety. Phytomedicine. 2019. 58: 152871.
- Xu S, et al. Tanshinone II-A inhibits oxidized LDL-induced LOX-1 expression in macrophages by reducing intracellular superoxide radical generation and NF-?B activation. Transl Res. 2012; 160: 114-24.
- Guan Z, et al. Tanshinone IIA induces ferroptosis in gastric cancer cells through p53-mediated SLC7A11 down-regulation. Biosci Rep. 2020. 40.
- Zhao J, L Li, G Fang. Salvianolic acid A attenuates cerebral ischemia/reperfusion injury induced rat brain damage, inflammation and apoptosis by regulating miR-499a/DDK1. Am J Transl Res. 2020. 12: 3288-3301.
- Piao CL, et al. Utilizing network pharmacology to explore the underlying mechanism of Radix Salviae in diabetic retinopathy. Chinese medicine. 2019; 14: 58 DOI: 10.1186/s13020-019-0280-7.
- Zhou Y, et al. Identification of Potential Molecular Targets and Active Ingredients of Mingmu Dihuang Pill for the Treatment of Diabetic Retinopathy Based on Network Pharmacology. Biomed Res Int. 2022; 2022: 2896185.
- Trott O, AJ Olson. AutoDock Vina:Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010; 31: 455-61.
- Cui Y, et al. Network Pharmacology Analysis on the Mechanism of Huangqi Sijunzi Decoction in Treating Cancer-Related Fatigue. J Healthc Eng. 2021; 2021: 9780677.
- Zhang L, et al. Exploring the mechanisms underlying the therapeutic effect of Salvia miltiorrhiza in diabetic nephropathy using network pharmacology and molecular docking. Biosci Rep. 2021; 41.
- Jiménez J, et al. DeepSite: Protein-binding site predictor using 3D-convolutional neural networks. Bioinformatics. 2017; 33: 3036-3042.
- Saeed M, et al. Identification of Putative Plant-Based ALR-2 Inhibitors to Treat Diabetic Peripheral Neuropathy. Curr Issues Mol Biol. 2022; 44: 2825-2841.
- Huang J, et al. Effects of Vitexin, a Natural Flavonoid Glycoside, on the Proliferation, Invasion, and Apoptosis of Human U251 Glioblastoma Cells. Oxid Med Cell Longev. 2022; 2022: 3129155.
- Saeed M, et al. Assessment of Antidiabetic Activity of the Shikonin by Allosteric Inhibition of Protein-Tyrosine Phosphatase 1B (PTP1B) Using State of Art: An In Silico and In Vitro Tactics. Molecules. 2021; 26.
- Clinic M. Diabetic retinopathy-Symptoms and causes. 2023.
- Antonetti DA, R Klein, TW Gardner. Diabetic retinopathy. N Engl J Med. 2012; 366: 1227-39.
- An X, et al. The Mechanism of Compound Danshen Dripping Pills in the Treatment of Diabetic Retinopathy Based on Network Pharmacology and Molecular Docking. 2021.
- Ding L, D Jin, X Chen. Luteolin enhances insulin sensitivity via activation of PPARγ transcriptional activity in adipocytes. J Nutr Biochem. 2010; 21: 941-7.
- Lu He, et al. Effects of luteolin on retinal oxidative stress and inflammation in diabetes. RSC Advances. 2015; 5: 4898-4904.
- Liu JF, et al. Reduction of lipid accumulation in HepG2 cells by luteolin is associated with activation of AMPK and mitigation of oxidative stress. Phytother Res. 2011; 25: 588-96.
- Yang Y, M Zhou, H Liu. Luteolin, an aryl hydrocarbon receptor antagonist, alleviates diabetic retinopathy by regulating the NLRP/NOX4 signalling pathway: Experimental and molecular docking study. Physiol Int. 2021.
- Zhang M, et al. Luteolin Attenuates Diabetic Nephropathy through Suppressing Inflammatory Response and Oxidative Stress by Inhibiting STAT3 Pathway. Exp Clin Endocrinol Diabetes. 2021; 129: 729-739.
- Zeng X, et al. Study on the Antioxidant Effect of Tanshinone IIA on Diabetic Retinopathy and Its Mechanism Based on Integrated Pharmacology. Evid Based Complement Alternat Med. 2022; 2022: 9990937.
- Gao H, et al. Total tanshinones exhibits anti-inflammatory effects through blocking TLR4 dimerization via the MyD88 pathway. Cell Death & Disease. 2017; 8: 3004-e3004.
- Guo ZL, et al. Sodium Tanshinone IIA Silate Alleviates High Glucose Induced Barrier Impairment of Human Retinal Pigment Epithelium through the Reduction of NF-?B Activation via the AMPK/p300 Pathway. Current Eye Research. 2020; 45: 177-183.
- Alzhrani RM, et al. Tanshinone IIA Inhibits VEGF Secretion and HIF-1a Expression in Cultured Human Retinal Pigment Epithelial Cells under Hypoxia. Current Eye Research. 2017; 42: 1667-1673.
- Zhang Z, H Liu, J Liu. Akt activation: A potential strategy to ameliorate insulin resistance. Diabetes Research and Clinical Practice. 2019; 156: 107092.
- Kang C, D LeRoith, E J Gallagher. Diabetes, Obesity, and Breast Cancer. Endocrinology. 2018; 159: 3801-3812.
- Jacot JL, D Sherris. Potential Therapeutic Roles for Inhibition of the PI3K/Akt/mTOR Pathway in the Pathophysiology of Diabetic Retinopathy. J Ophthalmol. 2011; 2011: 589813.
- Lin FL, et al. Gene Therapy Intervention in Neovascular Eye Disease: A Recent Update. Molecular Therapy. 2020; 28: 2120-2138.
- Ferrara N, AP Adamis. Ten years of anti-vascular endothelial growth factor therapy. Nat Rev Drug Discov. 2016; 15: 385-403.
- Staff, D. Why Does High Blood Sugar Damage Blood Vessels? diabetestalk.net. 2017.
- Gurel Z, et al. Identification of O-GlcNAc Modification Targets in Mouse Retinal Pericytes: Implication of p53 in Pathogenesis of Diabetic Retinopathy. PLOS ONE. 2014; 9: e95561.
- Huang X, et al. The PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci. 2018; 14: 1483-1496.
- Yang X, et al. Combination therapy with semaglutide and rosiglitazone as a synergistic treatment for diabetic retinopathy in rodent animals. Life Sci. 2021; 269: 119013.
- Zhang Y, W Wang, A Yang. The involvement of ACO3 protein in diabetic retinopathy through the PI3k/Akt signaling pathway. Adv Clin Exp Med. 2022; 31: 407-416.
- Chang CZ, et al. Arctigenin, a Potent Ingredient of Arctium lappa L. Induces Endothelial Nitric Oxide Synthase and Attenuates Subarachnoid Hemorrhage-Induced Vasospasm through PI3K/Akt Pathway in a Rat Model. Biomed Res Int. 2015; 2015: 490209.
- Cui J, et al. Gambogic acid ameliorates diabetes-induced proliferative retinopathy through inhibition of the HIF-1a/VEGF expression via targeting PI3K/AKT pathway. Life Sci. 2018; 192: 293-303.
- Ji Z, et al. miR-7a Targets Insulin Receptor Substrate-2 Gene and Suppresses Viability and Invasion of Cells in Diabetic Retinopathy Mice via PI3K-Akt-VEGF Pathway. Diabetes Metab Syndr Obes. 2021; 14: 719-728.
- Zhang C, et al. Association Between Atherosclerosis and Diabetic Retinopathy in Chinese Patients with Type 2 Diabetes Mellitus. Diabetes Metab Syndr Obes. 2020; 13: 1911-1920.
- Ellis PT, et al. Diabetic Retinopathy and Atherosclerosis: is there a Link? Current Diabetes Reviews. 2013; 9(2): 146-160.
- Papadaki M, et al. Fluid shear stress as a regulator of gene expression in vascular cells: Possible correlations with diabetic abnormalities. Diabetes Research and Clinical Practice. 1999; 45: 89-99.
- Molins B, et al. Shear stress modulates inner blood retinal barrier phenotype. Experimental Eye Research. 2019; 187: 107751.
- Ding X, et al. Luteolin Attenuates Atherosclerosis Via Modulating Signal Transducer And Activator Of Transcription 3-Mediated Inflammatory Response. Drug Des Devel Ther. 2019; 13: 3899-3911.
- Yang C, et al. Tanshinone IIA: A Chinese herbal ingredient for the treatment of atherosclerosis. Front Pharmacol. 2023; 14: 1321880.
- Pan D, L Xu, M Guo. The role of protein kinase C in diabetic microvascular complications. Frontiers in Endocrinology. 2022; 13.
- Cai J, M Boulton. The pathogenesis of diabetic retinopathy: Old concepts and new questions. Eye. 2002. 16: 242-260.
- Li S, B Zhang. Traditional Chinese medicine network pharmacology: Theory, methodology and application. Chin J Nat Med. 2013; 11: 110-20.
- Theoharides T, S Asadi, S Panagiotidou. A case series of a luteolin formulation (NeuroProtek®) in children with autism spectrum disorders. SAGE Publications Sage UK: London, England. 2012; 317-323.
- Zhou ZY, et al. Sodium tanshinone IIA sulfonate: A review of pharmacological activity and pharmacokinetics. Biomedicine & Pharmacotherapy. 2019; 118: 109362.
- Mansour SE, et al. The Evolving Treatment of Diabetic Retinopathy. Clin Ophthalmol. 2020; 14: 653-678.
