
Research Article
Austin J Pharmacol Ther. 2022; 10(2).1164.
Postconditioning Efficacy of Natural Carnosine and a Carnosine-Containing Galanin Receptor Agonist in Myocardial Ischemia/Reperfusion Injury
Pisarenko O*, Studneva I, Serebryakova L, Konovalova G, Lankin V, Veselova O, Dobrokhotov I, Timoshin A, Abramov A, Avdeev D and Sidorova M
National Medical Research Center of Cardiology, 121552 Moscow, Russia
*Corresponding author: Oleg Pisarenko, National Medical Research Center of Cardiology, 121552 Moscow, Russia
Received: October 12, 2022; Accepted: November 09, 2022; Published: November 16, 2022
Abstract
The mechanisms of protective action of peptide WTLNSAGYLLGPβAHOH (Gal), the pharmacological agonist of galanin receptor GalR2, against myocardial Ischemia/Reperfusion Injury (IRI) remain obscure. This work was designed to compare anti-ischemic and antioxidant effect of Gal and the dipeptide carnosine (βAH), the C-terminal part of the Gal molecule.Gal was obtained by automatic solid phase synthesis using the Fmoc methodology. The effect of Gal and carnosine was studied in a rat model of acute Myocardial Infarction (MI) when administered intravenously at the onset of reperfusion in the dose range of 0.5-5.0 mg/kg. Administration of the optimal dose of Gal (1 mg/kg) reduced the size of MI, decreased the plasma activity of creatine kinase-MB and lactate dehydrogenase at the end of reperfusion and improved preservation of the energy state of the Area at Risk (AAR) of the heart to a significantly greater extent than carnosine. In addition, Gal more effectively reduced the formation of hydroxyl radicals spin adducts and lipid peroxidation products in the AAR compared to carnosine. The optimal dose of carnosine (1 mg/kg) caused a significantly greater increase in the activity of catalase and glutathione peroxidase in the AAR than Gal. The results suggest the possibility of using Gal as an adjuvant therapy to reduce myocardial reperfusion injury and oxidative stress.
Keywords: Galanin; Carnosine; Myocardial infarction; Lipid peroxidation; Antioxidant enzymes; Reactive oxygen species
Abbreviations
AAR: Area at Risk; CAT: Catalase; CK-MB: Creatine Kinase- MB; Cu,Zn-SOD: Cu,Zn-Superoxide Dismutase; DMPO: 5,5-Dimethyl-1-Pyrrolin N-Oxide; DMSO: Dimethyl Sulfoxide; Gal: WTLNSAGYLLGPβAH-OH; GSH-Px: Glutathione Peroxidase; IRI: Ischemia/Reperfusion Injury; IS: Infarct Size; LAD: Left Anterior Descending Coronary Artery; LDH: Lactate Dehydrogenase; LPO: Lipid Peroxidation; LV: Left Ventricle; PCr: Phosphocreatine; PPC: Pharmacological Postconditioning; ROS: Reactive Oxygen Species; TBARS: Thiobarbituric Acid Reactive Substances; βAH: L-Carnosine, ΣAN: Total Adenine Nucleotide Pool; ΣCr: Total Creatine.
Introduction
Myocardial ischemia and reperfusion induce various forms of cell and coronary microcirculation damage, which contribute to myocardial infarction [1]. Pharmacological Post Conditioning (PPC) for cardioprotection is attracting researchers and physicians with a wide range of reperfusion therapeutic interventions to limit Ischemia/Reperfusion Injury (IRI). The mechanisms of PPC are implemented through the involvement of many triggers, mediators and end effectors involved in cardio protective effects [2]. The neuropeptide galanin, consisting of 29 amino acid residues (30 amino acid residues in humans), is involved in the regulation of vital processes - memory, food intake, body weight, alcohol dependence, neuropathic pain, ionic homeostasis and osmosis [3]. Like other natural G-protein-coupled receptor ligands, galanin is able to activate intracellular signaling cascades, triggering the mechanisms of programmed cell survival under conditions of insufficient supply of oxygen and energy substrates [4]. In peripheral organs and tissues, galanin acts not only through neuronal mechanisms, but also by activating receptors GalR1, GalR2 and GalR3.We have previously shown that exogenous N-terminal galanin fragments (2- 11) and (2-15) (WTLNSAGYLL-NH2 and WTLNSAGYLGGPHAOH, respectively), which preferentially bind to the GalR2 receptor, decrease the formation of superoxide radicals in mitochondria and reduce apoptosis and necrosis during myocardial I/R [5,6]. Subsequently, a number of proteolytically stable analogs of galanin fragment (2-11) and (2-15) were synthesized, which retained the pharmacophore amino acid residues responsible for binding to the GalR2 receptor [7]. The chimeric molecule representing the sequence of galanin (2-13) supplemented with the natural dipeptide carnosine WTLNSAGYLLGPβAH-OH (Gal) turned out to be the most effective. Gal improved the parameters of rat heart function, the integration of cell membranes, and the energy state of cardiomyocytes, as well as reduced the formation of Reactive Oxygen Species (ROS) and Lipid Peroxidation (LPO) products, and increased the activity of antioxidant enzymes in the heart when modeling oxidative stress in vitro and in vivo [8]. It cannot be ruled out that beneficial effects of Gal could be due to C-terminal carnosine. The effectiveness of carnosine has been shown in various pathophysiological conditions, including myocardial I/R injury, and is primarily due to its direct antioxidant effect, as well as the properties of a modulator of superoxide dismutase, an antiglycating agent, a metal ion chelator, and a molecular chaperone [9-11]. The aim of this work was to compare the anti-ischemic and antioxidant effects of Gal and carnosine in in vivo and in vitro models of I/R injury of the rat heart. For this purpose we evaluated the effect of these peptides as postconditioning agents on myocardial Infarct Size (IS), damage to cardiomyocyte membranes, ROS generation, the formation of LPO products, and the activity of Cu,Zn-Superoxide Dismutase (Cu,Zn-SOD), Glutathione Peroxidase (GSH-Px) and Catalase (CAT).
Materials and Methods
Animals
Age-matched male Wistar rats weighing 300+/-2 g were provided by the Animal House Stolbovaya of Scientific Center for Biomedical Technologies (Moscow, Russia). All rats were kept in individual cages at 20-250C with a natural light-dark cycle and had free access to water and standard pelleted diet (Aller Petfood, St. Petersburg, Russia). The animal experiments were performed in accordance with the European Convention for the Protection of Vertebrate Animals Used for Experimental and other Scientific Purposes (No 123 of 18 March 1986). The Bioethical Committee of National Medical Research Center of Cardiology, Moscow approved the experimental protocols (permission No. 9 of 12 September 2021).
Peptides
Gal was produced using a stepwise solid-phase synthesis with Fmoc methodology [7]. It was purified using reverse phase High Performance Liquid Chromatography (HPLC), and its structure was characterized with 1H-NMR-spectroscropy and MALDI TOF (Matrix-assisted laser desorption/ionization-time of flight) massspectrometry. The chemical structure of the peptide, its mass spectrum and HPLC conditions are shown in (Figure S1, S2 and S3 in Supplementary data). Characteristics of Gal peptide are given in (Table 1). L-carnosine (βAH) was purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA).
Sequence
MW, g/mol
Yield*, %
MALDI-TOF, m/z
HPLC **
Rt, min
Purity,%
WTLNSAGYLLGPβAH-OH
1499.67
46.3
1499.76
1521.73[M+Na]+
1537.72[M+K]+14.66
98.2
Rt - Retention Time. *The yields are given relatively to the first amino acid, which is attached to the polymer. ** The analytical HPLC was performed on a Kromasil 100-5 C18 column (4, 6 × 250 mm), the size of sorbent particles was 5 μm. Buffer A (0.1% trifluoracetic acid) and buffer B (80% acetonitrile in buffer A) were used as eluents. The column was eluted at a flow rate of 1 ml/min with a concentration gradient of buffer B in buffer A from 20 to 80% within 30 min at 220 nm.
Table 1: Characteristics of the synthesized Gal peptide.
Chemicals and Kits
Chemicals and enzymes for myocardial metabolite determination were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Kits for assessment of plasma Creatine Kinase-MB (CK-MB) and Lactate Dehydrogenase (LDH) activity were purchased from BioSystems S.A. (Barcelona, Spain). Commercial preparations of Cu,Zn-SOD and GSH-Px from bovine erythrocytes and of CAT from bovine liver (Sigma-Aldrich Chemical Co. (St. Louis, MO, USA) were used in vitro model systems. A spin trap 5, 5-dimethyl- 1-pyrrolin N-oxide (DMPO) was purchased from Abcam (UK). All other chemicals of the analytical grade were purchased from Serva Chemicals (Germany) and Fluka Chemie (Switzerland). Solutions were prepared using deionized water (Millipore Corp. Bedford, MA, USA).
Experimental Design
Rats were randomly allocated into four groups (n = 8): Initial State (I.s.), Control (Cont), Carnosine (C) and peptide Gal (G). A 30- min period of stabilization of hemodynamic parameters (the initial state, i.s.) followed the animal's preparation. In controls, a 40-minute Left Anterior Descending (LAD) coronary artery occlusion followed by a 60-minute reperfusion was performed after i.s. in the C and G group, the peptides were administered intravenously (iv) as a bolus at doses of 0.5; 1.0; 2.0; 3.0 or 5.0 mg/kg at the onset of reperfusion. Rats of the control group were injected with the same volume of saline (0.5 ml). In a separate series of experiments the effect of the peptide solvent (0.2% Dimethyl Sulfoxide, DMSO) on the myocardial IS and plasma activities of CK-MB and LDH was examined.
Assessment of Myocardial Infarct Size
At the end of reperfusion, LAD coronary artery was occluded and 2 ml of 2% Evans Blue solution was injected through the jugular vein to distinguish the myocardial non-ischemic Area from the Area at Risk (AAR). The heart was excised and myocardial infarct size was determined using 2, 3, 5-triphenyltetrazolium chloride staining of the Left Ventricle (LV) [12]. The stained LV slices were placed between two transparent glasses and captured using a scanner at 600 d.p.i. resolution; the saved images were digitized by computerized planimetry using Image J software (NIH, USA). The AAR was expressed as a percentage of LV weight, the IS was expressed as a percentage of the AAR in each group.
Assessment of Cardiac Markers
At the end of the steady state and reperfusion, blood samples were collected for plasma separation and stored at -700C for further analysis. Plasma LDH and CK-MB activities were used as indicators of cardiomyocyte membrane damage.
Monitoring of ROS Production in the AAR of Rat Heart
The level of short-lived oxygen radicals in the AAR was monitored by micro dialysis technique and spin trapping with DMPO [13]. This compound can effectively interact with both superoxide and hydroxyl radicals producing relatively stable spin adducts that can be detected using Electron Paramagnetic Resonance (EPR) technique [14]. A Varian E-109 E X-band EPR spectrometer (Germany) was used for registration of EPR spectra of dialysates. EPR signals of DMPO spin adducts were recorded at room temperature with a magnetic field modulation of 0.1 mT, modulation of frequency of 100 kHz, microwave power of 10 mW, and microwave frequency of 9.15 GHz. Magnetic field was scanned with a center at g = 2.00 during signal recording.
Preparation of Tissue Homogenates
At the end of reperfusion, the AAR was quickly excised from the heart and freeze-clamped by a Wollenberger clamp cooled in liquid nitrogen. A portion of the frozen tissue samples was homogenized in 50 mM Na-phosphate buffer, pH 7.4 (1:10 wt/vol) using an Ultra- Turrax T-25 homogenizer (IKA-Labortechnik, Staufen, Germany). The homogenates were centrifuged at 1000xg for 10 min at 4oC in a Joan MR-23 centrifuge (France). The supernatants contained the cytosolic fraction were used for the estimation of Cu, Zn-SOD, CAT and GSHPx activities, Thiobarbituric Acid Reactive Substances (TBARS) and protein. The remaining frozen tissue samples were used to prepare protein-free extracts using cooled 6% HClO4 (10 ml/g) as previously described [5] for the determination of myocardial metabolites. Tissue dry weights were determined by weighing a portion of the pellets after extraction with 6% HClO4 and drying overnight at 1100C. In a separate series, the non-ischemic area was excised from the heart and freeze-clamped to determine the metabolites in the initial state.
Determination of Antioxidant Enzymes Activity and TBARS
Cu, Zn-SOD activity was determined from inhibition of blue nitrotetrazolium reduction by the superoxide radical generated in the system of xanthine-xanthine oxidase [15]. CAT activity was determined by the rate of H2O2 consumption at 200C during 1 min at λ = 240 nm [16]. Activity of GSH-Px was determined in the conjugated system of glutathione-glutathione reductase by oxidation of NADPH using tert-butyl hydroperoxide as a substrate [17]. Proteins in the supernatant of the cardiac muscle homogenate were precipitated with 10% trichloroacetic acid (1:1). The level of TBARS, the secondary LPO products, was determined in the reaction with 2-thiobarbituric acid by analyzing quantity of the produced trimethine complex at λ = 532 nm [18]. Protein in the supernatants was estimated by the method of Lowry.
Determination of Myocardial Metabolites
Concentrations of ATP, ADP, AMP, PCr, Cr and lactate in neutralized tissue extracts were determined by modified enzymatic methods [19] using a Shimadzu UV-1800 spectrophotometer (Japan).
Assessment of the Effect of Peptides on Activities of the Antioxidant Enzymes
Commercial preparations of Cu, Zn-SOD and GSH-Px and CAT dissolved in 50 mM phosphate buffer (pH 7.4) to a concentration of 250 μg/ml were used. Gal and carnosine dissolved in 50 mM phosphate buffer (pH 7.4) were added to the enzyme-containing solutions to final concentrations of 0.01 and 0.1 mM. The resulting mixtures were incubated at 40C for 24 h. Activities of Cu, Zn-SOD, CAT, and GSH-Px were determined on completion of incubation as described in [15-17].
Statistical Analysis
All data are presented as means ± SEM. Results were analyzed by one-way ANOVA followed by Bonferroni multiple range tests for estimation of differences between more than two groups. Comparisons between two groups involved use of the Student’s unpaired t-test. A P<0.05 was considered statistically significant.
Results
Effects of Gal and carnosine on myocardial IRI in rats in vivo
Histochemical analysis of LV sections after reperfusion did not reveal significant differences in the AAR between the control, G, C groups, and administration of 0.2% DMSO. For these groups, the average value of the ratio AAR/LV, % was 40.8±0.9%. In the control, myocardial IS was 43.3±2.1% (Figure 1A). The administration of the vehicle (0.2% DMSO) did not affect IS: in this case, the ratio of IS/ AAR, % was 41.6±3.1%. The administration of the studied doses of Gal resulted in a decrease in IS. The optimal dose of Gal 1.0 mg/kg reduced IS by an average of 39.7% compared with control (P<0.001). In the case of carnosine, the largest reduction in IS by 22.4% was found for a dose of 1.0 mg/kg (P<0.01). The difference in IS limitation between Gal and carnosine at 1 mg/kg was statistically significant (P=0.044).
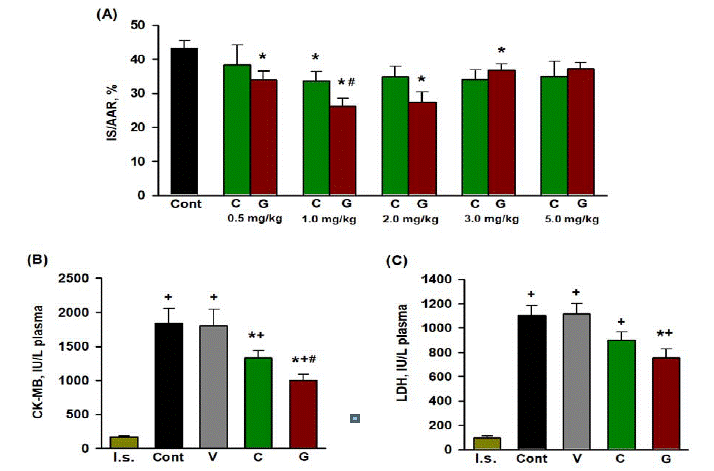
Figure 1: Effects of intravenous injections of Gal and carnosine on the parameters of myocardial I/R injury in rats in vivo.
(A) Dose-dependent effect of the peptides on myocardial infarct size (IS/AAR, %): Cont, control; C, carnosine; G, a pharmacological agonist of galanin receptor
GalR2. Data are means + SEM for each group of eight animals. A significant difference (P< 0.05) vs: *Cont, #C. Effect of optimal doses of the peptides on plasma
activities of CK-MB (B) and LDH (C) in rats at the end of reperfusion. I.s., initial state, Cont, control (injection of saline); V, vehicle, 0.2% DMSO; C (carnosine, 1 mg/
kg); G (agonist of galanin receptor GalR2, 1 mg/kg). Data are means + SEM for each group of eight animals. A significant difference (P<0.05) vs: + I.s, *Cont, # C.
Activity of Necrosis Markers in Blood Plasma
In the control, the plasma activity of necrosis markers CK-MB and LDH by the end of reperfusion were an order of magnitude higher than those in the initial state (Figure 1B, C). The administration of 0.2% DMSO did not affect the activity of both enzymes compared with the values in the control. The optimal dose of Gal (1.0 mg/kg) significantly reduced the activity of CK-MB and LDH compared with the control by 45.6 (P<0.005) and 31.6% (P<0.05), respectively. Carnosine reduced the plasma activity of CK-MB by an average of 27.6% (P<0.05) and the activity of LDH by 18.4% (P = 0.079) compared with the control at the end of reperfusion. A statistically significant difference between peptides was found for the reduction in CK-MB activity (P=0.030).
Changes in Metabolic State of the AAR Induced By Gal and Carnosine
The effect of optimal doses of Gal and carnosine on the parameters of the metabolic state of the AAR at the end of reperfusion is shown in (Figure 2). The administration of Gal increased the content of ATP (by 37.9% compared with the control, P= 0.012) and had almost no effect on the levels of ADP and AMP. This resulted in a significant increase in the total adenine nucleotide pool ΣAN=ATP+ADP+AMP (by 52.3% compared with the control, P<0.001). Under the influence of Gal, the total creatine (ΣCr=PCr+Cr) was retained in the AAR to a greater extent than in the control (on average by 27.6%, P<0.01). These changes were accompanied by a 3-fold decrease in the accumulation of lactate in the AAR compared with the control (P<0.001). Carnosine showed a clear tendency to increase ATP and ADP in the AAR compared with the values in the control, and did not affect the content of AMP. This led to a significant increase in ΣAN (P=0.024). Carnosine did not have a significant effect on the components of the PCr-Cr system compared to these indices in the AAR of the control group. The administration of carnosine reduced lactate in the AAR compared with the control (P<0.001). The overall effectiveness of both peptides on metabolic state of the reperfused AAR was approximately the same. A statistically significant difference between the peptide groups was observed only for ΣCr (Figure 2E): Gal preserved 81.9% of pre-ischemic Cr content, while carnosine 68.4% (P<0.01).
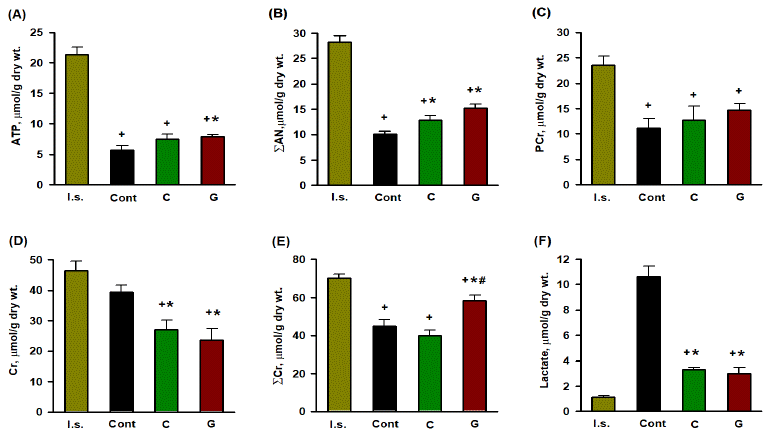
Figure 2: Effects of optimal dose of Gal and carnosine (1mg/kg) on metabolic state of the area at risk of rat heart at the end of reperfusion.
(A), (B), (C), (D), (E), (F) Myocardial contents of ATP, SAN=ATP+ADP+AMP, PCr, Cr, SCr=PCr+Cr, and lactate. I.s., initial state; Cont, control; C, iv administration
of carnosine, G, iv administration of the pharmacological agonist of galanin receptor GalR2. Data are means ±SEM from 8 experiments. A significant difference (P
<0.05) vs: + I.s., * Cont, # C.
Effect of Gal and Carnosine on ROS Production in the AAR
The EPR spectra of the dialysate samples were four narrow equidistant lines with an amplitude ratio of 1:2:2:1 (Figure S4, Supplementary data), characteristic of the paramagnetic DMPOOH adduct formed as a result of the interaction of DMPO with hydroxide [15]. Figure 3A presents changes in the dialysate DMPOOH concentration in the course of experiment. In the control, this parameter increased after 40 minutes of LAD occlusion, which was due to an increase in the level of ROS in the interstitium of the AAR. This could be due to a significant increase in the rate of ROS generation in the mitochondrial respiratory chain upon restoration of blood flow in the AAR. The administration of Gal at the onset reperfusion significantly reduced the concentration of DMPO-OH in the dialysate compared with the control. These data indicate a decrease in the formation of ROS in the reperfused area of the heart under the action of this peptide. There was no significant decrease in the DMPO-OH adduct in the dialysates of the AAR compared with the control under the influence of carnosine. Starting from 120th minute of the experiment until the end of reperfusion, the differences in the dialysate DMPO-OH concentration between the peptide groups were significant (P<0.05).
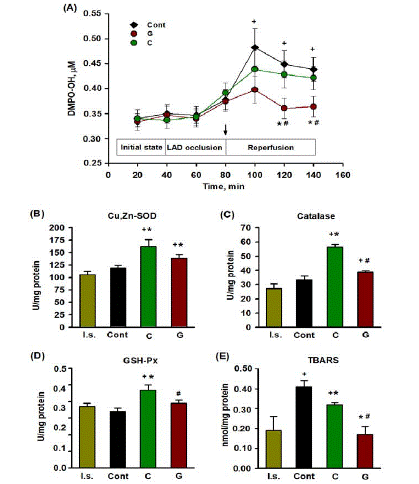
Figure 3: Antioxidant effect of optimal dose of Gal and carnosine (1mg/kg) in reperfusion of the area at risk of rat heart.
(A) Effect of peptides on the concentration of DMPO-OH adduct in dialysates from the area at risk of rat heart during the experiment. Cont, control; G, agonist of
galanin receptor GalR2; C, carnosine. The arrow shows iv administration of peptides. Data are means ±SEM from 5 experiments. A significant difference (P <0.05)
vs: + initial state, * Cont, # C.
(B), (C), (D) Activity of Cu,Zn-SOD, CAT and GSH-Px in the area at risk. (E) Content of TBARS in the area at risk. I.s., initial state; Cont, control; C, carnosine; G,
agonist of galanin receptor GalR2. Data are means ± SEM from 8 experiments. A significant difference (P <0.05) vs: + I.s., * Cont, # C.
TBARS Content and Activity of Antioxidant Enzymes in the AAR
The content of TBARS in the ARR of the control group at the end of reperfusion was two times higher than in the initial state (P=0.012, Figure 3E). The administration of Gal and carnosine significantly reduced the formation of TBARS in comparison with the control by 2.4 and 1.3 times, respectively. In the Gal group, this LPO index was almost two times lower than in the carnosine group (P<0.005). In the control, the activity of Cu, Zn-SOD, CAT and GSH-Px in the AAR did not differ from the values in the initial state (Figure 3B, C, D). The injection of Gal increased the activity of Cu, Zn-SOD (P=0.043) in the AAR, but did not have a significant effect on the activity of CAT and GSH-Px compared with the control. In the carnosine group, the activities of Cu, Zn-SOD, CAT and GSH-Px were higher by 1.3, 1.7 and 1.4 times, respectively, than in the control (P<0.02-0.001). In general, these experiments showed that carnosine increased the activity of CAT and GSH-Px in the AAR to a significantly greater extent than Gal.
The effect of peptides on the activity of Cu, Zn-SOD, CAT, and GSH-Px in myocardial reperfusion injury could be associated with their direct effect on enzymes. This situation was modeled by the incubation of Gal and carnosine with commercial Cu,Zn-SOD, GSHPx and CAT in in vitro systems. When choosing the concentrations of peptides in the incubation medium, the dose and volume of circulating blood in a rat with an average weight of 300 g were taken into account. In accordance with this, a concentration of peptides close to physiological (0.01 mM) and an order of magnitude higher was used. Varying the concentrations of peptides in the incubation medium had little effect on the activity of antioxidant enzymes (Table 2). Both peptides at a concentration of 0.01 mM and 0.1 mM significantly increased the activity of Cu, Zn-SOD by an average of 38 and 28% for carnosine and Gal, respectively, compared with the values in the control. Incubation with carnosine or Gal at concentrations of 0.01 mM and 0.1 mM caused a slight but significant decrease in CAT activity (on average by 5% for both peptides) and had no effect on GSH-Px activity compared with the control. These data show that changes in the activity of commercial antioxidant enzymes during incubation with carnosine and Gal are of a multidirectional nature, which is not consistent with the data of in vivo experiments (Figure 3).
Cu,Zn-SOD, U/ml
CAT, U/ml
GSH-Px, U/ml
Control
145.30+3.19
462.84+3.07
12.73+0.45
Carnosine,0.01mM
Carnosine,0.1 mM
199.32+1.19*
202.21+4.01*#
439.25+1.02*
446.77+4.70*
13.35+0.27
12.99+0.28
Gal, 0.01 mM
Gal, 0.1 mM193.73+3.72*
180.23+3.36*443.12+1.77*
438.36+2.68*13.82+0.34
13.12+0.10Data are presented as means + SEM for a series of 3 experiments. Significant difference P<0.05 from: * control, # Gal.
Table 2: Influence of Gal and carnosine on the activity of commercial antioxidant enzymes in vitro.
Discussion
In the present work, the post conditioning activity of Gal, the pharmacological agonist of the galanin receptor GalR2, and the dipeptide carnosine was studied in acute myocardial infarction in rats in vivo. Intravenous bolus administration of these peptides at the onset of reperfusion initiated anti-ischemic protection of the damaged myocardium. This was manifested in the limitation of myocardial IS, a reduction of cardiomyocyte membrane damage, and an improvement in the metabolic state of AAR at the end of reperfusion. Antioxidant properties of both peptides were also revealed in this model. They consisted in a decrease in the formation of DMPO-OH, the adduct of hydroxyl radicals, in the interstitium of the AAR during reperfusion, and an increase in the myocardial activity of Cu, Zn-SOD, CAT and GSH-PX, which was accompanied by a decrease in the formation of TBARS in the reperfused area of the heart.
One of the reasons for the increased activity of antioxidant enzymes in the AAR of the rat heart under the action of peptides could be upregulation of gene expression. This assumption is supported by the results of a study [20], which demonstrated an increase in Cu,Zn- SOD mRNA expression in the infarcted myocardium of galanintreated mice. The ability of carnosine to enhance the antioxidant capacity of the cell by increasing the expression of key antioxidant enzymes was also noted in various experimental objects [21,22]. Many peptides are known to be able to scavenge ROS and inhibit LPO [23]. These properties are well documented for carnosine [9- 11]. In a number of independent studies, including myocardial I/R injury, a suppression in the formation of ROS and LPO products (malonic dialdehyde, peroxyl radicals, and cytotoxic unsaturated aldehydes 4-hydroxy-trans-2-nonenal and acrolein) under the action of carnosine have been demonstrated [24,25]. There are no data on the direct antioxidant effect of Gal in the contemporary literature. However, recent studies have shown that its natural analog, the N-terminal fragment of galanin (2-15) WTLNSAGYLLGPHA-OH, reduced the formation of superoxide anion radical and hydrogen peroxide in mitochondria of isolated rat cardiomyocytes and cardiomyoblasts of the H9c2 cell line during hypoxia/reoxygenation, which was accompanied by a decrease in apoptosis [6]. We believe that the mechanisms of action of Gal in conditions characterized by increased levels of oxidative stress (I/R injury, diabetes, metabolic syndrome, neurodegenerative diseases) are an important task for future research.
The influence of Gal and carnosine on parameters of myocardial injury and oxidative stress is compared in (Table 3). Of note, Gal limited myocardial IS to a significantly greater extent than carnosine. This effect was accompanied by a greater decrease in the activity of both necrosis markers (CK-MB and LDH) in blood plasma and a more pronounced improvement in metabolic state of the AAR at the end of reperfusion compared with these indices for carnosine. These key differences directly point to the benefits of Gal as a postconditioning agent. The possibility of activation of various signal transduction pathways upon binding of N-terminal fragments of galanin to the GalR2 receptor and its consequences for damaged cardiomyocytes has been discussed earlier [8,26]. Coupling of the GalR2 receptor to various types of G proteins (Gi/o, Gq/11, and G12/13) can stimulate the uptake and oxidation of glucose by cardiomyocytes [27], inhibit proapoptotic BAD/BAX proteins and reduce the activity of caspase-3 and caspase-9 [3], block the opening of mitochondrial transient permeability pores (mPTP) and thus promote cell survival and motility [28]. It is highly probable that these mechanisms can be activated by Gal peptide. Along with this, Gal is able to enhance the antioxidant protection of the reperfused myocardium, which may not be directly related to the activation of GalR2 receptors. In accordance with previous results [10,24,25,29], carnosine showed a significantly more pronounced increase in the activity of antioxidant enzymes (CAT and GSH-Px) in the AAR compared to Gal.
Gal
Carnosine
Limitation in IS/AAR,% of control
39.7*
22.4
Decrease in plasma CK-MB activity, % of control
45.6*
27.6
Decrease in plasma LDH activity, % of control
31.6*
NS
Reduction of Cr loss in the AAR, μmol/g dry wt.#
12.7*
22.1
Increase in AN content in the AAR, μmol/g dry wt.+
5.24
2.81
Increase in PCr content in the AAR, μmol/g dry wt.+
4.54
1.56
Reduction of lactate accumulation in AAR, μmol/g dry wt.+
7.64
6.80
Decrease in DMPO-OH concentration in the interstitium of the AAR at the end of reperfusion, μM +
0.075*
0.017
Increase in Cu,Zn-SOD activity in the AAR, U/mg protein +
19.44
42.95
Increase in CAT activity in the AAR, U/mg protein +
5.39
22.93*
Increase in GSH-Px activity in the AAR,U/mg protein +
NS
0.13*
Reduction in TBARS content in the AAR, nmol/mg protein +
0.24*
0.09
The data presented in Figures 1-3 was used. Significant difference (P <0.05) from: *the comparison peptide; #the initial state; +the control. NS, no significant difference from control.
Table 3: Effect of postconditioning with Gal and carnosine on parameters of myocardial injury and oxidative stress in acute myocardial infarction in rats.
Conclusions
The pharmacological agonist of galanin receptor GalR2, peptide Gal WTLNSAGYLLGPβAH-OH, and its C-terminal fragment, the dipeptide carnosine βAH, exhibit similar metabolic and antioxidant effects in a model of regional ischemia and reperfusion of the rat heart in vivo. When using the same doses, Gal reduces the necrotic death of cardiomyocytes in the AAR during reperfusion and the release of the necrosis marker CK-MB into the bloodstream to a significantly greater extent than carnosine. As is known, the use of pharmacological agents in early reperfusion, capable of postconditioning the ischemic heart, is recognized as a useful addition to the generally accepted methods of protection against myocardial infarction and is well documented in the experiment. To date, a small number of such compounds have been studied in clinical trials. The results of this work suggest the possibility of using Gal as an adjuvant therapy to reduce myocardial reperfusion injury. In the future, it seems important to study the molecular mechanisms and signaling molecules, the activation of which by pharmacological agonists of galanin receptors is able to reproduce the beneficial effects of postconditioning.
Funding Sources
This study was supported by grants from the Russian Foundation for Basic Research (No. 18-015-00009) and the Russian Science Foundation (No. 22-15-00013) and the Ministry of Health of the Russian Federation (No. 121031700143-1).
Ethical Statement
All animal experiments were carried out in accordance with the recommendations for the care and handling of animals of the Bioethical Committee of the National Medical Research Center for Cardiology, Moscow (permission No. 9, September 12, 2021).
Competing Interests
The authors have declared that no competing interests exist.
Author Contribution
OP and MS designed and planned the study. LS and AA performed a simulation of acute myocardial infarction in rats. OV performed determination of TBARS and the statistical analyzes. ID performed blood glucose determination and biological sample preparation. GK determined the activity of antioxidant enzymes. AT studied the formation of ROS by the EPR method. IS carried out determination of myocardial metabolites and activity of necrosis markers. DA and MS synthesized and purified Gal. OP and VL wrote the manuscript. The final version of the manuscript was approved by all authors.
References
- Heusch G. Myocardial ischaemia–reperfusion injury and cardioprotection in perspective. Nature Reviews Cardiology. 2020; 17: 773-789.
- Wu Y, Liu H, Wang X. Cardioprotection of pharmacological postconditioning on myocardial ischemia/reperfusion injury. Life Sciences. 2021; 264: 118628.
- Lang R, Gundlach AL, Kofler B. The galanin peptide family: receptor pharmacology, pleiotropic biological actions, and implications in health and disease. Pharmacology & Therapeutics. 2007; 15: 177–207.
- West C, Hanyaloglu AC. Minireview: Spatial Programming of G Protein- Coupled Receptor Activity: Decoding Signaling in Health and Disease. Molecular Endocrinology. 2015; 29: 1095–1106.
- Timotin A, Pisarenko O, Sidorova M, Studneva I, Shulzhenko V, Palkeeva M, et al. Myocardial protection from ischemia/reperfusion injury by exogenous galanin fragment. Oncotarget. 2017; 8; 21241-21252.
- Pisarenko O, Timotin A, Sidorova M, Studneva I, Shulzhenko V, Palkeeva M, et al. Cardioprotective properties of N-terminal galanin fragment (2-15) in experimental ischemia/reperfusion injury. Oncotarget. 2017; 8: 101659- 101671.
- Palkeeva M, Studneva I, Molokoedov A, Serebryakova L, Veselova O, Ovchinnikov M, et al. Galanin/GalR1-3 system: a promising therapeutic target for myocardial ischemia/reperfusion injury. Biomedicine & Pharmacotherapy. 2019; 109; 1556-1562.
- Pisarenko OI, Studneva IM, Serebryakova LI, Timoshin AA, Konovalova GG, Lankin VZ, et al. Antioxidant Properties of Galanin and Its N-Terminal Fragments in in vitro and in vivo Oxidative Stress Modeling. Biochemistry (Moscow). 2021; 86; 496-505.
- Bellia F, Vecchio G, Cuzzocrea S, Calabrese V, Rizzarelli E. Neuroprotective features of carnosine in oxidative driven diseases. Molecular Aspects of Medicine. 2011; 32: 258–266.
- Boldyrev AA, Aldini G, Derave W. Physiology and pathophysiology of carnosine. Physiological Reviews. 2013; 93: 1803–1845.
- Johnson P, Hammer JL. Histidine dipeptide levels in ageing and hypertensive rat skeletal and cardiac muscles. Comparative Biochemistry and Physiology. 1992; 103: 981-984.
- Kitakaze M, Takashima S, Funaya H, Minamino T, Node K, Shinozaki Y, et al. Temporary acidosis during reperfusion limits myocardial infarct size in dogs. American Journal of Physiology. 1997; 272: H2071-H2078.
- Timoshin AA, Tskitishvili OV, Serebryakova LI, Kuzmin AI, Medvedev OS, Ruuge EK. Microdialysis study of ischemia-induced hydroxyl radicals in the canine heart. Experientia. 1994; 50: 677–679.
- Britigan BE, Cohen MS, Rosen GM. Detection of the production of oxygencentered free radicals by human neutrophils using spin trapping techniques: a critical perspective. Journal of Leukocyte Biology. 1987; 41: 349-362.
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and assay applicable to acrylamide gels. Analytical Biochemistry. 1971; 44: 276- 287.