
Research Article
Austin J Pharmacol Ther. 2022; 10(2).1165.
2,3,5,4'-Tetrahydroxystilbene-2-O-Β-D-Glucoside Inhibiting Hepatocytes Injury Through Reducing the Expression of Tumor Necrosis Factor-a in LPSStimulated Kupffer Cells via Regulating PPAR-γ/NFκB
Wei D1, Jin R1, Zhao H1 and Li Q1,2*
¹School of Public Health, Shanghai University of Traditional Chinese Medicine, China
²Shanghai Frontiers Science Center of TCM Chemical Biology; Institute of Interdisciplinary Integrative Medicine Research, Shanghai University of Traditional Chinese Medicine, China
*Corresponding author: Qian Li, School of Public Health, Shanghai University of Traditional Chinese Medicine, 1200 Cailun Road, Shanghai 201203, China
Received: October 20, 2022; Accepted: November 15, 2022; Published: November 22, 2022
Abstract
2,3,5,4'-Tetrahydroxystilbene-2-O-Β-D-Glucoside (TSG), a main bioactive component of polygonum multiflorum Thumb, exerts anti-oxidant, antitumor, anti-inflammatory effects. However, the protective effects of TSG on hepatocytes through anti-inflammatory effects have rarely been investigated. Mouse liver macrophages (Kupffer cells, KUP5 cells) were primed with various concentrations of TSG before Lipopolysaccharide (LPS) treatment, and AML12 hepatocytes were cultured with the supernatants of KUP5 cells. The results showed that TSG inhibits the expression of iNOS in KUP5 cells. Meanwhile, Tumor Necrosis Factor-a (TNF-a) and interleukin-1Β (IL-1Β) expression in KUP5 cells were reversed after TSG treatment. Additionally, the expression of Peroxisome Proliferators-Activated Receptors-γ (PPAR-γ) was up-regulated while the phosphorylation of p65 and IκB-a were decreased in KUP5 cells after TSG treatment. Furthermore, GW9662 (PPAR-γ antagonist) treatment could eliminate the anti-inflammatory effects of TSG. In AML12 hepatocytes, the level of AST, ALT, and apoptosis was decreased after being cultured with the supernatants of KUP5 cells pretreated with TSG. Moreover, TNF-a neutralization of KUP5 cells supernatants could decrease the level of AST, ALT, and AML12 hepatocyte apoptosis. These results indicate that TSG activates PPAR-γ, thereby decreasing nuclear factor kappa-B (NFκB) activation and reducing the release of TNF-a in macrophages to protect hepatocytes from injury. Our study may help further understand the protective effects of TSG on hepatocytes by suppressing liver inflammation.
Keywords: 2,3,5,4'-tetrahydroxystilbene-2-O-Β-D-glucoside; Kupffer cells; Tumor necrosis factor-a; PPAR-γ; NFκB; Hepatocyte
Abbreviations
TSG: 2,3,5,4'-Tetrahydroxystilbene-2-O-B-D-Glucoside; LPS: Lipopolysaccharide; Inos: Inducible Nitric Oxide Synthase; TNF-A: Tumor Necrosis Factor-A; IL-1Β: Interleukin-1Β; PPAR-Γ: Peroxisome Proliferators-Activated Receptors-G; AST: Aspartate Transaminase; ALT: Alanine Transaminase; CCK-8 Assay: Cell Counting Kit-8 Assay; MFI: Mean Fluoresce Intensity; Qpcr: Quantitative Polymerase Chain Reaction; ELISA: Enzyme-Linked Immunosorbent Assay Kcs: Kupffer Cells; Nfκb: Nuclear Factor Kappa-B; IL-6: Interleukin-6
Introduction
Inflammation plays an important role in many diseases [1]. The liver is a target of inflammatory cytokines and can release a wide number of proteins to control the inflammatory response [2]. Moreover, multiple hepatic disorders may become more severe as a result of excessive inflammation, which can lead to a significant loss of hepatocytes [3]. Chronic liver inflammation leads to fibrosis and cirrhosis, which is the 12th leading cause of death in the United States [4]. Great attention and efforts have been devoted to discovering drugs against liver inflammation-induced liver injury. What is more, it has been reported that several herbal medicines can be used to treat liver injury [5].
Polygonum multiflorum Thumb could exert multiple effects, including anti-inflammation, immunomodulation, antioxidant, and anti-cancer activities [6]. 2,3,5,4'- Tetrahydroxystilbene -2-O-Β-D-Glucoside (TSG) is a main bioactive component of polygonum multiflorum Thumb and has several functions, including immunosuppressive, anti-tumor, anti-inflammatory, and anti-fatty liver effects [7]. What is more, TSG effectively protects the liver against acute alcoholic liver injury [8]. Accumulating evidence has shown that TSG decreases the production of pro-inflammatory cytokines [9]. However, the protective effects of TSG on hepatocytes through suppressing liver inflammation have rarely been investigated.
Kupffer Cells (KCs) are liver-derived macrophages and account for 80–90% of tissue macrophages in the body [10]. KCs counteract the aforementioned stimuli and play a fundamental role in the liver inflammatory response. KCs secrete pro-inflammatory cytokines including TNF-a and IL-6 after stimulation with LPS, which leads to liver inflammation [11]. Notably, TNF-a participates in the occurrence and process of liver diseases and induces hepatocyte apoptosis, which is the most important event in the mechanism and a common feature of hepatic failure [12]. Moreover, TNF-a can induce multiple mechanisms to initiate apoptosis in hepatocytes leading to subsequent liver injury [13]. It has been reported that TNF-a is involved in the pathophysiology of viral hepatitis, alcoholic liver disease, and ischemia-reperfusion injury [14].
Peroxisome Proliferation-Activated Receptors (PPARs) are nuclear receptors that include three subfamilies encoded by distinct genes: a, Β, and γ. Among PPARs, PPAR-γ matters considerably in the immune response [15]. PPAR-γ regulates the polarization of macrophages and the production of TNF-a was increased in PPAR-γ KO macrophages [16]. Moreover, PPAR-γ exerts antiinflammatory effects by reducing NFκB activation. After binding to toll-like receptors, LPS activates the NFκB signaling pathway. NFκB trans-locates into the nucleus, and then pro-inflammatory genes are activated [17].
Therefore, the protective effects of TSG on hepatocytes through anti-inflammatory effects and its mechanism were explored in the present study. KCs (KUP5 cells) were stimulated with LPS, and then the anti-inflammatory effects of TSG were tested. Moreover, AML12 hepatocytes were cultured with the supernatants of KUP5 cells to evaluate the role of TNF-a. We further explore the involvement of PPAR-γ and NFκB in LPS-induced inflammation of KUP5 cells. Our study demonstrated that TSG activates PPAR-γ, thus inhibiting the activation of NFκB, and then reduces the production of TNF-a of KCs, thereby protecting hepatocytes from injury. Our study provides the basis for the clinical application of TSG in the treatment of liver inflammation-induced liver injury.
Materials and Methods
Reagents and Chemicals
2,3,5,4'-tetrahydroxystilbene-2-O-Β-D-glucoside (analytical standard, purity 98.0%) was purchased from Yuanye Bio-technology (Shanghai, China); Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum (FBS) and penicillin/streptomycin were obtained from Thermo (Thermo Scientific, CA, USA). 1% insulin-transferrinselenium (ITS), LPS, and GW9662 (PPAR-γ antagonist) were from Sigma (St. Louis, MO, USA).
Cell Culture and Treatment
Mouse liver macrophages (KUP5 cells) and AML12 hepatocytes were obtained from RIKEN Cell Bank (Japan) and the Cell Bank of the Chinese Academy of Science (Shanghai, China), respectively. Both cell types were cultured in DMEM containing 10% FBS, and 1% penicillin/streptomycin in a 5% CO2 incubator at 37°C. AML12 hepatocytes were cultured in DMEM/F12 medium with 1% Insulin- Transferrin-Selenium (ITS), 10% FBS, 40 ng/mL dexamethasone, and 1% penicillin/streptomycin at 37°C with 5% CO2. KUP5 cells were primed with TSG before LPS stimulation, and AML12 hepatocytes were cultured with the supernatants of KUP5 cells. KUP5 cells were pretreated with GW9662 (PPAR-γ antagonist) for testing the rule of PPAR-γ, and TNF-a was neutralized in the supernatants of KUP5 cells for testing the rule of TNF-a.
Cell Viability Assay
Cell counting kit-8 assay (CCK-8, beyotime, China) was performed to evaluate the cytotoxicity of TSG and LPS. KUP5 cells were pretreated with various concentrations of TSG (0, 60, 120, 240, and 480 μM) for 1h before the treatment with LPS (1 μg/ml) for 24h. The mediums were removed, and 110μl CCK-8 detection solution (100μl DMEM containing 10μl CCK-8 solution) was added into each well. After incubation for 1h, the optical density at 450nm was measured using a microplate reader (Biotek, USA). The concentration of TSG used in this study (0, 60, 120, 240, and 480 μM) was based on the previous research [18].
RNA Isolation and qPCR Analysis
Total RNA was isolated using TRIzol regent (Invitrogen, CA, USA), and the RNA was reversely transcribed into cDNA using the cDNA Synthesis Kit (Invitrogen, CA, USA). The mRNA amplifications were conducted with a qPCR system (Thermo Fisher Scientific, Vantaa, Finland). The experimental results were analyzed using the 2-ΔΔCt method. GAPDH was employed as the internal standard. The primer sequences, iNOS, forward 5'-ATGTCCGAAGCAAACATCAC-3', reverse 5' -TAATGTCCAGGAAGTAGGTG-3', GAPDH forward 5'- TTCAACGGCACAGTCAAGGC -3', reverse 5' GACTCCACGACATACTCAGCACC -3'.
Detection of TNF-a, IL-1Β, and IL-6 (ELISA)
KUP5 cells were primed with TSG for 1h and then stimulated with LPS for 24h. The supernatants were collected after centrifuging at 10,000g for 15min. The level of TNF-a and IL-1Β was assessed by ELISA Kit according to the instructions (Biolegend, San Diego, USA).
Flow Cytometry
The antibodies included FITC-anti-NOS2 (5C1B52, Thermo Scientific, CA, USA), PPAR-γ (81B8) Rabbit mAb, p-p65 Rabbit mAb (93H1), p-IκB-a Rabbit mAb (14D4) (Cell Signaling Technology, MA, USA), and Alexa Fluor 488 Donkey anti-rabbit IgG (poly4064) (BioLegend, San Diego, USA). KUP5 cells were treated with TSG for 1h after the treatment with LPS (1μg/ml) for 1h or 24h. Then, cells were harvested and placed into the cell plate for detection. Cells were first fixed/permeabilized with nuclear staining kit (Thermo Scientific, CA, USA) for 1h. For the measurement of iNOS, cells were incubated with the diluted antibody of iNOS for 1h. In the case of detecting PPAR-γ, p-p65, and p-IκB-a, the cells were incubated with diluted primary antibodies for 1h and then incubated with secondary antibodies for another 1h. AML12 cells were cultured with supernatants of KUP5 cells. AML12 apoptosis was detected using Annexin V / PI apoptosis kit (Beyotime Biotechnology, China). After staining, cells were detected using a BD flow cytometer (BD Biosciences, San Jose, USA) and data analysis was performed with Flowjo (9.3.2, FlowJo LLC, Ashland, USA).
Biochemical Tests
AML12 cell supernatants were collected for detection after centrifugation. The level of AST and ALT in supernatants of AML12 cells was measured using the Reitman-Frankel assay (Nanjing Jiancheng Institute, China).
TNF-a Neutralization
To test the effect of TNF-a on the apoptosis of hepatocytes, 100μl anti-TNF-a (final concentration, 1μg/ml) (clone: XT3.11) or control antibody (clone: MOPC-21) (BioXcell, West Lebanon, NH BE0058) was added into the supernatants of LPS-treated KUP5 cells for TNF-a neutralization. AML12 cells were then cultured with the supernatants.
PPAR-γ Inhibition
GW9662 was used for PPAR-γ inhibition. KUP5 cells were treated with 10 μM GW9662 for 30 min and incubated with TSG for 1 h, and then cells were treated with 1μg/mL LPS.
Statistical Analysis
Data analysis was performed using GraphPad Prism 9 (GraphPad Software Inc., CA, USA). One-way ANOVA and Tukey's post hoc test were used for the comparison between groups. Data were presented as the means ± Standard Deviation (SD) of three independent experiments, and P < .05 was considered significant.
Results
The Cytotoxicity of TSG and LPS in KUP5 Cells
The viability of KUP5 cells was not decreased after the treatment with different concentrations of TSG and LPS. Moreover, cytotoxic effects were not observed after the co-treatment with TSG and LPS, indicating that the effects of TSG on KUP5 cells were not attributed to cytotoxic effects (Figure 1). Therefore, 60, 120, and 240μM of TSG were selected for the following experiments.
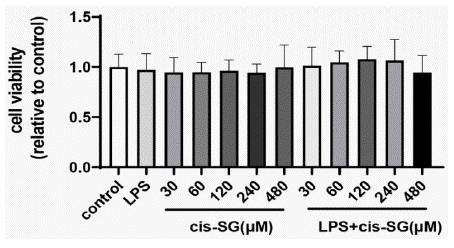
Figure 1: TSG and LPS cytotoxicity in KUP5 cells. KUP5 cells were
treated with various concentrations of TSG (0, 30, 60, 120, 240, and 480
μM) or LPS (1μg/ml) alone, or primed with TSG for 1h, and then treated with
LPS for another 24h. CCK-8 assay was used to measure the cell viability, OD
value of the control group was set as 1. Data were presented as mean ± SD.
TSG Down Regulates the Expression of iNOS and Proinflammatory Cytokines in KUP5 Cells
LPS induces macrophages toward pro-inflammatory (M1) polarization [19]. To evaluate the anti-inflammatory effects of TSG, the expression iNOS (the M1 marker) was measured. As shown in (Figure 2A), relative mRNA expression of iNOS was increased after LPS treatment, while iNOS expression was decreased after 120μM and 240μM TSG treatment, respectively. Similarly, the pretreatment with 120μM and 240μM TSG reduced the relative protein expressions of iNOS (Figure.2B, 2C). In addition, we found that TNF-a and IL-1Β were decreased after TSG pretreatment (Figure.2D, 2E). The results indicate that TSG suppresses KUP5 towards M1 polarization.
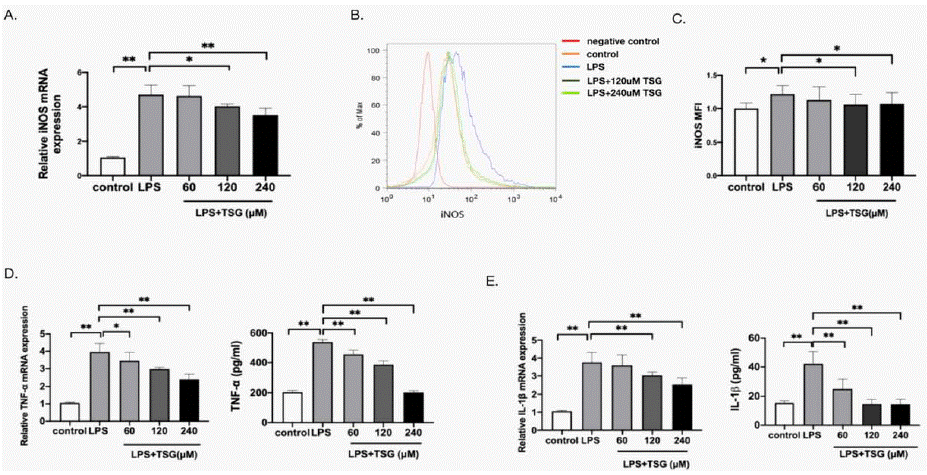
Figure 2: TSG downregulates LPS-induced iNOS expression and proinflammatory cytokines in KUP5 cells. KUP5 cells were primed with TSG for 1 h (60,
120, 240 μM) and then treated by LPS (1 μg/ml) for another 24 h. The expression of iNOS, TNF-a, and IL-1Β was measured. A. Relative mRNA expression of iNOS
in KUP5 cells. B. Representative flow plot for iNOS expressions of KUP5 cells. C. Relative MFI of iNOS. D. Relative mRNA expression and the concentration of
TNF-a. E. Relative mRNA expression and the concentration of IL-1Β. Data are expressed as mean ± SD, * p < 0.05, and ** p < 0.01 compared with the groups as
marked, respectively.
TSG Increases PPAR-G Expression While Reducing Nfκb Activation in KUP5 Cells
Considering the vital role of PPAR-γ in immune responses, the effect of TSG on PPAR-γ was examined to further explore the molecular mechanism. As indicated in (Figure 3A), PPAR-γ was reduced after stimulation with LPS, while the treatment of TSG increased the expression of PPAR-γ. Besides, the expression of p-p65 and p-IκB-a was detected to explore whether TSG prevents the activation of NFκB. The expression of p-p65 and p-IκB-adecreased after TSG treatment (Figure 3B and 3C). These results indicate that TSG activates PPAR-γ while reducing NFκB activation.
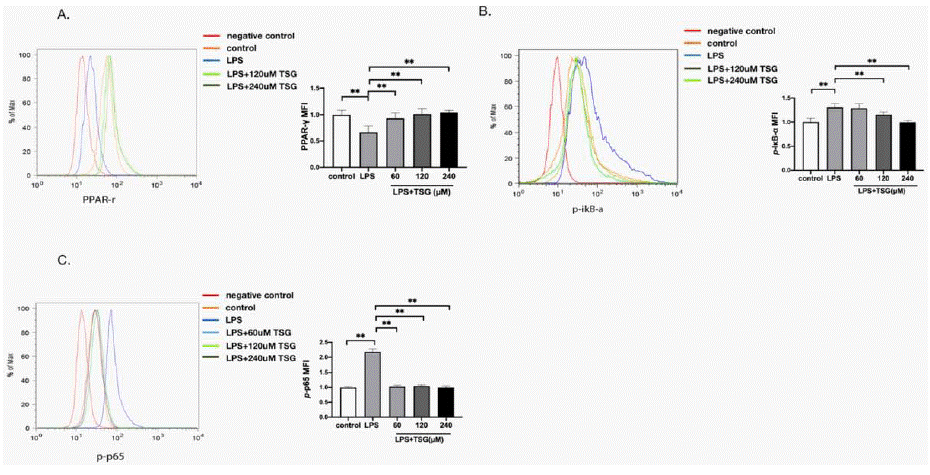
Figure 3: TSG increases PPAR-γ expression while reducing NFκB activation in KUP5 cells. KUP5 cells were treated with TSG for 1 h followed by treatment
with LPS (1μg/ml) for another 1 h. Flow cytometry was used to detect the level of PPAR-γ, p-p65 and p-IκB-a.
A. Representative flow plot and MFI for PPAR-γ expression.
B. Representative flow plot and MFI for p-IκB-a expression.
C. Representative flow plot and MFI for p-p65 expressions. Data are shown as mean ± SD. ** p < 0.01 compared with groups as marked.
PPAR-γ Mediates the Anti-Inflammatory Effects of TSG
With the increase in PPAR-γ level, attempts were made to explore the involvement of PPAR-γ in TSG inhibiting inflammatory response. GW9662 (PPAR-γ antagonist) was used before the TSG treatment. The expressions of iNOS and the concentrations of TNF-a were reduced by TSG. GW9662 was found capable of abolishing the antiinflammatory effect of TSG (Figure 4A and B). p-p65 and p-IκB-a were activated by GW9662 (Figure 4C and D). These results indicate that the inhibition of TSG on LPS-induced inflammatory response was mediated by PPAR-γ.
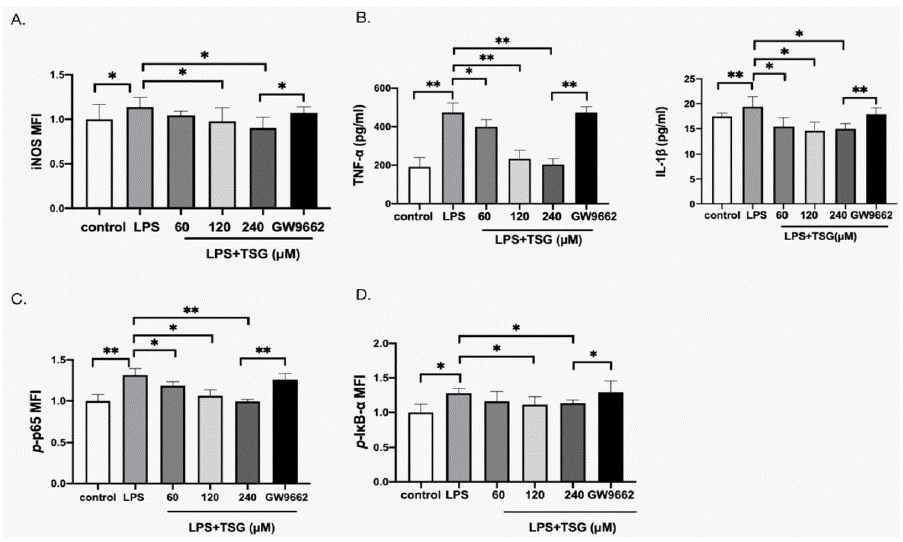
Figure 4: PPAR-γ mediates the anti-inflammatory effects of TSG.
KUP5 cells were treated with TSG (60, 120, and 240μM) for 1h, or 10uM GW9662 for 30min before TSG treatment (240μM) and stimulated with LPS for 24h. The
expression of iNOS was measured using flow cytometry. The concentration of TNF-a and IL-1Β were analyzed with ELISA, and the expression of p-p65 and p-IκB-a
was measured by flow cytometry. A. Relative iNOS MFI of KUP5 cells. B. Concentration of TNF-a and IL-1Β. C. Relative p-p65 MFI of KUP5 cells. D. Relative
p-IκB-a MFI of KUP5 cells. Data were expressed as mean ± SD. * p < 0.05 and ** p < 0.01 compared with other groups.
The Injury of AML Hepatocytes Was Restored After Being Cultured With the Supernatants of KUP5 Cells Pretreated With TSG
AML hepatocytes were cultured with supernatants of KUP5 cells for testing the efficiency of TSG in the case of protecting hepatocytes through anti-inflammatory effects. As shown in (Figure 5), the level of AST, ALT, and the apoptosis of hepatocytes was increased after being cultured with the supernatants of LPS-stimulated RAW264.7 cells. However, AST, ALT levels, and the apoptosis of hepatocytes were restored after being cultured with the supernatants of KUP5 cells pretreated with TSG. These results indicate that TSG protects hepatocytes through anti-inflammatory effects.
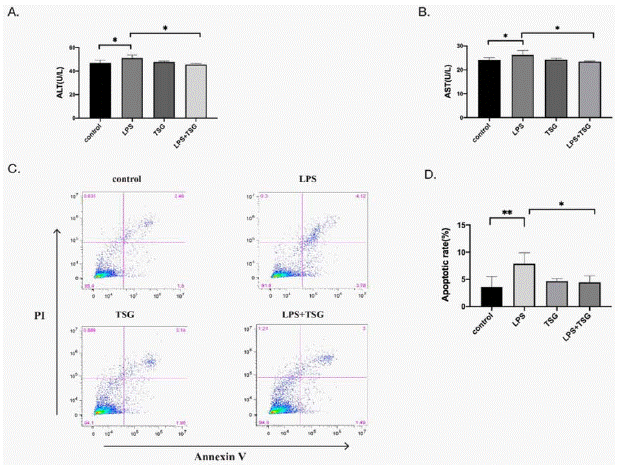
Figure 5: The injury of hepatocytes restored after being cultured with the supernatants of KUP5 cells pretreated with TSG. KUP5 cells were pretreated
with TSG (240μM) for 1h with or without LPS stimulation, and then, AML hepatocytes were cultured with the supernatants of KUP5 cells for another 24h. The AML
hepatocytes supernatants were collected for detecting the ALT and AST levels. AML12 cell apoptosis was measured by flow cytometry. A. AST level in AML12 cells
supernatants. B. ALT level in AML12 cells supernatants. C. Representative flow plots for apoptosis of AML12 cells. D. Apoptosis rate of AML12 cells as shown in
C. Data were expressed as mean ± SD. *p < 0.05 and **p < 0.01 compared with other groups.
TSG Reduces TNF-a Expression in Macrophages to inhibit the Injury of Hepatocytes by Regulating PPAR-γ
Given that TSG downregulates LPS-induced TNF-a expression, it is more likely that TNF-a mediates the hepatocytes injury. As shown in (Figure 6), the level of AST, ALT, and the apoptosis of AML12 cells were restored after TNF-neutralization. These findings indicated that TNF-a mediates the apoptosis of AML12 cells. It was also found that the level of AST, ALT, and AML12 cell apoptosis was increased after being cultured with the supernatants of KUP5 cells pretreated with GW9662. These results indicate that TSG activates PPAR-γ, thereby reducing TNF-a and hepatocyte apoptosis.
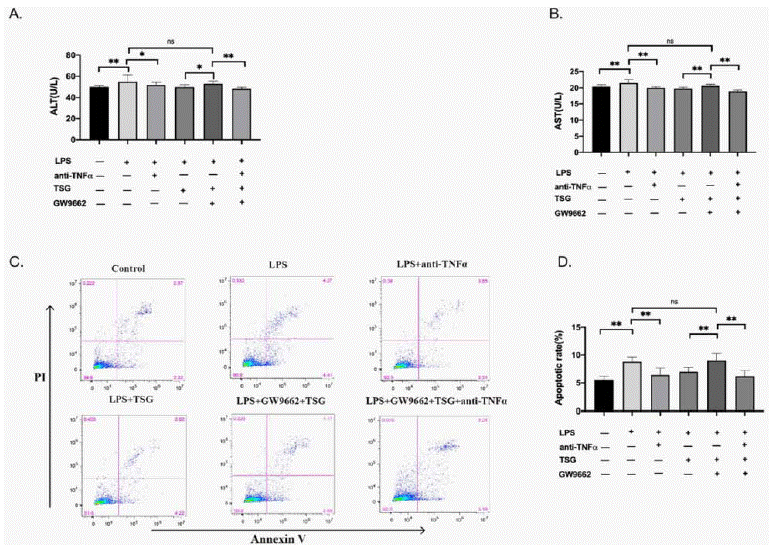
Figure 6: TSG reducing TNF-a expression to inhibit the injury of hepatocytes via regulating PPAR-γ. KUP5 cells were divided into 6 groups, control group,
LPS + isotype control group, LPS + anti-TNF-a group, LPS + TSG group, LPS+ TSG + GW9662 group and LPS+ TSG + GW9662+anti-TNF-a group. For the LPS
+ isotype control group and LPS + anti-TNF-a group, KUP5 cells were pretreated with 1μg/ml LPS for 24h and anti-TNF-a (1μg/ml) or isotype control was added to
the supernatants, and then AML12 cells were cultured with the supernatants for 24h. KUP5 cells were primed with TSG (240μM) for 1h, or with GW9662 (10μM) for
30 min for LPS + TSG group, LPS+ TSG + GW9662 group and LPS+ TSG + GW9662+anti-TNF-a group. Cells were treated with LPS for 1h, and 100μl anti-TNF-a
(1μg/ml) was added to the supernatants of KUP5 cells for the anti-TNF-a group. The same volume of isotype control was also used. AML12 cells were cultured with
these supernatants for 24h. The ALT, AST level, and AML12 cell apoptosis were measured. A. AST level in AML12 cells supernatants. B. ALT level in AML12 cells
supernatants. C. Representative Flow plots for apoptosis of AML12 cells. D. Apoptotic rate of AML12 cells. Data were expressed as mean ± SD. *p < 0.05 and **p
< 0.01 compared with other groups. ns indicates no difference between the groups.
Discussion
TSG, one of the main extractions from polygonum multiflorum Thumb, has been reported to exert anti-tumor, anti-inflammation, and anti-oxidant effects [20]. Whether TSG can protect hepatocytes through anti-inflammatory effects remains unknown. Our study suggests that TSG reduces the injury of hepatocytes by suppressing the inflammatory response of liver macrophages. Further research confirms that TSG activates PPAR-γ, suppressing NF-κB and decreasing the expression of TNF-a of KCs, thereby reducing liver injury.
The inflammatory response is an important self-defense mechanism. Immune cells are recruited to the infection site releasing inflammatory factors and removing pathogens when cells encounter harmful stimuli [21]. However, inflammation leads to a series of pathological reactions, such as arthritis, heart disease, Alzheimer's disease, atherosclerosis, cancer, and liver disease [21]. Inflammation is considered one of the hallmarks of acute and chronic liver disease [22].
As liver resident macrophages, KCs play an important role in live inflammatory responses. In response to pathogen invasion, KCs express a range of polarized phenotypes. Considerable studies have proven the essential role of M1 macrophages in the inflammation process [23]. After being stimulated by LPS, KCs secret pro-inflammatory cytokines, thus promoting the process of inflammation-related diseases [24]. Of note, we found that iNOS (the marker of M1 macrophages) expression was increased after LPS (1μg/ml) stimulation in KUP5 cells, while TSG was found to significantly reduce iNOS expression in KCs, suggesting that the anti-inflammatory effects of TSG might be related to the inhibition of M1 KCs. Indeed, inhibiting TNF-a is beneficial to the treatment of inflammation-related diseases [25]. It is observed that hepatocyte apoptosis may be related to the release of TNF-a secreted by KCs in our study.
TNF-a is involved in chronic inflammation and cell apoptosis [25]. Additionally, the activation of apoptotic pathways leads to the proliferation of hepatocytes that develops into fibrosis, cirrhosis, and even liver cancer [26]. The present study justifies that TSG suppresses TNF-a production. Additionally, after neutralizing the TNF-a of KUP5 supernatants, the level of AST, ALT, and the injury of hepatocytes were decreased. In this case, the protective effect of TSG on hepatocytes may be attributable to the inhibition of TNF-a. Furthermore, TNF-a activates NF-κB signaling, MAPK signaling, and Caspase-8 signaling, and thus regulates cell survival and proliferation [27,28], which also regulates the inflammation processes by interacting with the Wnt family, and the TGF-Β superfamily [29,30]. Therefore, the mechanism of TNF-a in liver disease needs to be further studied.
Besides, PPAR-γ inhibits inflammatory cytokines to exert antiinflammatory effects [31]. Indeed, some herbal extractions exercise protective effects by activating PPAR-γ [32]. We found that PPAR-γ expression was significantly up-regulated by TSG. In addition, NFκB signaling participates in regulating inflammation induced by extracellular stimulation [33]. We found that GW9662 reverses the activation of NFκB and abolishes the anti-inflammation effects of TSG. Thus, our study revealed that TSG attenuates liver injury by suppressing liver inflammation via PPAR-γ/ NFκB pathway in KCs.
Conclusion
In summary, our study demonstrated that TSG reduces the inflammatory response in KCs, thereby protecting hepatocytes from injury. The promising mechanism is that TSG activates PPAR-γ, and then downregulates NFκB activation and the release of TNF-a in KCs, thereby protecting hepatocytes from injury (Figure 7). This study may help further understand the protective effects of TSG on hepatocytes through downregulating liver inflammation.
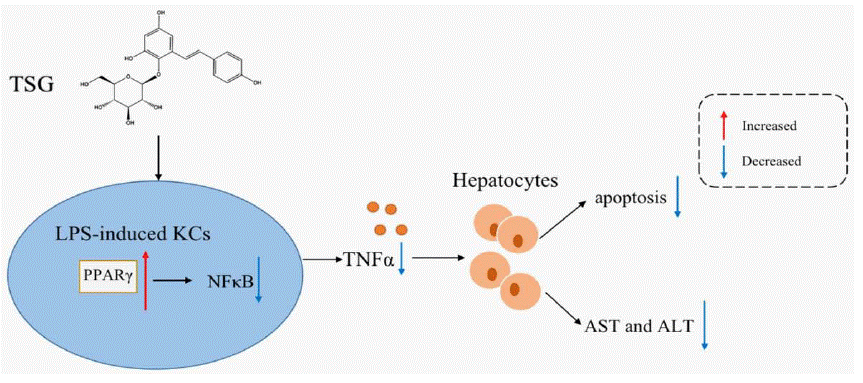
Figure 7: The diagrammatic sketch of TSG inhibiting hepatocytes injury through reducing the expression of TNF-a in LPS-stimulated Kupffer cells via regulating
PPAR-γ/NFκB. TSG activates PPAR-γ, and then downregulates NFκB activation and the release of TNF-a in KCs, thereby protecting hepatocytes from injury.
Declarations of Competing Interests
The authors declare the existence of no competing interests.
Funding
This work was supported by the Special Program for Discipline Construction of the School of Public Health (Grant number GJ202103) and the Project of Shanghai University of Traditional Chinese Medicine (Grant number 2021LK006).
References
- Conese M, Assael BM. Bacterial infections and inflammation in the lungs of cystic fibrosis patients. Pediatr Infect Dis J. 2001; 20: 207-13.
- Khansari N, Shakiba Y, Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat Inflamm Allergy Drug Discov. 2009; 3: 73-80.
- Robinson MW, Harmon C, O'Farrelly C. Liver immunology and its role in inflammation and homeostasis. Cell Mol Immunol. 2016; 13: 267-76.
- Koyama Y, Brenner DA. Liver inflammation and fibrosis. J Clin Invest. 2017; 127: 55-64.
- Stickel F, Schuppan D. Herbal medicine in the treatment of liver diseases. Dig Liver Dis. 2007; 39: 293-304.
- Park HJ, Zhang N, Park DK. Topical application of Polygonum multiflorum extract induces hair growth of resting hair follicles through upregulating Shh and beta-catenin expression in C57BL/6 mice. J Ethnopharmacol. 2011; 135: 369-75.
- Zhang YZ, Shen JF, Xu JY, Xiao JH, Wang JL. Inhibitory effects of 2,3,5,4'-tetrahydroxystilbene-2-O-beta-D-glucoside on experimental inflammation and cyclooxygenase 2 activity. J Asian Nat Prod Res. 2007; 9: 355-63.
- Wang W, He Y, Lin P, Li Y, Sun R, Gu W, et al. In vitro effects of active components of Polygonum Multiflorum Radix on enzymes involved in the lipid metabolism. J Ethnopharmacol. 2014; 153: 763-70.
- Jang KJ, Choi SH, Yu GJ, Hong SH, Chung YH, Kim CH, et al. Antiinflammatory potential of total saponins derived from the roots of Panax ginseng in lipopolysaccharide-activated RAW 264.7 macrophages. Exp Ther Med. 2016; 11: 1109-15.
- Li P, He K, Li J, Liu Z, Gong J. The role of Kupffer cells in hepatic diseases. Mol Immunol. 2017; 85: 222-9.
- Tsutsui H, Nishiguchi S. Importance of Kupffer cells in the development of acute liver injuries in mice. Int J Mol Sci. 2014; 15: 7711-30.
- Fan JH, Feng GG, Huang L, Tang GD, Jiang HX, Xu J. Naofen promotes TNF-alpha-mediated apoptosis of hepatocytes by activating caspase-3 in lipopolysaccharide-treated rats. World J Gastroenterol. 2014; 20: 4963-71.
- Dong J, Jimi E, Zeiss C, Hayden MS, Ghosh S. Constitutively active NFkappaB triggers systemic TNFalpha-dependent inflammation and localized TNFalpha-independent inflammatory disease. Genes Dev. 2010; 24: 1709- 17.
- Ezquerro S, Mocha F, Fruhbeck G, Guzman-Ruiz R, Valenti V, Mugueta C, et al. Ghrelin Reduces TNF-alpha-Induced Human Hepatocyte Apoptosis, Autophagy, and Pyroptosis: Role in Obesity-Associated NAFLD. J Clin Endocrinol Metab. 2019; 104: 21-37.
- Christofides A, Konstantinidou E, Jani C, Boussiotis VA. The role of peroxisome proliferator-activated receptors (PPAR) in immune responses. Metabolism. 2021; 114: 154338.
- Silveira LS, Biondo LA, de Souza Teixeira AA, de Lima Junior EA, Castoldi A, Camara NOS, et al. Macrophage immunophenotype but not anti-inflammatory profile is modulated by peroxisome proliferator-activated receptor gamma (PPARgamma) in exercised obese mice. Exerc Immunol Rev. 2020; 26: 10- 22.
- Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004; 18: 2195- 224.
- Yu W, Zhang X, Wu H, Zhou Q, Wang Z, Liu R, et al. HO-1 Is Essential for Tetrahydroxystilbene Glucoside Mediated Mitochondrial Biogenesis and Anti- Inflammation Process in LPS-Treated RAW264.7 Macrophages. Oxid Med Cell Longev. 2017; 2017: 1818575.
- Zhang X, Mosser DM. Macrophage activation by endogenous danger signals. J Pathol. 2008; 214: 161-78.
- Huang C, Wang Y, Wang J, Yao W, Chen X, Zhang W. TSG (2,3,4' ,5-tetrahydroxystilbene 2-O-beta-D-glucoside) suppresses induction of proinflammatory factors by attenuating the binding activity of nuclear factorkappaB in microglia. J Neuroinflammation. 2013; 10: 129.
- Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018; 9: 7204- 18.
- Hou XJ, Ye F, Li XY, Liu WT, Jing YY, Han ZP, et al. Immune response involved in liver damage and the activation of hepatic progenitor cells during liver tumorigenesis. Cell Immunol. 2018; 326: 52-9.
- Jetten N, Verbruggen S, Gijbels MJ, Post MJ, De Winther MP, Donners MM. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis. 2014; 17: 109-18.
- Lu TF, Yang TH, Zhong CP, Shen C, Lin WW, Gu GX, et al. Dual Effect of Hepatic Macrophages on Liver Ischemia and Reperfusion Injury during Liver Transplantation. Immune Netw. 2018; 18: e24.
- Zelova H, Hosek J. TNF-alpha signalling and inflammation: interactions between old acquaintances. Inflamm Res. 2013; 62: 641-51.
- Elsharkawy AM, Mann DA. Nuclear factor-kappaB and the hepatic inflammation-fibrosis-cancer axis. Hepatology. 2007; 46: 590-7.
- Borghi A, Verstrepen L, Beyaert R. TRAF2 multitasking in TNF receptorinduced signaling to NF-kappaB, MAP kinases and cell death. Biochem Pharmacol. 2016; 116: 1-10.
- Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008; 133: 693-703.
- Yoshimatsu Y, Wakabayashi I, Kimuro S, Takahashi N, Takahashi K, Kobayashi M, et al. TNF-alpha enhances TGF-beta-induced endothelialto- mesenchymal transition via TGF-beta signal augmentation. Cancer Sci. 2020; 111: 2385-99.
- Vallee A, Lecarpentier Y. Crosstalk Between Peroxisome Proliferator- Activated Receptor Gamma and the Canonical WNT/beta-Catenin Pathway in Chronic Inflammation and Oxidative Stress During Carcinogenesis. Front Immunol. 2018; 9: 745.
- Bouhlel MA, Derudas B, Rigamonti E, Dievart R, Brozek J, Haulon S, et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007; 6: 137-43.
- Shou X, Zhou R, Zhu L, Ren A, Wang L, Wang Y, et al. Emodin, A Chinese Herbal Medicine, Inhibits Reoxygenation-Induced Injury in Cultured Human Aortic Endothelial Cells by Regulating the Peroxisome Proliferator-Activated Receptor-gamma (PPAR-gamma) and Endothelial Nitric Oxide Synthase (eNOS) Signaling Pathway. Med Sci Monit. 2018; 24: 643-51.
- Karin M. NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol. 2009; 1: a000141.