
Research Article
Austin J Pharmacol Ther. 2023; 11(1): 1168.
Identification of Potential Drugs for Colorectal Cancer Chemoprevention through Computational Analysis
Kioko B*and Kagia R
Department of Pharmacology and Pharmacognosy, Kabarak University, Kenya
*Corresponding author: Kioko BenardDepartment of Pharmacology and Pharmacognosy, Kabarak University, Kenya
Received: December 07, 2022; Accepted: January 20, 2023; Published: January 27, 2023
Abstract
Introduction: Colorectal cancer is one of the common causes of hospitalizations, readmission, and poor quality of life due to disability, pain, and death. Most drugs identified to provide chemoprevention in colorectal cancer, such as NSAIDs, have a high level of toxicity. There is need to find novel drugs targeting colorectal cancer with favorable clinical profiles.
Objective: The study aimed to identify possible colorectal cancer prevention drugs by comparing the docking scores (representing potential biologic activity) of Aspirin, Sulindac, and Celecoxib with their structurally similar analogs. Materials and Methods: Ligand-based virtual screening and structure-based virtual screening were done for aspirin, sulindac and celecoxib to identify potential drug-like compounds. Compounds that passed the screening, pharmacokinetic profiling, and toxicity testing were considered possible drugs for colorectal cancer chemoprevention.
Results: The study identified 7 drug-like compounds from the ZINC database. ZINC02570895, with a better docking score than celecoxib coupled with favorable toxicity and metabolic profiles, was the most appropriate drug candidate for the inhibition of PDK-1. ZINC22309227, with a better docking score and favorable pharmacokinetic profile than sulindac was the most appropriate compound for further development into a MAP Kinase inhibitor. ZINC39406706, ZINC26469982, ZINC01847506, ZINC3382343, and ZINC01682308 had favorable toxicity profiles compared to aspirin and were most suitable for development of cyclooxygenase inhibitors in colorectal cancer prevention.
Conclusion: In-vivo and in-vitro tests are needed to ascertain the biological activity, synthesizability and clinical use of the compounds.
Keywords: Colorectal Cancer; NSAIDs; MAP Kinase 3; PDK-1; Cyclooxygenase Enzyme; Chemoprevention.
Abbeviations: PDK-1: 3-Phosphoinositide-Dependent Kinase-1; COX 2: Cyclooxygenase 2; COX 1: Cyclooxygenase 1; NSAIDs: Non-Steroidal Anti-Inflammatory Drugs; CRC: Colorectal Cancer; SBVS: Structure-Based Virtual Screening; LBVS: Ligand Based Virtual Screening; MAPK: Mitogen-Activated Protein Kinase; HBAs: Hydrogen Bond Acceptors; HBDs: Hydrogen Bond Donors
Introduction
Cancer is one of the most common causes of hospitalizations, readmission, and poor quality of life due to disability and pain, and death [1]. Cancer occurs due to dysregulation of various checkpoints in cell differentiation and replication. It is one of the major causes of mortality worldwide having accounted for approximately 10 million deaths in 2020 [2]. One of the major mechanisms of oncogenesis is inflammation [1]. Colorectal Cancer (CRC) cases are increasing at an alarming rate. In CRC, uncontrolled inflammation has been linked to development of adenomatous polyps which progress to neoplasm. Non-Steroidal Anti-Inflammatory Drugs (NSAIDS) have been shown to prevent colorectal cancer by limiting production of various inflammatory mediators [3,4].
The current cancer treatment modalities have helped prolong the survival of cancer patients without altering mortality [5]. In some cases, there is no definitive treatment resulting in watchful waiting like in the case of early-stage prostate cancer [6]. Treatment modalities like surgery and some chemotherapy agents leave the patients weaker due to adverse effects [5]. Due to this, there is a need for cancer management to focus on prevention rather than treatment. This is especially true in developing countries, where cancer is diagnosed at later stages. There are two primary modalities of cancer prevention: lifestyle modification and chemoprevention in high-risk patients. Lifestyle modification fails at times in high-risk patients. For example, mutation of the p53 gene confers a 29% chance of developing cancer regardless of lifestyle modification [7]. Therefore, people with a higher risk of colorectal cancer need prevention more than lifestyle modifications.
Chemoprevention is a promising field in colorectal cancer prevention. For decades, epidemiological studies have shown Sulindac, Aspirin, and Celecoxib to have a preventive action against CRC [7,8]. Sulindac and Celecoxib are beneficial in preventing the development of colorectal cancer in persons with the Adenomatous Polyposis Coli (APC) gene mutation [9]. Patients with the APC gene mutation present with adenomas in the colon, which progress into colon cancer if resection is not done [10]. According to Yin et al. [11], Aspirin significantly reduces the incidence of colorectal cancer.
Despite the many epidemiological studies on cancer chemoprevention, no definitive mechanism has been elucidated to show how NSAIDs prevent CRC. Some researchers have strongly suggested that COX inhibition and COX-independent pathways are responsible for the action of NSAIDs against CRC [12]. Cyclooxygenase (COX) enzyme is a major molecular target in CRC. Increased expression of cyclooxygenase in CRC causes an increase in prostaglandins which promote autocrine and paracrine signaling, causing unlimited proliferation and survival of cells [13]. Tumor cells can also produce excess PGE2, which acts in a paracrine/autocrine mechanism to promote angiogenesis through increased production of VEGF [14]. However, some reports have indicated that the effects of the drugs on CRC are more dependent on COX-independent pathways than COX-dependent pathways [9]. The claim is supported by a report that high levels of prostaglandin in-vitro and in-vivo inhibit cancer growth. Mitogen Activated Protein Kinase-3 (MAPK) and 3-Phosphoinositide-Dependent Protein Kinase 1 (PDK-1) are some of the most studied COX-independent pathways in CRC oncogenesis [15,16].
Chemoprevention is the use of chemical compounds to alter the course of disease with low toxicity [12]. Aspirin, Sulindac, and Celecoxib significantly alter CRC oncogenesis. However, chronic use of these drugs is marred by COX-Inhibition-Associated Adverse Effects (CIAAEs) such as gastrointestinal ulceration and bleeding for Aspirin and sulindac and cardiac toxicity for Celecoxib [13]. Also, the doses required for chemoprevention are higher than those used for anti-inflammatory and analgesic purposes which pose more toxicity.
The current study identifies potential drug-like molecules for preventing colorectal cancer by comparing the biological activity (expressed as docking scores) of Aspirin, Sulindac, and Celecoxib with their analogs in silico. The results are analyzed and interpreted based on binding energies to various target molecules involved in CRC development. The study acts as a foundation for cell-based high throughput screening which can be done to ascertain anticancer activity of the analogues.
Materials and Methods
Materials
PubChem online database was used to download structures of Aspirin, Celecoxib, and Sulindac. Avogadro software was used to optimize the structures of the NSAIDs and their analogues. Chimera software was used to dock the NSAIDs to their molecular targets. Protein databank was used to obtain the structure of the molecular targets of Aspirin, Celecoxib, and Sulindac. PubChem sketcher online tool was used to draw compound structures based on canonical smiles. Swiss similarity was used to perform ligand-based virtual screening. Swiss ADME online tool was used to predict pharmacokinetic profiles. Protox Server online tool was used to predict the toxixty profiles of the drugs and their analogues based on LD50.
Methods
An experimental quantitative study carried out through computational analysis. Both structure based virtual screening and ligand based virtual screening were used. Ligand-based virtual screening is based on the principle that compounds with a similar pharmacophore have a similar structure-activity relationship. In contrast, structure-based virtual screening is based on the principle that compounds with the highest docking score have the most increased activity [17]. Binding energies estimate the affinity of compounds to targets based on compound conformation and complementarity with the features of the binding pocket. Combination of both techniques has proved to be more accurate in identification of drug-like compounds than any of them used alone. Numerical data were collected, analyzed, and interpreted insilico. Aspirin, Sulindac, and Celecoxib were screened against the Zinc Drug-like database to obtain similar compounds that were screened against various targets in colorectal cancer. Similarity scores were based on the combination of the Tanimoto coefficient and Electroshape 3-D similarity [18].
Ligand-Based Virtual Screening
The canonical smiles of Aspirin, Sulindac, and Celecoxib were downloaded from PubChem (https://pubchem.ncbi.nlm.nih.gov/)and entered into the Swiss Simimilarity online tool. 40 similar analogs that met the sampling requirements for each drug were sketched using the PubChem sketcher tool (https://pubchem.ncbi.nlm.nih.gov//edit3/index.html), and their corresponding Molfiles downloaded. PDB format structures of Aspirin, Sulindac, and Celecoxib were downloaded and saved.
Structure-Based Virtual Screening
The drugs and their respective analogues were converted to the 3-D format, and the MMFF94s as the force field were optimized using the Avogadro software and minimized using the chimera software. The respective molecular targets (COX-2, PDK-1 & MAPK) were downloaded from the protein databank database (https://www.rcsb.org/ ) and saved. Using the chimera software, the nonstandard residues in the molecular targets were removed, and the resulting structure was saved. Surface binding analysis was carried out between the downloaded analogues and their respective molecular targets using the Auto Dock Vinatool on Chimera software. Surface binding analysis was done on Aspirin, Sulindac, and Celecoxib to act as positive controls. The docking scores of each compound were recorded.
Pharmacokinetics
The SWISSADME online tool (http://www.swissadme.ch/) was used to forecast the pharmacokinetic profiles of the drug-like compounds. Parameters such as molrcular weight, log P, hydrogen bond acceptors, hydrogen bond donors, number of rotatable bonds, gastrointestinal absorption, and susceptibility to p-glycoproteins was assessed and recorded.
Toxicity Profile
The protox server tool (https://tox-new.charite.de/protox II/index.PHP?site=compound input) was used to forecast the toxicity of the drug-like compounds. Toxicity was determined as a measure of Lethal Dose (LD50). The results obtained were recorded in the format of a table.
Data Presentation and Analysis
Data was presented in tables, created through Microsoft Word, showing the different NSAIDs and their analogues versus their respective docking scores, toxicity profiles and pharmacokinetic profiles. Percentages were used to relate the similarity of an analogue to the respective drug. Docking scores were numerical data representing the binding energy of an analogue or drug to the respective molecular target. Data recorded in tabular form was analyzed and interpreted numerically to give numerical comparisons of the docking scores of each analogue and its respective drug. This data was then interpreted descriptively. Data on the pharmacokinetic profiles of selected analogues were analyzed and interpreted non-numerically.
Results & Discussion
Forty structurally similar compounds each were identified for Aspirin, Sulindac, and Celecoxib. Analysis and selection of the potential drug-like compounds was made based on docking scores, toxicity profile (LD50 mg/kg), pharmacokinetic profile (GI absorption, metabolic profile, p-glycoprotein substrate), and adherence to the Lipinski rule of 5 relative to parent compounds (Aspirin, Sulindac, and Celecoxib).
Celecoxib and its Analogues
5 out of 40 compounds had a higher docking score than Celecoxib for PDK-1. ZINC01431703 (LD50 = 1300 mg/kg) and ZINC26673721 (LD50 = 280 mg/kg) were more toxic than celecoxib while ZINC02570895, ZINC13761811, ZINC02047040 had same LD50 (1400 mg/kg). No single compound had a combined higher docking score, better toxicity, and pharmacokinetic profile than Celecoxib. ZINC02570895 had the same toxicity profile and pharmacokinetic profile as Celecoxib but with a better docking score. ZINC13761811 had the same toxicity profile and binding energy but a better pharmacokinetic profile. ZINC13761811 showed the best pharmacokinetic profile. None of the compounds was a p-glycoprotein substrate (Table 3).
COMPOUND
DOCKING SCORE
% similarity
LD 50 (Mg/Kg) and Toxicity class
Lipinski rule
(v = no violation; X=violation)GI Absorption
P-gp
SubstrateMetabolism
ZINC02570895
-8.9
99.7
1400
v
High
No
CYP (1A2, 2C9) Inhibitor
CELECOXIB
-8.8
100
1400
v
High
No
CYP (1A2, 2C9) Inhibitor
ZINC13761811
-8.8
88.2
1400
v
High
No
No effect on CYP enzymes
ZINC02047040
-8.8
99.2
1400
v
High
No
CYP (1A2, 2C19,2C9) Inhibitor
ZINC01431703
-9.1
48.8
1300
v
Low
No
CYP (1A2, 2C19, C29, 2D6, 3A4) Inhibitor
ZINC26673721
-8.8
77.3
280
v
Low
Yes
CYP (1A2, 2C19,2C9, 3A4) Inhibitor
Table 1: Properties of selected compounds relative to Celecoxib.
COMPOUND
DOCKING SCORE
% SIMILARITY
LD 50 (Mg/Kg)
Lipinski rule of 5
P-gp substrate
GI absorption
CYP Metabolism
ZINC22309227
-7.2
72.3
264
v
No
High
CYP (1A2, 2C9) inhibitor
ZINC12341529
-8.0
99.0
264
v
No
High
CYP (1A2, 2C19, 2C9) Inhibitor
ZINC13654467
-9.2
40.4
1190
v
Yes
resistanceHigh
CYP (2C19, 2C9, 2D6, 3A4) Inhibitor
InteractionsSULINDAC
-7.0
100
264
v
No
High
CYP (2C19, 2C9, 3A4) Inhibitor
Table 2: Properties of selected compounds relative to sulindac.
COMPOUND
Binding energy to COX-1
Binding energy to COX-2
% Similarity
LD 50 Mg/Kg
Lipinski rule of 5
P-gp substrate
GIT absorption
Metabolism
ZINC39406706
-5.7
-7.4
98.4
3200
v
No
High
No effect on CYP enzymes
ZINC26469982
-6.8
-8.0
98.7
1240
v
No
High
No effect on CYP enzymes
ZINC01847506
-6.6
-7.6
98.1
1240
v
No
High
No effect on CYP enzymes
ZINC33823423
-6.5
-7.6
99.6
1240
v
No
High
No effect on CYP enzymes
ZINC01682308
-6.5
-7.4
98.5
1240
v
No
High
CYP 1A2 inhibitor
ZINC19405119
-10.6
-11.3
98.6
250
v
No
High
No effect on CYP enzymes
Aspirin
-6.3
-6.0
100
250
v
No
High
No effect on CYP enzymes
Table 3: Properties of selected compounds relative to Aspirin.
ZINC01431703, ZINC02570895, ZINC13761811, ZINC02047040, and ZINC266737721 can inhibit PDK-1 and prevent the development or progression of colorectal cancer and hence were selected as potential drugs. However, one of the major causes of treatment failure is the efflux of drugs and extreme toxicity [19]. The current study used susceptibility to efflux proteins and toxicity profiles as major parameters in identifying drug-like compounds. P-glycoproteins are responsible for the efflux of drugs which reduces intracellular drug accumulation leading to treatment failure [20]. P-glycoprotein susceptibility for each of the selected compounds was estimated from the SwissADME online tool, and none of the compounds was a substrate of p glycoproteins. LD50 (mg/kg) was used to estimate the toxicity of the compounds identified. Celecoxib-induced cardiotoxicity is a major cause for drug withdrawal. The selected compounds had the same or higher toxicity compared to celecoxib.
The ability of a drug to achieve a positive therapeutic activity is based on its ability to achieve adequate concentration in the site of action. While various factors determine this, among them the route of administration, most drug design and discovery drives aim at obtaining orally active drugs [21]. The Lipinski rule of 5 stipulates specific requirements that must be met for a compound to possess drug-like properties that enable it to be orally active, i.e., the compound must have a molecular weight of less than 500 Daltons, log P of less than 5, no more than 5 HBDs, no more than 10 HBAs, and less than ten rotatable bonds [22]. All five compounds selected for Celecoxib adhered to the Lipinski rule of 5. However, ZINC01431703 showed low GI absorption and high inhibition of metabolic enzymes predisposing it to significant drug-drug interactions. ZINC02570895 is a CYP 1A2, & 2C9 inhibitor which makes it less likely to interact with many drugs due to the low number of drugs metabolized by both CYP 1A2, & 2C9 enzymes. ZINC02570895, with a better docking score than Celecoxib coupled with favorable toxicity and metabolic profiles, was the most appropriate drug candidate for the inhibition of PDK-1. Like Celecoxib, ZINC02570895 interacts with PDK-1 using van der Waals forces, conventional hydrogen bonding, T-shaped pi stacking, and pi cation bonding. However, ZINC02570895 interacts with the receptor using an additional pi-pi stacking as shown in (Figure 7).
Sulindac and its Analogues
All 40 compounds had a higher docking score compared to sulindac. 24 of the 40 compounds had a poor toxicity profile (LD50 < 240 mg/kg). Eight compounds did not comply with the Lipinski rule of 5, while six had poor GI absorption. Fourteen compounds were P-gp substrates. All compounds were inhibitors of CYP 450 enzymes. ZINC22309227 and ZINC12341529 had better docking scores and pharmacokinetic profiles than sulindac (Table 4). Despite ZINC13654467 having the highest docking score (-9.2), it was a p-gp substrate and, therefore, susceptible to drug resistance. Also, ZINC13654467 was a CYP (2C19, 2C9, 2D6, 3A4) inhibitor which predisposes it to significant drug-drug interactions.
Sulindac interacts with MAP kinase using pi-sulfur bonding, van der Waals forces, conventional hydrogen bonding, pi-sigma bonds, and amide pi-stacking (Figure 8). ZINC22309227 interacts with MAP Kinase through pi-anion binding, van der Waals forces, amide pi-stacking, and pi-alkyl bonding (Figure 8). Interaction with MAP kinase regulates intestinal epithelial differentiation. The extracellular signal-regulated (ERK) MAP kinases are responsible for intestinal epithelial proliferation in the development and progression of colorectal cancer [23]. Several growth factors and proto-oncogenes promote growth and differentiation through the ERK MAP kinase pathway [24]. Therefore, ZINC22309227 is potential chemopreventive drugs targeting the ERK MAP kinases. The compounds are orally active, have less potential for metabolic drug-drug interactions, and are not a p-gp substrate.
Aspirin and its Analogues
Out of 40 compounds, 6 had better docking scores and toxicity profiles coupled with favourable pharmacokinetic profiles than Aspirin (Table 5). The compounds in Table 5 below are potential candidates for further testing. Most of the compounds had a better toxicity profile and higher biological activity (better docking scores) compared to Aspirin.ZINC39406706 had a relatively higher docking score on COX-2, was 12 X less toxic than Aspirin, and had an excellent pharmacokinetics profile.ZINC19405119 had the highest docking score but the same toxicity as Aspirin. All the compounds obeyed the Lipinski rule of five and had a high gastrointestinal absorption. None of the selected compounds was a p-glycoprotein substrate.
Selectivity in COX-1 inhibition is associated with a poor toxicity profile. Several structurally similar compounds were found to have better binding energy on COX-2 than Aspirin. ZINC19405119, ZINC39406706, ZINC26469982, ZINC01847506, ZINC3382343, and ZINC01682308 had higher binding energies on both COX-1 and COX-2 compared to Aspirin. Therefore, these drug-like compounds can be used to prevent colorectal cancer by targeting prostaglandin-mediated polyposis, especially in patients who have inherited the mutated APC gene. ZINC19405119 had the highest binding energy for COX-1 and COX-2 but was relatively as toxic as Aspirin. ZINC39406706 had the best toxicity and pharmacokinetic profiles. The compound was 12 times less toxic than Aspirin, adhered to the Lipinski rule of five, and was not a p-glycoprotein substrate. This made it the best compound for development into a chemopreventive agent targeting the Cyclooxygenase enzyme. ZINC39406706 interacts with COX-2 through T-shaped pi-pi stacking, hydrogen bonding, and van der Waals forces (Figure 3).
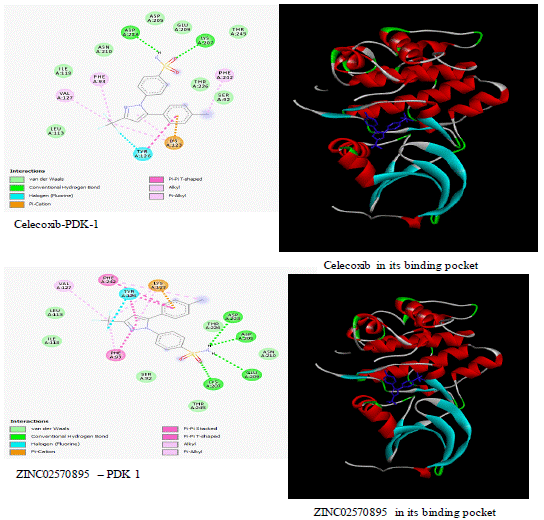
Figure 1: PDK-1 interactions with Celecoxib and ZINC02570895.
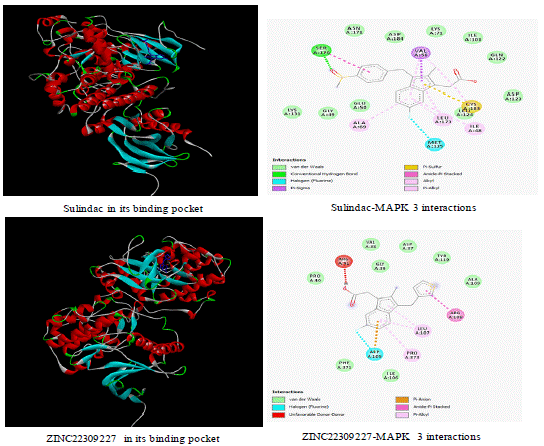
Figure 2: MAPK 3 interactions with Sulindac and ZINC22309227.
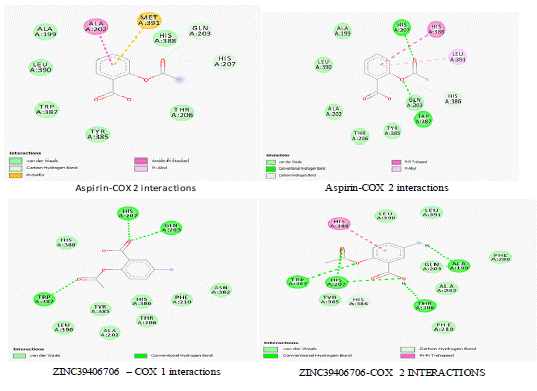
Figure 3: Cyclooxygenase enzyme interactions with Aspirin and ZINC39406706.
Aspirin interacts with COX-2 through hydrogen and van der Waals forces only. The additional T-shaped pi-pi stacking explains why ZINC39406706 has a better docking score than Aspirin (Figure 9). Aspirin is associated with extreme gastrointestinal toxicity. Therefore, toxicity profiles were the most significant parameter used to choose the drug-like compounds. ZINC39406706, ZINC26469982, ZINC01847506, ZINC3382343, and ZINC01682308 had favorable toxicity profiles compared to aspirin. This gives flexibility in dosing and duration of therapy making the compounds suitable for clinical use in CRC chemoprevention. Also, the selected compounds had no inhibitory effect on Cytochrome P450 enzymes and thus are suitable for patients with comorbidities.
Conclusion
The study identified 7 drug-like compounds from the ZINC database based on biological activity (docking scores), toxicity and pharmacokinetic profiles. ZINC02570895, with a better docking score than celecoxib coupled with favorable toxicity and metabolic profiles, was the most appropriate drug candidate for the inhibition of PDK-1. ZINC22309227, with a better docking score and favorable pharmacokinetic profile than sulindac was the most appropriate compound for further development into a MAP Kinase inhibitor. ZINC39406706, ZINC26469982, ZINC01847506, ZINC3382343, and ZINC01682308 had favorable toxicity profiles compared to aspirin and were most suitable for development cyclooxygenase inhibitors in colorectal cancer prevention.Further tests are needed to ascertain the biological activity, synthesizability and clinical use of the compounds. The identified compounds could be helpful in patients with mutations that predispose them to colorectal cancer and in the prevention of secondary polyps and adenomas in patients cured of CRC. For example, patients who have inherited the APC gene are more likely to get colorectal cancer and could benefit from preventive drugs [25]. Also, patients treated with CRC are susceptible to secondary tumours.
Recommendations
In vitro and in vivo studies to be carried out on the 7 drug-like compounds identified from the ZINC database to assess chemopreventive activity against colorectal cancer.
ZINC02570895 to undergo SAR and physicochemical optimization in readiness for in vivo and invitro testing against PDK-1 in colorectal cancer
ZINC22309227 to undergo SAR and physicochemical optimization in readiness for invivo and in vitro testing against MAPK 3 in colorectal cancer.
ZINC39406706, ZINC26469982, ZINC01847506, ZINC33823423, ZINC01682308 to undergo SAR and physicochemical optimization in readiness for invivo and invitro testing against COX enzyme in colorectal cancer.
References
- Kolawole OR, Kashfi K. NSAIDs and Cancer Resolution: New Paradigms beyond Cyclooxygenase. International Journal of Molecular Sciences. 2022; 23: 1432.
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2021; 71: 209-49.
- Alfonso L, Ai G, Spitale RC, Bhat GJ. Molecular targets of aspirin and cancer prevention. British journal of cancer. 2014; 111: 61-7.
- Cai Y, Yousef A, Grandis JR, Johnson DE. NSAID therapy for PIK3CA-Altered colorectal, breast, and head and neck cancer. Advances in biological regulation. 2020; 75: 100653.
- Hanna TP, King WD, Thibodeau S, Jalink M, Paulin GA, et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. bmj. 2020; 371: m4087.
- Trevor AJ, Katzung BG, Masters SB, Kruidering-Hall M. Pharmacology examination & board review. New York: McGraw-Hill Medical; 2010.
- Tsao AS, Kim ES, Hong WK. Chemoprevention of cancer. CA: a cancer journal for clinicians. 2004; 54: 150-80.
- Gurpinar E, Grizzle WE, Piazza GA. COX-independent mechanisms of cancer chemoprevention by anti-inflammatory drugs. Frontiers in oncology. 2013; 3: 181.
- Ganduri V, Rajasekaran K, Duraiyarasan S, Adefuye MA, Manjunatha N. Colorectal Carcinoma, Cyclooxygenases, and COX Inhibitors. Cureus. 2022; 14: e28579.
- Singh AD, Bhatt A, Joseph A, Lyu R, Heald B, et al. Natural history of ampullary adenomas in familial adenomatous polyposis: a long-term follow-up study. Gastrointestinal Endoscopy. 2022; 95: 455-67.
- Yin T, Wang G, Ye T, Wang Y. Sulindac, a non-steroidal anti-inflammatory drug, mediates breast cancer inhibition as an immune modulator. Scientific reports. 2016; 6: 1-8.
- Stolfi C, De Simone V, Pallone F, Monteleone G. Mechanisms of action of non-steroidal anti-inflammatory drugs (NSAIDs) and mesalazine in the chemoprevention of colorectal cancer. International journal of molecular sciences. 2013; 14: 17972-85.
- Alberts DS, Hixson L, Ahnen D, Bogert C, Einspahr J, et al. Do NSAIDs exert their colon cancer chemoprevention activities through the inhibition of mucosal prostaglandin synthetase?. Journal of Cellular Biochemistry. 1995; 59: 18-23.
- Hamoya T, Fujii G, Miyamoto S, Takahashi M, Totsuka Y, Wakabayashi K, Toshima J, Mutoh M. Effects of NSAIDs on the risk factors of colorectal cancer: a mini review. Genes and Environment. 2016; 38: 1-7.
- Wellbrock C, Arozarena I. The complexity of the ERK/MAP-kinase pathway and the treatment of melanoma skin cancer. Frontiers in cell and developmental biology. 2016; 4: 33.
- Xu Z, Liao B, Zhang R, Yao J, Shi R, Wang L. Expression of 3-phosphoinositide-dependent protein kinase 1 in colorectal cancer as a potential therapeutic target. Medical Oncology. 2015; 32: 1-98.
- Gillet V. Ligand-based and structure-based virtual screening. University Lec. 2013.
- Zoete V, Daina A, Bovigny C, Michielin O. Swiss Similarity: a web tool for low to ultra high throughput ligand-based virtual screening. J Chem Inf Model. 2016; 56: 1399-404.
- Ma X, Cai Y, He D, Zou C, Zhang P, et al. Transient receptor potential channel TRPC5 is essential for P-glycoprotein induction in drug-resistant cancer cells. Proceedings of the National Academy of Sciences. 2012; 109: 16282-7.
- Bellamy WT. P-glycoproteins and multidrug resistance. Annual review of pharmacology and toxicology. 1996; 36: 161-83.
- Katsuno K, Burrows JN, Duncan K, Van Huijsduijnen RH, Kaneko T, Kita K, Mowbray CE, Schmatz D, Warner P, Slingsby BT. Hit and lead criteria in drug discovery for infectious diseases of the developing world. Nature Reviews drug discovery. 2015; 14: 751-8.
- Hassan Baig M, Ahmad K, Roy S, Mohammad Ashraf J, Adil M, et al. Computer aided drug design: success and limitations. Current pharmaceutical design. 2016; 22: 572-81.
- Kudaravalli S, den Hollander P, Mani SA. Role of p38 MAP kinase in cancer stem cells and metastasis. Oncogene. 2022: 1-9.
- Nandi S, Bagchi MC. Exploring CDKs, Ras-ERK, and PI3K-Akt in Abnormal Signaling and Cancer. Journal of Cancer Research Updates. 2022; 11: 63-9.
- Zhang L, Shay JW. Multiple roles of APC and its therapeutic implications in colorectal cancer. JNCI: Journal of the National Cancer Institute. 2017; 109: djw332.