
Opinion
Austin J Pharmacol Ther. 2023; 11(3): 1179.
“Calcium Influx Pathways in Breast Cancer: Opportunities for Pharmacological Intervention”
Hari Sonwani*
Apollo College of Pharmacy, Anjora, Durg, India
*Corresponding author: Hari Sonwani Apollo College of Pharmacy, Anjora, Durg, India. Email: harisonwani10@gmail.com
Received: November 06, 2023 Accepted: December 07, 2023 Published: December 14, 2023
Abstract
Numerous cellular processes, including the release of neurotransmitters and the contraction of muscles, are largely triggered and regulated by Ca2+ inflow via Ca2+ permeable ion channels. In addition, Ca2+ influx regulates cellular migration and proliferation, two mechanisms linked to cancer. This study focuses on calcium influx in breast cancer cells and discusses how future drugs for breast cancer therapy may be pharmacological modulators of particular Ca2+ influx channels. Certain breast tumors have altered expression of particular calcium permeable ion channels. Such alterations may occasionally be connected to the prognosis and subtype of breast cancer. These days, models both in vivo and in vitro have assisted in identifying particular Ca2+ channels that are crucial for the growth and invasiveness of of cancerous breast cells. Nonetheless, additional research is still needed to fully understand several features of Ca2+ influx in breast cancer. These include figuring out the processes behind the changed expression and the best treatment plan to target breast cancer cells via particular Ca2+ channels. In the upcoming ten years, research should concentrate on the function of Ca2+ influx in mechanisms other than the migration and proliferation of breast cancer cells.
Keywords: Breast cancer; Calcium channels; Calcium influx; Calcium signalling; Oncology
Abbreviations: N-cyano-N"-[(1S)-1-phenylethyl]; [Ca2+]CYT, cytoplasmic-free calcium; IP3, inositol 1,4,5-trisphosphate; JNJ41876666, 3-[7-trifluoromethyl-5- (2-trifluoromethyl-phenyl)]; ErbB2 (also known as HER2), human EGF receptor 2; EC, endothelial cells; EMT, epithelial to mesenchymal transition; ER+, oestrogen positive; ERa, oestrogen receptor a;Azol-2-yl-1H-benzimid[4.5]dec -1-oxa-2-aza-spiro2-eneHydrochloride; NNC 55-0396, (1S,2S); NFAT, nuclear factor for activated T-cells[(3-benzimidazol-2-yl)propyl] -2-(2-(N-]The methylamino)ethyl grouptetrahydro-6-fluoro-1,2,3, 4-Pyr3, 1-[4-[(2,3,3-trichloro-1-oxo-2-propen-1-yl)amino]phenyl]; PMCA, plasma membrane Ca2+-ATPase; -1-isopropyl-2-naphtyl cyclopropanecarboxylate dihydrochlorideTrifluoromethyl, or 5-4-carboxylic acid pyrazole -1H; SCID stands for severe combined immune deficiency; SB-209712 is 1,6,bis{1-[4-(3-phenylpropyl) piperidinyl]}hexane; transient receptor potential; TRP, secretory route Ca2+-ATPase
Introduction
With intracellular free Ca2+ levels almost 20 000 times lower than in the external environment (100 nM vs. 1.8 mM), cells maintain a significant gradient of free Ca2+ across the plasma membrane (Carafoli, 1987; Clapham, 2007). Utilizing this Ca2+ gradient, cells frequently use Ca2+ influx to start and control cellular signals, typically by opening Ca2+ permeable ion channels. Numerous varied routes are regularly muscle contraction, gene transcription, cell division, and neurotransmitter release are all triggered by increases in intracellular cytoplasmic-free calcium ([Ca2+]CYT) [14]. For a number of ailments, Ca2+ permeable ion channels may be useful pharmacological targets. Among these disorders include hypertension, for which nifedipine and other L-type voltage-gated Ca2+ channel blockers are used clinically [5], and chronic pain. Ziconotide, an N-type channel inhibitor, is employed (Malmberg and Yaksh, 1995). The research that has evaluated calcium influx routes in the development of breast cancer and identified calcium permeable ion channels as pharmacological targets for breast cancer therapy will be the main emphasis of this review.
Calcium Signaling: The Critical Function of Calcium Influx
Numerous reviews [14 Leybaert and Sanderson, 2012) describe how mammalian cells control [Ca2+]CYT levels and the significance of the nature of variations in [Ca2+]CYT (such as [Ca2+]CYT oscillations and localized changes in Ca2+). As Figure 1 showsa few of the primary calcium exchangers, pumps, and channels in the pathways that signal calcium. In summary, the active efflux of Ca2+ from the cell through the plasma membrane Ca2+-ATPases (PMCAs) maintains [Ca2+]CYT levels at low levels. Activation of these enzymes, together with Na+/Ca2+ exchangers and sarco/endoplasmic reticulum Ca2+ ATPases, lowers [Ca2+]CYT. There are various mechanisms that can lead to increases in [Ca2+]CYT. For instance, several GPCRs, via means of activation of Through IP3-activated Ca2+ channels, PLC and the production of inositol 1,4,5-trisphosphate (IP3) release Ca2+ from internal calcium reserves, such as the sarco/endoplasmic reticulum [14]. The recently discovered mitochondrial Ca2+ uniporter (Kirichok et al., 2004) and the Na+/Ca2+ exchanger NCLX (Palty et al., 2010) are two more organelles that are involved in Ca2+ signaling. and the Golgi, which uses the secretory route Ca2+-ATPases (SPCAs) to sequester intracellular Ca2+. The opening of calcium permeable ion channels on the plasma membrane also results in increases in [Ca2+]CYT. Many distinct physiological activities, especially those involving excitable cells, such the release of neurotransmitters in neurons and the excitation–contraction coupling in skeletal muscle (Rios and Brum, 1987) [26], depend critically on calcium influx. (Tsien et al., 1988). In cells in the epithelium, calcium influx is also crucial for processes like the intestinal epithelial cells' absorption of Ca2+ [7,61]. We will give a quick summary of the many kinds of calcium permeable materials in the next section of this review.
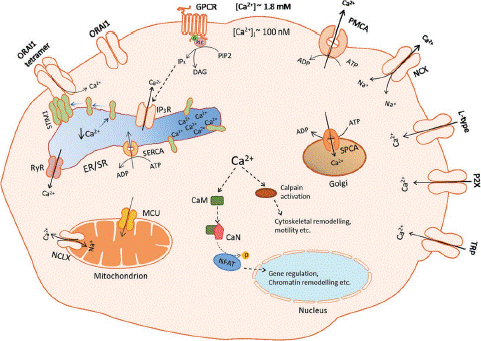
Figure 1: Schematic depiction of some of the Ca2+ channels, pumps and exchangers involved in Ca2+ signalling in mammalian cells. Ca2+ influx channels include the ORAI1 channel (an example of a store-operated Ca2+ entry channel), L-type Ca2+ channels (an example of a voltage-gated Ca2+ channel), P2X receptor channel (an example of a ligand-gated Ca2+ channel) and TRP channels (channels that vary in their Ca2+ selectivity). GPCRs increase [Ca2+]CYT via PLC-mediated generation of IP3 and activation of IP3R. [Ca2+]CYT levels are sustained at low levels through the active efflux of Ca2+ by PMCAs and Na+/Ca2+ exchangers on the plasma membrane. Sequestration of Ca2+ into the ER Ca2+ store is mediated by SERCA, into the mitochondria by Mitochondrial Ca2+ Uniporter (MCU) and into the Golgi by secretory pathway Ca2+-ATPase (SPCA). Increases in [Ca2+]CYT can result in the activation of calcineurin (CaN) that phosphorylates the transcription factor NFAT, which after translocation into the nucleus regulates gene transcription [30]. Calcium can also activate many cytosolic proteins with Ca2+-sensitivity confirmation and activities such as calpain, which can regulate a number of important cellular processes including cytoskeletal remodelling and motility (Storr et al., 2011).
Human Cell Mechanisms for Calcium Influx
Many other types of calcium permeable ion channels are also expressed in intracellular organelles, including the isoforms of IP3 receptors (IP3R1, IP3R2, and IP3R3) and the ryanodine receptors (RyR1, RyR2, and RyR3), which are mediators of calcium-induced calcium release [22]. That is expressed on human cells' plasma membranes. The processes of intracellular Ca2+ signaling are shown in Figure 1, and some of the major Ca2+ influx pathways and examples of their naturally occurring activators are shown in Figure 2. The general kinds of calcium permeable ion channels are briefly described below, with special emphasis on some of the ion channels that will be covered.

Figure 2: Ca2+ influx pathways. Examples of influx pathways and naturally occurring-activation pathways. CaV3.2 is an example of a voltage-gated Ca2+ channel that is activated by membrane depolarization (Panner and Wurster, 2006); ORAI1 is an example of a store-operated Ca2+ channel that is activated upon depletion of endoplasmic reticulum Ca2+ stores (Lewis, 2011); P2X5 is an example of a purine receptor that facilitates the flow of Ca2+ across the plasma membrane in response to extracellular ATP (Surprenant and North, 2009); examples of TRP channels include the canonical mechanosensitive cation channel TRPC1, which can be activated by membrane stretch (Maroto et al., 2005), the vanilloid TRPV1 channels activated by high temperatures [13], the melastatin TRPM8 channel activated by lower temperatures (Prevarskaya et al., 2007), the sole member of ankyrin TRPA family TRPA1, which is a key chemoreceptor responsive to reactive chemicals (Moran et al., 2011), TRPM7, which can be directly activated by mechanical stress (Numata et al., 2007), and TRPV6, which has constitutive activity at low [Ca2+]i and physiological membrane potential [52].
Calcium Permeable Ion Channels That are Voltage-Gated
Voltage-gated calcium permeable ion channels are characterized by their sensitivity to changes in membrane potential, as their name suggests. Members of this class can, however, differ greatly in their physiological, pharmacological, and regulatory traits, as has been discussed elsewhere [24]. Calcium channels that are voltage-gated include the L-type, N-type, T-type, R-type, and P/Q-type. Various subunits make up these channels, but the calcium-selective pore is formed by the a1 subunit [40. For L-types, the a1 is encoded by CACNA1S, CACNA1C, CACNA1D, and CACNA1F genes; for C-types, CACNA1A, For P/Q, N, and R kinds, use CACNA1B and CACNA1E; for T types, use CACNA1G, CACNA1H, and CACNA1I [16]. Studies evaluating CaV1 channels in T-lymphocytes have shown that, despite being primarily associated with excitable cells like those in the central nervous system and muscle tissue, voltage-gated calcium channels also have significant functions in other cell types [42,53].
TRP Channels, or Transient Receptor Potentials
Numerous TRP channels, the majority of which are permeable to Ca2+ ions, have been discovered in mammalian cells since the discovery of the first TRP channel in Drosophila [58]. These findings have been reported by Wes et al. (1995), Caterina et al. (1997), Clapham (2002), Story et al. (2003), and Ramsey et al. (2006). The families of TRP channels that are expressed in human cells are TRPC, TRPA, TRPV, TRPM, TRPML, and TRPP. A lot Some of these channels function as sensors. For example, TRPM8 is activated by lower temperatures (Peier et al., 2002; Prevarskaya et al., 2007) while TRPV1 is triggered by higher temperatures [13,23]. Certain members of this class are also triggered by substances that can be found in nature, such as menthol, which cools the body, and capsaicin, which is the spicy part of chilli peppers. These compounds activate the aforementioned TRPM8 and TRPV1 channels, respectively [29]. The disorders linked to mutations in these ion channels, as well as the functional roles and mechanical, chemical, and temperature sensing characteristics of TRP channels, have all been well examined (Minke, 2006; Nilius, 2007; Prevarskaya et al., 2007). Apart from the function of TRP mutations Certain TRP channel overexpression is linked to certain malignancies in humans, including those of the breast and prostate (Prevarskaya et al., 2007; Ouadid- Ahidouch et al., 2013).
Ca2+ Ligand-Gated Channels
Certain endogenous ligands directly activate specific calcium permeable ion channels. Ion channels like NMDA and a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, which are triggered by the neurotransmitter glutamate [20] (Watkins and Jane, 2006), as well as P2X receptors, a class of purine receptors that react to extracellular ATP by promoting the flow of Ca2+ across the plasma membrane (Surprenant and North, 2009), are among those that are expressed on their membrane. The P2X ion channel family consists of seven members that are crucial to a wide range of procedures, such as blood coagulation and neural signaling (Pankratov et al., 1998) [59].
Despite being ligand-gated, IP3 receptors are not generally linked to Ca2+ influx since they are primarily expressed on the internal Ca2+ store of the endoplasmic reticulum. Nonetheless, findings of IP3R3 plasma membrane expression in ciliated cells [8] and IP3R1 plasma membrane expression in B lymphocytes [34] support the idea that the IP3 receptor is a ligand-gated ion channel that facilitates calcium influx.
Store-Based Ca2+ Entrance System
The phenomenon known as capacitive calcium entry was initially described in 1986 and refers to increases in Ca2+ influx following the depletion of intracellular calcium reserves (Putney, 1986). Still, the Not until 2006 was the full chemical identity of the elements causing this significant Ca2+ influx mechanism discovered. At this point, the mutation causing a severe combined immune deficiency syndrome linked to decreased store-operated Ca2+ entry was found, and a functional small interfering RNA (siRNA) screen was used to identify the calcium channel ORAI1 [47] (Zhang et al., 2006). Numerous reviews have been written about the now-well-characterized mechanism for store-operated Ca2+ entry (Parekh and Putney, 2005; Varnai et al., 2009; [53] (Putney, 2011). In summary, the endoplasmic reticulum Ca2+ sensor STIM1 is redistributed in response to the depletion of Ca2+ stores in the endoplasmic reticulum. It oligomerizes to regions of the endoplasmic reticulum that are near the plasma membrane, allowing the N-terminal portion of ORAI1 proteins to engage with the CRAC activation domain of STIM1 (Lewis, 2011). Through a calcium channel created by ORAI1 oligomers, this interaction facilitates the influx of Ca2+ (Mignen et al., 2008). Owing to its increased affinity for endoplasmic reticulum luminal Ca2+ levels, the STIM1-related isoform STIM2 seems to be a crucial modulator of basal Ca2+ influx in cells through ORAI1 [21].
Cancer and Calcium Signaling
Apoptosis, proliferation, migration, invasion, and other processes relevant to cancer are all regulated by calcium signaling (Roderick and Cook, 2008; Lee et al., 2011; Prevarskaya et al., 2011). Numerous calcium channels and pumps have been linked to various types of cancer. These connections have often been established via the finding that a calcium channel or pump is overexpressed in cancer, or the finding that a particular calcium channel or pump plays a part in a particular cancer-related process. Previous reviews (Roderick and Cook, 2008; Prevarskaya et al., 2011; Monteith et al., 2012) have examined the connection between calcium signaling and cancer as well as the significance of particular calcium pumps and channels in various cancer types. Here, our attention will be on the research that has looked specifically at calcium signaling in breast cancer.
Cancer of the Breast
According to Schulman et al. (2010), breast cancer incidence is rising in developing economies, while it remains one of the leading causes of death in the developed world. Breast cancer is actually a group of diseases, despite being referred to as one.
Breast Cancer
Breast cancer incidence is rising in developing economies, and it remains one of the leading causes of death in the developed world (Shulman et al., 2010). Breast cancer is essentially a group of disorders with widely distinct prognoses and ideal treatment regimens, while being referred to as a single illness frequently (Sorlie, 2009; Vargo-Gogola and Rosen, 2007). Because they respond well to hormonal therapy that targets the oestrogen receptor, such as tamoxifen and selective oestrogen receptor modulators, breast cancers that express the oestrogen receptor are generally associated with a relatively good long-term prognosis (Zhang et al., 2000; Park and Jordan, 2002). The treatment of breast tumors that overexpress human epidermal growth factor has been completely transformed by the discovery of the monoclonal antibody trastuzumab.
Factor receptor 2 (ErbB2 receptor) [9], commonly referred to as the HER2 receptor. On the other hand, "triple negative" breast cancers are typically linked to a poor prognosis and a dearth of long-term effective medicines. This is partly because these tumors overexpress the oestrogen and progesterone receptors and lack ErbB2 receptors (Schneider et al., 2008). Microarray analysis of breast cancer samples also demonstrates the heterogeneity of the disease. Hierarchical clustering has been utilized in these research to identify different molecular subtypes of breast cancer. These consist of the recently described Claudin-low, basal-like, luminal A, luminal B, ErbB2, and luminal (Sorlie et al., 2001) [60]. Triple negative and basal-like and Claudin-low breast cancer subtypes significantly overlap (Prat et al., 2010) and have a dismal prognosis; novel, efficient treatments are most urgently needed for these tumors (Perou et al., 2000).
Certain modifiers of calcium signaling, in particular regulators of calcium influx, have been identified in recent research as possible new targets for the treatment of breast cancer.
Inflow of Calcium and Lactation
The relationship between calcium and the breast is evident. One essential component of milk is calcium, which the breast produces as part of its physiological role to nourish newborns. Three crucial and connected mechanisms are thought to be involved in the transport of calcium from the maternal blood supply into milk: the entry of calcium into breast epithelial cells, the sequestration of Ca2+ into the secretory pathway and later release into milk, as well as the Ca2+'s direct outflow into milk (Neville, 2005; Lee et al., 2006). During lactation, extra calcium transport is made possible by highly specialized Ca2+ transporters. Studies using expression and mutant animals have directly demonstrated the function of PMCA2, a calcium efflux pump commonly linked to neurons, in the movement of Ca2+ from the cytoplasm of mouse mammary epithelial cells into milk (Reinhardt et al., 2000; 2004). Based on expression studies, Golgi Ca2+ accumulation during lactation and the subsequent release of Ca2+ into milk may be caused by an isoform of the SPCA, called SPCA2, which likewise has restricted tissue distribution [41]. Apart from its potential functions during breastfeeding, PMCA2 and SPCA2 expression is linked to cell death and/or proliferation, respectively, in certain human breast cancer cell lines and is raised in some human breast malignancies [46] (VanHouten et al., 2010). Therefore, some breast cancers are associated with secretory pathway calcium pumps that are up-regulated during lactation and the plasma membrane. This might also apply to proteins that play a key role in controlling the Ca2+ influx during lactation. ORAI1 isoform up-regulation was found to be a characteristic of breastfeeding in studies evaluating store-operated Ca2+ entry in mice at various stages of mammary gland development (McAndrew et al., 2011). An elegant potential mechanism to balance the supply and demand for Ca2+ outflow and sequestration in milk could be store-operated calcium influx (Ca2+ inflow via ORAI1). Since As will be covered later, ORAI1 has recently been recognized by a number of groups as a possible target for breast cancer treatment.
Ca2+ Homeostasis Changes in Breast Cancer
Numerous critical processes in carcinogenesis, including invasion, migration, angiogenesis, cell death, and proliferation, are regulated by calcium signaling. This function has been thoroughly examined and is well-established [14] (Monteith et al., 2007). Furthermore, it is now widely known that certain malignancies are defined by changes in particular calcium signaling components. Such alterations are observed in various cancers, such as prostate cancer (Lehen'kyi et al., 2007) where increased Ca2+ entry mediated by TRPV6 is linked to enhanced transcription factor nuclear factor for activated T-cell (NFAT) activation and proliferation, and ovarian cancer (Yang et al., 2009b) where increases in Ca2+ influx mediated by TRPC3 lead to increased proliferation. However, It seems that changes in calcium signaling do not initiate breast tumorigenesis; instead, these changes may be pharmacologically manipulated to decrease the growth and spread of breast cancer or even stimulate the death of breast cancer cells. distinct subtypes of breast cancer seem to alter calcium signaling in distinct ways, which can be mediated by very different mechanisms and have different outcomes. For instance, basal-like breast cancers have much higher levels of the secretory route Ca2+ ATPase I isoform (SPCA1), and in the basal-like breast cancer cell line MDA-MB-231, silencing SPCA1 lowers proliferation. According to Graci et al. (2010), this functional outcome is linked to suppression of the synthesis of active insulin-like growth factor 1 receptor. This is by a method that probably includes the modification of Golgi lumen-resident pro-protein convertases that are Ca2+-dependent [46]. It seems that breast tumors that are positive for the ErbB2 receptor are more closely linked to the overexpression of the calcium efflux pump PMCA2 (VanHouten et al., 2010). The resistance to cell death that T-47D breast cancer cells exhibit when exogenous PMCA2 is overexpressed implies that PMCA2 inhibitors may facilitate the pathways leading to cell death in breast malignancies that overexpress PMCA2. The remaining sections of this study will concentrate on the challenges surrounding the targeting of specific Ca2+ channels in the therapy of breast cancer, as well as the Ca2+ influx pathways that are remodeled in some cases of breast cancer.
Ca2+ Influx Channels are altered in Breast Cancer
Evidence of the Ca2+ influx remodeling is present. Breast cancer and ligand-gated Ca2+ channels Certain research that aim to comprehend key pathways and processes in breast cancer focus on ligand-activated Ca2+ channels. Still, further research is needed. Studies evaluating P2X7 receptors provide as examples of this, as they have connected this receptor to the invasiveness of cancer cells (Jelassi et al., 2011) and the anti-invasive characteristics of the anthraquinone emodin (Jelassi et al., 2013). MDA-MB-435S cells, a basal breast cancer cell with strong melanoma-like properties, have been used in the majority of these researches [1,25]. Research on alternative P2X receptor isoforms and basal-like and non-basal-like breast cancer cell lines may help determine which subtype(s) of breast cancer and which P2X receptors may have the greatest therapeutic promise for the control of the disease of metastases from breast cancer.
Mechanisms Responsible for Altered Plasma Membrane Ca2+ Channel Expression in Breast Cancer Cells
Although neglected for some time, recent studies have begun to explore the mechanisms by which specific Ca2+ channels are overexpressed in some breast cancers. One possible mechanism for the overexpression of some calcium permeable ion channels is through hormone receptors, such as receptor a for estrogen (ERa). In human MCF-7 breast cancer cells, silencing ERa lowers the levels of ORAI3 mRNA and protein but has no effect on ORAI1 levels (Motiani et al., 2013). This suggests a possible molecular connection between breast cancer cells expressing ERa and ORAI3 overexpression. In MCF-7 cells, ERa silencing also lowers TRPM8 levels, while 17-Β-oestradiol raises them [27]. The discovery that progesterone inhibits the expression of the Ca2+ permeable ion channel TRPV4 in T-47D breast cancer cells (Jung et al., 2009) further implies that hormonal mechanisms may be responsible for the altered expression of certain calcium channels in breast malignancies. Additional evaluation of this process for additional calcium channels and the effects of antioestrogen treatment it now seems appropriate to focus on the expression of calcium channels in clinical breast cancer.
It is widely acknowledged that gene amplification has a role in breast cancer. The humanized monoclonal antibody trastuzumab takes advantage of the gene amplification of ErbB2 receptors in many aggressive breast tumors [9]. The likelihood that calcium channel gene amplification contributes to breast cancer has not been examined in many studies. The overexpression of TRPV6 in SK-BR-3, ZR-75-1, and T-47D breast cancer cell lines, where copy numbers range from 6 to 9, and in certain breast cancers, where an elevated copy number of TRPV6 is linked to oestrogen receptor negative, triple negative, and basal-like breast cancers, suggests that TRPV6 gene amplification may be one possible mechanism for this overexpression (Peters et al., 2012). Others Gene methylation is one example of an epigenetic modification that is one of the causes for altered expression in breast cancer that has not yet been well investigated. According to Palmieri et al. (2012), DNA demethylation causes a substantial increase in CACNA2D3 levels in MDA-MB-453 breast cancer cells. The gene for the voltage-gated calcium channel regulatory subunit, CACNA2D3, is linked to greater methylation in breast cancers with metastases to the central nervous system. The methylation of the CACNA2D3 gene is suggested as a potential biomarker for the development of metastases, however its importance for calcium signaling and breast cancer pathways is yet unknown (Palmieri et al., 2012). Future research on this and other putative mechanisms for altered Ca2+ channel expression in breast cancer cells ought to receive more attention.
Control in Ca2+ Channel Function
Research has started to show that the control of calcium channels in breast cancer cells is complex. It is possible that enhanced activation of a calcium channel (in this case, through overexpression of another protein) rather than overexpression of the calcium channel itself is the driving force for tumor progression in some cases, as suggested by the ability of the SPCA2 calcium pump's N-terminal domain to activate Ca2+ influx via ORAI1 and promote activation of NFAT [46]. Kim et al.'s discovery that the tumor suppressor Numb1 is a negative regulator of TRPV6 activity lends more credence to the significance of these indirect processes (Kim et al., 2013b). Proliferation is increased by number one silencing. where it directly interacts with TRPV6 through basal Ca2+ influx in MCF-7 breast cancer cells (Kim et al., 2013b). Changes in calcium channel location add another layer of complexity to the involvement of calcium influx channels in cancer. Bidaux et al. showed that a portion of the overexpressed TRPM8 protein is found on the endoplasmic reticulum in prostate cancer cells. This location is linked to the advancement of prostate cancer via changing the calcium level of internal stores [17]. To find out if comparable localization changes happen in breast cancer cells, more research is needed. It is known that in MDA-MB-468 breast cancer cells, suppressing TRPC1 reduces the high levels of basal Ca2+ influx mediated by ORAI1 in this cell line [32]. Observations suggest that TRPC1 expression on the endoplasmic reticulum of MDA-MB-468 breast cancer cells and the stimulation of Ca2+ leakage from that calcium storage are partially responsible for this. To determine whether non-plasmalemmal localization of TRPC1 and other calcium channels is a characteristic of some breast tumors, more research is necessary.
Targeting Calcium Influx Pathways with Pharmaceuticals in Breast Cancer
One of the main potential benefits of using calcium influx regulators as new cancer treatment targets is their obvious capacity to create pharmacological modulators of Ca2+ permeable ion channels, as this review has explained. Many of the calcium permeable ion channels that are known to have activators and inhibitors are listed in Table 2. connected to malignancies. Most of the investigations included in this review have used pharmacological inhibitors, siRNA or short hairpin (sh)RNA-mediated silencing, or both, to identify particular calcium permeable ion channels as possible therapeutic targets. Considering the part that calcium signaling plays in promoting cellular motility and proliferation, such strategies are obviously appropriate. Indeed, pharmacological inhibitors of calcium influx pathways have been shown in vivo studies to prevent invasion and/or proliferation of breast cancer (Taylor and Simpson, 1992) [12]. The induction of cancer cell death is an additional mechanism of oncology therapy. Studies on this feature of calcium influx in breast cancer are scarce, despite the fact that sustained high levels of [Ca2+]CYT might promote apoptosis and Necrosis can even be induced by significant elevations in [Ca2+]CYT [49]. Hence, administering a channel activator to create a prolonged calcium influx strong enough to cause cell death is one recommended strategy to target an overexpressed calcium permeable ion channel. As was previously mentioned, many prostate tumors overexpress TRPM8, and preliminary research using the prostate cancer cell line LNCaP indicates that menthol, a TRPM8 activator, may cause apoptosis (Zhang and Barritt, 2004). Although TRPM8 has been found to be overexpressed in certain types of breast cancer (Tsavaler et al., 2001) [27,35], the effects of activating this and other calcium permeable ion channels on breast cancer cells have not been thoroughly investigated.
It is most likely that calcium causes cell death channel opening only take place in breast cancer cells when the channel is sufficiently overexpressed to allow an activator to generate enough calcium influx to encourage cell death pathways. Among the possible dangers of utilizing an activator to induce the death of breast cancer cells is the consequence of activation in cells that have only a moderate overexpression of the ion channel. In this latter scenario, channel activation could actually promote proliferation and/or invasion. Clinically, this could result in an initial reduction in tumor volume (via cell death), followed by a period of accelerated proliferation and metastasis. In vitro and in vivo experiments are required to address this possibility. However, another outcome of channel activation in breast cancer cells could be a reduction in proliferation and invasion due to a change in the nature of [Ca2+]CYT changes. Sustained Ca2+ influx induced by a channel activator in breast cancer cells could interfere with processes such as proliferation and motility. Studies of this possible phenomenon may be hampered in breast cancer cells, as many of the calcium influx channels overexpressed in breast cancer cells (Table 1) do not have both widely available selective inhibitors and activators However, such studies, particularly in vivo, would greatly advance our understanding of the best therapeutic strategies for targeting calcium channels in breast cancer.
New Developments in the Mechanisms of Calcium Influx in Breast Cancer
Numerous investigations by many research groups have significantly advanced our understanding of calcium influx in breast cancer cells. Naturally, the majority of research has concentrated on determining the mechanisms underlying the increased expression of particular calcium permeable channels in breast cancer cells, as well as the involvement of calcium signaling in significant events in the evolution of the disease. On the other hand, some recent research is starting to pinpoint particular Ca2+ permeable channels in different settings, which might indicate new fields that could advance quickly over the course of the next ten years. Chemotherapeutic resistance is one of these topics. Recent research by Ma et al. demonstrated that adriamycin-resistant MCF-7 breast cancer cells can become adriamycin-sensitive again when TRPC5 is silenced (Ma et al., (2012)). This study offers evidence that focusing on a particular Ca2+ channel could be a potential strategy for reversing breast cancer cells' resistance to certain chemotherapies.
Apart from the direct correlation between particular calcium permeable ion channels and invasiveness and cellular migration, research has also started to link these channels to other critical processes in breast cancer metastasis, including the Epithelial to Mesenchymal Transition (EMT) [32,63]. Growth factors, such as EGF, and hypoxia are known to trigger EMT in breast cancer cells (Lester et al., 2007; Lo et al., 2007). During EMT, a variety of proteins express themselves differently, giving rise to enhanced migratory and invasive characteristics as well as resistance to cell death. Current Research has shown that Ca2+ influx routes may undergo modifications due to epithelial-mesenchymal transition. P2X5 mRNA levels are elevated and purine receptor Ca2+ signaling is changed in response to EGF-induced EMT in MDA-MB-468 breast cancer cells [31]. Research employing the identical model demonstrate that EMT diminishes basal, agonist, and store-operated Ca2+ calcium signaling. This is demonstrated by the correlation between EMT, which is brought about by the transcription factor Oct4 being down-regulated, and alterations in store-operated Ca2+ entry in MCF-7 cells [63]. In MDA-MB-468 breast cancer cells, calcium signaling is also a critical step in the development of EGF and hypoxia-mediated EMT, with TRPM7 contributing to the induction of some EMT markers by EGF. This most likely happens as a result of interactions with signal transducer and transcription activator 3's phosphorylation [32].
Hanahan and Weinberg (2011) outlined the hallmarks, developing predictors, and enabling aspects of cancer in their most current review. They also emphasized the significance of the microenvironment and the cellular heterogeneity of tumors. While calcium influx has been extensively researched and linked to some cancer characteristics, as previously said, the field of calcium signaling research in certain tumor biology domains is still in its early stages. For instance, there are surprisingly few research examining calcium signaling between breast cancer cells in tumors, despite the obvious significance of this process in reactions to growth factors in the tumor microenvironment. interactions with the surrounding cells (immune inflammatory cells, for example) [57]. Additionally, research on the function of calcium signaling in cancer stem cells is particularly lacking. This is most likely partially caused by the technological challenges associated with measuring Ca2+ in vivo and in three-dimensional culture models. But recent developments in genetically targeted Ca2+ sensors and imaging could result in investigations that broaden our knowledge of the potential roles that Ca2+ influx pathways may play in the development of tumors.
In Summary
Certain breast cancer cells have changes in the expression and/or activity of Ca2+ permeability ion channels. With certain molecular insights, our knowledge of why these variations in expression occur is progressively becoming clearer. The indubitably demonstrated sensitivity Certain Ca2+ channels are appealing targets for breast cancer treatment due to their selectivity towards pharmacological modulators. While studies conducted in vitro and in vivo frequently lend support to this method, further research is needed to identify the best course of treatment and identify potential resistance pathways to these drugs.
References
- Afrasiabi E, Hietamäki M, Viitanen T, Sukumaran P, Bergelin N, Törnquist K. Expression and significance of HERG (KCNH2) potassium channels in the regulation of MDA-MB-435S melanoma cell proliferation and migration. Cell Signal. 2010; 22: 57-64.
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, McGrath JC, et al. The Concise Guide to PHARMACOLOGY 2013/14: overview. Br J Pharmacol. 2013; 170: 1449-58.
- Amantini C, Mosca M, Nabissi M, Lucciarini R, Caprodossi S, Arcella A, et al. Capsaicin-induced apoptosis of glioma cells is mediated by TRPV1 vanilloid receptor and requires p38 MAPK activation. J Neurochem. 2007; 102: 977-90.
- Amantini C, Ballarini P, Caprodossi S, Nabissi M, Morelli MB, Lucciarini R, et al. Triggering of transient receptor potential vanilloid type 1 (TRPV1) by capsaicin induces Fas/CD95-mediated apoptosis of urothelial cancer cells in an ATM-dependent manner. Carcinogenesis. 2009; 30: 1320-9.
- Aoki K, Yoshida T, Kato S, Tazumi K, Sato I, Takikawa K. Hypotensive action and increased plasma-renin activity by Ca2+ antagonist (nifedipine) in hypertensive patients. Jpn Heart J. 1976; 17: 479-84.
- Aydar E, Yeo S, Djamgoz M, Palmer C. Abnormal expression, localization and interaction of canonical transient receptor potential ion channels in human breast cancer cell lines and tissues: a potential target for breast cancer diagnosis and therapy. Cancer Cell Int. 2009; 9: 23.
- Barley NF, Howard A, O’Callaghan D, Legon S, Walters JRF. Epithelial calcium transporter expression in human duodenum. Am J Physiol Gastrointest Liver Physiol. 2001; 280: G285-90.
- Barrera NP, Morales B, Villalón M. Plasma and intracellular membrane inositol 1,4,5-trisphosphate receptors mediate the Ca2+ increase associated with the ATP-induced increase in ciliary beat frequency. Am J Physiol Cell Physiol. 2004; 287: C1114-24.
- Baselga J, Norton L, Albanell J, Kim YM, Mendelsohn J. Recombinant humanized anti-HER2 antibody (Herceptin(TM)) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts (vol 58, pg 2825, 1998). Cancer Res. 1999; 59: 2020.
- Basora N, Boulay G, Bilodeau L, Rousseau E, Payet MD. 20-hydroxyeicosatetraenoic acid (20-HETE) activates mouse TRPC6 channels expressed in HEK293 cells. J Biol Chem. 2003; 278: 31709-16.
- Bates-Withers C, Sah R, Clapham DE. TRPM7, the Mg2+ inhibited channel and kinase. Adv Exp Med Biol. 2011; 704: 173-83.
- Belpomme D, Gauthier S, Pujade-Lauraine E, Facchini T, Goudier MJ, Krakowski I, et al. Verapamil increases the survival of patients with anthracycline-resistant metastatic breast carcinoma. Ann Oncol. 2000; 11: 1471-6.
- Benham CD, Gunthorpe MJ, Davis JB. TRPV channels as temperature sensors. Cell Calcium. 2003; 33: 479-87.
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003; 4: 517-29.
- Bertolesi GE, Shi CJ, Elbaum L, Jollimore C, Rozenberg G, Barnes S, et al. The Ca2+ channel antagonists mibefradil and pimozide inhibit cell growth via different cytotoxic mechanisms. Mol Pharmacol. 2002; 62: 210-9.
- Bidaud I, Mezghrani A, Swayne LA, Monteil A, Lory P. Voltage-gated calcium channels in genetic diseases. Biochim Biophys Acta. 2006; 1763: 1169-74.
- Bidaux G, Flourakis M, Thebault S, Zholos A, Beck B, Gkika D, et al. Prostate cell differentiation status determines transient receptor potential melastatin member 8 channel subcellular localization and function. J Clin Invest. 2007; 117: 1647-57.
- Bohlen CJ, Priel A, Zhou S, King D, Siemens J, Julius D. A bivalent tarantula toxin activates the capsaicin receptor, TRPV1, by targeting the outer pore domain. Cell. 2010; 141: 834-45.
- Bolanz KA, Hediger MA, Landowski CP. The role of TRPV6 in breast carcinogenesis. Mol Cancer Ther. 2008; 7: 271-9.
- Bortolotto ZA, Bashir ZI, Davies CH, Collingridge GL. A molecular switch activated by metabotropic glutamate receptors regulates induction of long-term potentiation. Nature. 1994; 368: 740-3.
- Brandman O, Liou J, Park WS, Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell. 2007; 131: 1327-39.
- Carafoli E, Santella L, Branca D, Brini M. Generation, control, and processing of cellular calcium signals. Crit Rev Biochem Mol Biol. 2001; 36: 107-260.
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997; 389: 816-24.
- Catterall WA. Voltage-gated calcium channels. Cold Spring Harb Perspect Biol. 2011; 3: a003947.
- Chambers AF. MDA-MB-435 and M14 cell lines: identical but not M14 melanoma? Cancer Res. 2009; 69: 5292-3.
- Cheng H, Lederer MR, Xiao RP, Gómez AM, Zhou YY, Ziman B, et al. Excitation-contraction coupling in heart: new insights from Ca2+ sparks. Cell Calcium. 1996; 20: 129-40.
- Chodon D, Guilbert A, Dhennin-Duthille I, Gautier M, Telliez MS, Sevestre H, et al. Estrogen regulation of TRPM8 expression in breast cancer cells. BMC Cancer. 2010; 10: 212.
- Chung SC, Mcdonald TV, Gardner P. Inhibition by SK&F-96365 of Ca2+ current, IL-2 production and activation in T lymphocytes. Br J Pharmacol. 1994; 113: 861-8.
- Clapham DE. Signal transduction. Hot and cold TRP ion channels. Science. 2002; 295: 2228-9.
- Crabtree GR. Generic signals and specific outcomes: signaling through Ca2+, calcineurin, and NF-AT. Cell. 1999; 96: 611-4.
- Davis FM, Kenny PA, Soo ET, van Denderen BJ, Thompson EW, Cabot PJ, et al. Remodeling of purinergic receptor-mediated Ca2+ signaling as a consequence of EGF-induced epithelial-mesenchymal transition in breast cancer cells. PLOS ONE. 2011; 6: e23464.
- Davis FM, Peters AA, Grice DM, Cabot PJ, Parat MO, Roberts-Thomson SJ, et al. Non-stimulated, agonist-stimulated and store-operated Ca2+ influx in MDA-MB-468 breast cancer cells and the effect of EGF-induced EMT on calcium entry. PLOS ONE. 2012; 7: e36923.
- Davis FM, Azimi I, Faville RA, Peters AA, Jalink K, Putney JW, et al. Induction of epithelial-mesenchymal transition (EMT) in breast cancer cells is calcium signal dependent. Oncogene. 2014; 33: 2307-16.
- Dellis O, Rossi AM, Dedos SG, Taylor CW. Counting functional inositol 1,4,5-trisphosphate receptors into the plasma membrane. J Biol Chem. 2008; 283: 751-5.
- Dhennin-Duthille I, Gautier M, Faouzi M, Guilbert A, Brevet M, Vaudry D, et al. High expression of transient receptor potential channels in human breast cancer epithelial cells and tissues: correlation with pathological parameters. Cell Physiol Biochem. 2011; 28: 813-22.
- Donà M, Dell’Aica I, Pezzato E, Sartor L, Calabrese F, Della Barbera M, et al. Hyperforin inhibits cancer invasion and metastasis. Cancer Res. 2004; 64: 6225-32.
- Donnelly-Roberts DL, Namovic MT, Surber B, Vaidyanathan SX, Perez-Medrano A, Wang Y, et al. [3H]A-804598 ([3H-3]2-cyano-1-[(1S)-1-phenylethyl]-3-quinolin-5-ylguanidine) is a novel, potent, and selective antagonist radioligand for P2X7 receptors. Neuropharmacology 2009; 56: 223–229.
- El Hiani Y, Ahidouch A, Roudbaraki M, Guenin S, Brûlé G, Ouadid-Ahidouch H. Calcium-sensing receptor stimulation induces nonselective cation channel activation in breast cancer cells. J Membr Biol. 2006; 211: 127-37.
- El Hiani Y, Ahidouch A, Lehen’kyi V, Hague F, Gouilleux F, Mentaverri R, et al. Extracellular signal-regulated kinases 1 and 2 and TRPC1 channels are required for calcium-sensing receptor-stimulated MCF-7 breast cancer cell proliferation. Cell Physiol Biochem. 2009; 23: 335-46.
- Ertel EA, Campbell KP, Harpold MM, Hofmann F, Mori Y, Perez-Reyes E, et al. Nomenclature of voltage-gated calcium channels. Neuron. 2000; 25: 533-5.
- Faddy HM, Smart CE, Xu R, Lee GY, Kenny PA, Feng M, et al. Localization of plasma membrane and secretory calcium pumps in the mammary gland. Biochem Biophys Res Commun. 2008; 369: 977-81.
- Fanger CM, Neben AL, Cahalan MD. Differential Ca2+ influx, KCa channel activity, and Ca2+ clearance distinguish Th1 and Th2 lymphocytes. J Immunol. 2000; 164: 1153-60.
- Faouzi M, Hague F, Potier M, Ahidouch A, Sevestre H, Ouadid-Ahidouch H. Down-regulation of Orai3 arrests cell-cycle progression and induces apoptosis in breast cancer cells but not in normal breast epithelial cells. J Cell Physiol. 2011; 226: 542-51.
- Faouzi M, Kischel P, Hague F, Ahidouch A, Benzerdjeb N, Sevestre H, et al. ORAI3 silencing alters cell proliferation and cell cycle progression via c-myc pathway in breast cancer cells. Biochim Biophys Acta. 2013; 1833: 752-60.
- Farrell AW, Gadeock S, Pupovac A, Wang B, Jalilian I, Ranson M, et al. P2X7 receptor activation induces cell death and CD23 shedding in human RPMI 8226 multiple myeloma cells. Biochim Biophys Acta. 2010; 1800: 1173-82.
- Feng M, Grice DM, Faddy HM, Nguyen N, Leitch S, Wang Y, et al. Store-independent activation of Orai1 by SPCA2 in mammary tumors. Cell. 2010; 143: 84-98.
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006; 441: 179-85.
- Fixemer T, Wissenbach U, Flockerzi V, Bonkhoff H. Expression of the Ca2+-selective cation channel TRPV6 in human prostate cancer: a novel prognostic marker for tumor progression. Oncogene. 2003; 22: 7858-61.
- Frandsen SK, Gissel H, Hojman P, Tramm T, Eriksen J, Gehl J. Direct therapeutic applications of calcium electroporation to effectively induce tumor necrosis. Cancer Res. 2012; 72: 1336-41.
- Gehring MP, Pereira TCB, Zanin RF, Borges MC, Braga Filho A, Battastini AMO, et al. P2X7 receptor activation leads to increased cell death in a radiosensitive human glioma cell line. Purinergic Signal. 2012; 8: 729-39.
- https://pubmed.ncbi.nlm.nih.gov/18183945/
- van de Graaf SFJ, Hoenderop JGJ, Bindels RJM. Regulation of TRPV5 and TRPV6 by associated proteins. Am J Physiol Ren Physiol. 2006; 290: F1295-302.
- Grice DM, Vetter I, Faddy HM, Kenny PA, Roberts-Thomson SJ, Monteith GR. Golgi calcium pump secretory pathway calcium ATPase 1 (SPCA1) is a key regulator of insulin-like growth factor receptor (IGF1R) processing in the basal-like breast cancer cell line MDA-MB-231. J Biol Chem. 2010; 285: 37458-66.
- Guilbert A, Dhennin-Duthille I, Hiani YEL, Haren N, Khorsi H, Sevestre H, et al. Expression of TRPC6 channels in human epithelial breast cancer cells. BMC Cancer. 2008; 8: 125.
- Guilbert A, Gautier M, Dhennin-Duthille I, Haren N, Sevestre H, Ouadid-Ahidouch H. Evidence that TRPM7 is required for breast cancer cell proliferation. Am J Physiol Cell Physiol. 2009; 297: C493-502.
- Hammadi M, Chopin V, Matifat F, Dhennin-Duthille I, Chasseraud M, Sevestre H, et al. Human ether a-gogo K+ channel 1 (hEag1) regulates MDA-MB-231 breast cancer cell migration through Orai1-dependent calcium entry. J Cell Physiol. 2012; 227: 3837-46.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011; 144: 646-74.
- Hardie RC, Minke B. The TRP gene is essential for a light-activated Ca2+ channel in drosophila photoreceptors. Neuron. 1992; 8: 643-51.
- Hechler B, Lenain N, Marchese P, Vial C, Heim V, Freund M, et al. A role of the fast ATP-gated P2X, cation channel in thrombosis of small arteries in vivo. J Exp Med. 2003; 198: 661-7.
- Herschkowitz JI, Simin K, Weigman VJ, Mikaelian I, Usary J, Hu ZY, et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007; 8: R76.
- Hoenderop JG, Nilius B, Bindels RJ. Calcium absorption across epithelia. Physiol Rev. 2005; 85: 373-422.
- Honore P, Donnelly-Roberts D, Namovic MT, Hsieh G, Zhu CZ, Mikusa JP, et al. A-740003 [N-(1-{[(cyanoimino)(5-quinolinylamino)methyl]amino}-2,2-dimethylpropyl)-2-(3,4-methoxyphenyl)acetamide], a novel and selective P2X(7) receptor antagonist, dose-dependently reduces neuropathic pain in the rat. J Pharmacol Exp Ther. 2006; 319: 1376-85.
- Hu JJ, Qin KH, Zhang, Gong JB, Li N, Lv D, et al. Downregulation of transcription factor Oct4 induces an epithelial-to-mesenchymal transition via enhancement of Ca2+ influx in breast cancer cells. Biochem Biophys Res Commun. 2011; 411: 786-91.