
Mini Review
Austin J Pharmacol Ther. 2024; 12(2): 1188.
Retinopathy and Their Mechanism
Ifrah Saroosh¹; Aisha Shakir¹; Tehseen Faiz²; Haim Sajid¹; Tehseen Faiz²; Muhammad Aetesam Nasir¹; Maham Ifikhar¹; Muhammad Adil Umar¹; Fatima Mazhar³; Waqar Mazhar*
1Department of Medicine and Surgery, Hitec-Institute of Medical Sciences Taxila Cantt, Pakistan
2Department of Ophthalmology, Hitec-Institute of Medical Sciences Taxila Cantt, Pakistan
3Department of Microbiology, Muhammad Nawaz Sharif University of Agriculture, Multan, Pakistan
*Corresponding author: Waqar Mazhar Department of Medicine and Surgery, Hitec-Institute of Medical Sciences Taxila Cantt, Pakistan. Tel: +923012222861 Email: waqarmazhar631@gmail.com,
Received: March 08, 2024 Accepted: April 18, 2024 Published: April 25, 2024
Abstract
Retinopathy is the emerging disease in Pakistan over the age of 30 in diabetic patients. In diabetic patients this disease causes blindness, visual loss, and hazy eyesight. Several suggested metabolic mechanisms that connect micro vascular problems to hyperglycemia. Activation of Protein Kinase C (PKC), oxidative stress, polyol buildup, and the production of Advanced Glycation End products (AGEs) are a few of these. The anti-VEGF drugs, laser, eye surgery is the treatment of Retinopathy. The patient HBA1C lab test below than 6.5% will be preferred for surgery.
Keywords: Retinopathy; Diabetic macular edema; Neo vascular glaucoma; Retinal detachment
Introduction
Retinopathy is a disease that can cause blindness and vision loss in aged peoples. It mostly occurs in diabetic patients so it called Diabetic Retinopathy [13]. Worldwide, the number of people with diabetes is more than 135 million. Over 18.2 million Americans, or 6.3% of the population, have diabetes, and 800,000 new instances of type 2 diabetes are diagnosed in the country each year [8]. It affects the blood vessels in the light sensitive layer of tissues of the retina. In early stages of this disease have no symptoms. But some people face problems in reading and seeing faraway objects. In the later stage of disease, the bleeding from blood vessels of retina in the form of gel like fluid. If this happens you see dark, floating spots like cobwebs. If the treatment on time it will benefit the patient but after time without time it become worse [12].
Diabetic Macular Edema (DME)
Approximately 1 in 15 diabetics will eventually develop DME. DME occurs when fluid leaks from retinal blood vessels into the macula, a region of the retina required for crisp, centre vision. This results in hazy eyesight [6].
Neo Vascular Glaucoma
Unusual blood vessels that emerge from the retina as a result of diabetic retinopathy may obstruct the flow of fluid out of the eye. One kind of glaucoma, which is a collection of eye conditions that can result in blindness and visual loss, is brought on by this [7].
Glaucoma: A class of eye conditions known as glaucoma can result in blindness and visual loss by harming the optic nerve, a nerve located at the back of the eye. You might not notice the symptoms at first since they might appear so slowly [1]. A thorough dilated eye exam is the only method to determine if you have glaucoma. While there is no known cure for glaucoma, vision protection and damage may frequently be stopped with early intervention.
Retinal Detachment
The back of your eye may develop scars as a result of diabetic retinopathy. Tractional retinal detachment refers to the condition when your retina pulls away from the back of your eye due to scarring [11].
Pathophysiology: There are several suggested metabolic mechanisms that connect micro vascular problems to hyperglycemia. Activation of Protein Kinase C (PKC), oxidative stress, polyol buildup, and the production of Advanced Glycation End products (AGEs) are a few of these. Through their influence on cellular metabolism, signaling, and growth factors, these mechanisms are hypothesized to modify the disease process [5].
Polyol accumulation of polyol occurs in experimental hyperglycemia, which in rats and dogs is associated with the development of basement thickening, pericyte loss, and micro aneurysm formation [3]. High concentrations of glucose increase flux through the polyol pathway with the enzymatic activity of aldosereductase, leading to an elevation of intracellular sorbitol concentrations. This rise in intracellular sorbitol accumulation has been hypothesized to cause osmotic damage to vascular cells [4]. Aldose Reductase Inhibitors (ARIs) have been evaluated for the prevention of retinal and neural damage in diabetes. However, three clinical trials of ARIs in humans have not shown efficacy in preventing the incidence or progression of retinopathy. The efficacy of new, more potent ARIs remains to be evaluated in clinical trials [10]. AGEs another well-characterized pathway is damage resulting from accumulation of AGEs. High serum glucose can lead to nonenzymatic binding of glucose to protein side chains, resulting in the formation of compounds termed AGEs. After 26 weeks of induced hyperglycemia, the retinal capillaries of diabetic rats have marked accumulation of AGEs as well as a loss of pericytes [14]. Furthermore, diabetic rats treated with amino guanidine (AGE formation inhibitor) have reduced AGE accumulation and reduced histological changes, including micro aneurysm formation and pericyte loss. An ongoing clinical trial is investigating the effect of amino guanidine in humans. Preliminary results suggest that amino guanidine reduces the progression of retinopathy but is associated with anemia.
Polyol accumulation
In rats and dogs, experimental hyperglycemia leads to the accumulation of polyol and is linked to the development of micro aneurysm formation, pericyte loss, and basement thickening. Elevated intracellular sorbitol concentrations result from high glucose concentrations increasing flow via the polyol pathway with the enzymatic activity of aldose reductase [3]. It has been proposed that vascular cells may sustain osmotic injury as a result of this increase in intracellular sorbitol buildup. Aldosereductase Inhibitors (ARIs) have been studied in relation to preventing diabetic nerve and retinal damage. Nonetheless, according to three human clinical trials, ARIs are ineffective at stopping the onset or development of retinopathy. Clinical studies need to be conducted to determine the effectiveness of new, more powerful ARIs [15].
AGEs
Damage brought on by a buildup of AGEs is another well-established route. Elevated blood glucose levels have the potential to cause non enzymatic glucose binding to side chains of proteins, which can lead to the creation of substances known as AGEs [2]. Following 26 weeks of induced hyperglycemia, diabetic rats' retinal capillaries show a significant buildup of AGEs and a pericyte loss. Additionally, diabetic rats given amino guanidine, an inhibitor of AGE synthesis, showed decreased buildup of AGE and decreased histological alterations, such as the development of micro aneurysms and the loss of pericytes [9]. The effects of amino guanidine in humans are being studied in a clinical study that is now underway. According to preliminary findings, amino guanidine slows the development of retinopathy but is linked to anaemia.
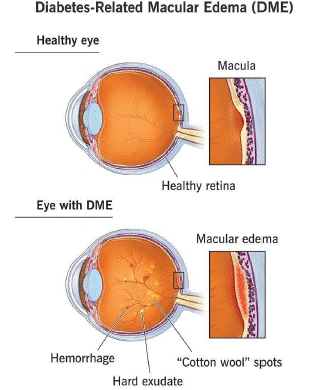
Figure 1: Diabetic Macular Edema.
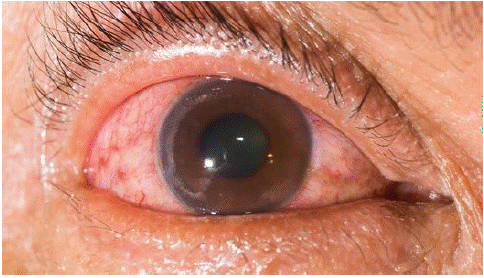
Figure 2: Neo vascular glaucoma.
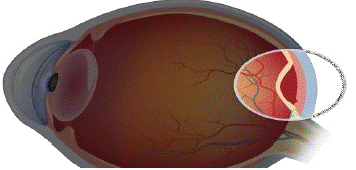
Figure 3: Retinal detachment.
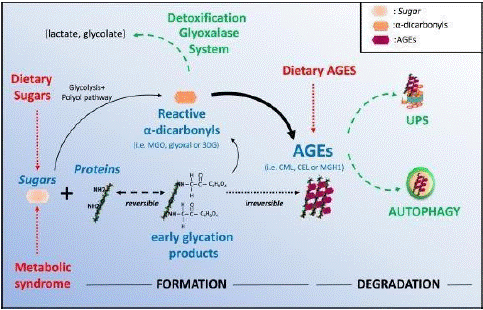
Figure 4: Advanced glycation end products mechanism in eye.
Conclusion
In Pakistan, over the age of 30 the retinopathy become leading disease in diabetic patient. Several mechanisms like PKC, AGES play role in this disease. In the patient the diseases cause color blindness, overall blindness, hazy eyesight, and visual loss. The doctor suggests the patient towards surgery and maintain hemoglobin A1C tests below than 6.5. The Laser and VEGF are alternative treatment against surgery in this disease. After surgery patient have care from sunlight exposure.
Author Statements
Availability of Data and Materials
Availability of data and materials on request by corresponding author.
Competing Interests
The authors have no competing interest.
Authors' Contributions
All authors contribute equally.
Acknowledgements
I am grateful to all of those with whom I have had the pleasure to work during this and other related projects.
References
- Bhowmik D, Kumar KS, Deb L, Paswan S, Dutta A. Glaucoma-A Eye Disorder Its Causes, Risk Factor, Prevention and Medication. The Pharma Innovation. 2012; 1: 66-81.
- Chilelli N, Burlina S, Lapolla A. AGEs, rather than hyperglycemia, are responsible for microvascular complications in diabetes: a “glycoxidation-centric” point of view. Nutrition, Metabolism and Cardiovascular Diseases. 2013; 23: 913-919.
- Obrosova GI, Kador PF. Aldose reductase/polyol inhibitors for diabetic retinopathy. Current pharmaceutical biotechnology. 2011; 12: 373-385.
- Garg SS, Gupta J. Polyol pathway and redox balance in diabetes. Pharmacological Research. 2022; 182: 106326.
- González P, Lozano P, Ros G, Solano F. Hyperglycemia and Oxidative Stress: An Integral, Updated and Critical Overview of Their Metabolic Interconnections. International Journal of Molecular Sciences. 2023; 24: 9352.
- Holekamp NM. Overview of diabetic macular edema. Am J Manag Care. 2016; 22: s284-s291.
- Hu CX, Zangalli C, Hsieh M, Gupta L, Williams AL, Richman J, et al. What do patients with glaucoma see? Visual symptoms reported by patients with glaucoma. The American journal of the medical sciences. 2014; 348: 403-409.
- Jonnalagadda SS. Effectiveness of medical nutrition therapy: importance of documenting and monitoring nutrition outcomes. Journal of the American Dietetic Association. 2004; 104: 1788-1792.
- Luo D, Fan Y, Xu X. The effects of aminoguanidine on retinopathy in STZ-induced diabetic rats. Bioorganic & medicinal chemistry letters. 2012; 22: 4386-4390.
- Mara L, Oates PJ. The polyol pathway and diabetic retinopathy. Diabetic Retinopathy. 2008; 159-186.
- Peate I. Retinal detachment. British Journal of Health care Assistants. 2022; 16: 236-241.
- Sayres R, Taly A, Rahimy E, Blumer K, Coz D, Hammel N, et al. Using a deep learning algorithm and integrated gradients explanation to assist grading for diabetic retinopathy. Ophthalmology. 2019; 126: 552-564.
- Shah CA. Diabetic retinopathy: A comprehensive review. Indian journal of medical sciences. 2008; 62: 500-519.
- Smit AJ, Lutgers H. The clinical relevance of advanced glycation endproducts (AGE) and recent developments in pharmaceutics to reduce AGE accumulation. Current medicinal chemistry. 2004; 11: 2767-2784.
- Zhu C. Aldose reductase inhibitors as potential therapeutic drugs of diabetic complications, chapter. 2013.