Research Article
Austin J Pharmacol Ther. 2014; 2 (4). 1022
Early Life Exposure to Methylmercury Influences Later Life of Development and Maternal Toxicity in Rats
Gandhi DN1* and Dhull Dinesh K2
11Department of Neurobehavioral Toxicology, National Institute of Occupational Health, Meghaninagar, Ahmedabad-380016, India
22Department of Neurophysiology ( Glial Cells), National Institute of Mental Health and Neurosciences, (NIMHANS), Banglore- 560029, India
*Corresponding author: : Gandhi DN, Department of Neurobehavioral Toxicology, National Institute of Occupational Health, Meghaninagar, Ahmedabad-380 016, Gujarat, India
Received: February 15, 2014; Accepted: March 02, 2014; Published: May 03, 2014
Abstract
Methylmercury (MeHg) is recognized as one of the most hazardous environmental pollutants. The aim of the present study was to find out whether and how early life of exposure to this neurotoxicant influences later life of developmental and maternal toxicity. The study was carried out on Wistar rats, the progeny of rat mothers exposed to MeHg (0.5, 1.0 and 1.5mg ⁄kg ⁄day) from gestational day (GD) 5 to till parturition (PND0). The following reproductive and developmental parameters were assessed: body weight, body weight gain(%), deaths, abortions or early deliveries, implantations, postimplantation, resorption, gestational length percentage viability, gait abnormality and hyper activity. The results obtained in the study showed no deaths, abortions, or early deliveries, enhanced maternal toxicity, which included deaths and decreased body weight gain (30.2%) and food consumption. The number of nonviable implants decreased significantly following exposure to 1.5mg⁄kg⁄day MeHg-treatment group, with the percentage of postimplantation loss (44.8%). In contrast, MeHg caused up to 33.3% of resorbed litters without showing sign of maternal toxicity such as gait alterations and hyperactivity in rats treated with 1.5mg⁄ kg⁄day. Average gestation length (days) was significantly affected with 1.0 and 1.5mg⁄ kg⁄day MeHg-treatment groups. The data suggests that gestational exposure would enhance the dose-dependent MeHg–induced embryo⁄fetal and maternal toxicity as a form of teratogenic action. Further studies of exposure to MeHg at present dose levels during critical windows of development induce a number of adverse health outcomes for offspring. Such effects may contribute to increased disease risks observed in human population.
Key words: Methylmercury (MeHg); Gestational exposure; Postnatal development; Maternal toxicity; Rat
Introduction
In the 1950s and 60s neurological disease was noted in many people living around Minamata Bay in Japan. People of all ages were affected, but effects were most severe in infants and children. The disease was traced to methylmercury (MeHg) pollution in the bay that accumulated to high levels in fish (10–40 ppm). The principal sources of exposure to Hg in the general population are ingestion and inhalation of Hg compounds. Due to its ubiquitous presence in the environment, health concerns are increasing. Methyl mercury (MeHg), an organic methylated form of mercury, exists in aquatics receiving industrial wastes containing mercury. The health impact of water contamination of MeHg continues to draw concern, since accidental poisoning that occurred in Minamata, Japan, Niigata and Iraq [1]. Faroe Islands cohort study reported MeHg related deficits in neurological and cognitive functions in school–age children [2]. The epidemiological and animal studies demonstrate that the fetuses are more vulnerable than mothers, as the sensitivity of the nervous system to MeHg toxicity is the highest during developmental stages [3,4].
Methylmercury is an embryotoxic and can induce teratogenic effects in golden hamsters [5,6], cats [7], rats [6,8], and mice [9–12]. Exposure events during critical windows of fetal and postnatal (PN) development pose a serious risk for adverse health outcomes later life [13]. Exposure to toxic elements such as mercury or arsenic during gestation and lactation may potentially cause adverse effects on the development of foetuses and neonates [14–17]. Developmental delays in acquiring motor skills associated with low to moderate prenatal MeHg exposure are known [18]. The fetus is especially susceptible to MeHg–induced embryo⁄fetal toxicity, and neurobehavioral effects including learning deficits in healthy animals exposed during gestation [3]. Behavioural alterations were observed, even at MeHg levels below those causing morphological abnormalities [19]. Several studies on the developmental effects of MeHg on rats and mice have provided specific hypotheses regarding the mechanisms of action of MeHg. It is likely that maternal fish intake–related MeHg exposure during pregnancy, at levels safe for mothers, may affect the developing nervous system of the foetus. This possibility is supported by the data from studies of the victims of mass MeHg poisonings in Japan [1].
In addition, the dam is primarily determined the development of major regulatory system underlying behaviour and physiology in the neonatal rat [20]. However, our earlier study [21] indicates maternal and embryo⁄foetal toxicity when high dose of MeHg (2.0mg⁄kg⁄ day) was given by gavages during GD5 till parturition to pregnant rats caused hundred percentage of resorption of the F1 generationoffspring. So it further worsened MeHg toxicity even before birth, adding up the impact throughout life. However, the impact depends on exposure, duration, route as well as form of exposure. Our goal was to develop a rat model of early life MeHg exposure through which we could identify critical windows of exposure that might result in adverse impacts on the development of the nervous system later in life. Thus, the study presented here was designed to further explore the adverse developmental outcomes following early life low dose MeHg exposure has detrimental impact on later life of development and maternal toxicity leads to change in neurobehavioral outcomes.
Materials and methods
Study design
Mated female Wistar rats were dosed daily with MeHg from GD5 to till parturition. The effects of exposure observed during the pre weaning period (PND1 to PND21) of life in offspring.
Ethical issues
In all the experiments was performed in accordance the guidelines of the Committee for the Purpose of Control and Supervision of Experimental Animals (CPCSEA), India. The Institutional Animal Ethics Committee approved the study design.
Animals
All experiments were performed on rats, white WISTARS. Sixty–four 64; mature male and female Wistar albino rats weighing 180–200g were obtained from the institutes breeding colony. After one–week acclimation in the laboratory, female rats were mated with males [2:1] overnight and examined the following morning for vaginal smears. Vaginal smears were taken daily between 9 a.m. to 10 a.m. from mated female rats. On the day when spermatozoa in the vaginal smear were found, the female was weighted and this day was regarded as the first day of gestation (GD0). During the experimental period, animal room was maintained at temperature 22 ± 2°c; relative humidity 65 ± 5%, (12h light⁄dark cycle), with free access to food and water.
Randomization, Mating and Treatment
Pregnant rats
Proestrus virgin female rats were mated proven–fertile male rats (2:1) overnight. The day of mating confirmed by the presence of sperm–positive vaginal smears was designated as gestation day (GD) 0. At GD0 female’s pregnant animals (dams) were randomly assigned to four groups of rats, and housed individually. Out of thirty GD 0 females, twenty–eight pregnant females delivered the pups; rest of the two females in higher dose did not deliver. Date of birth was designated as postnatal day (PND) 0. (Table: 1).
Preparation of dose formulation
The dose formulation for each group was prepared separately in order to maintain a constant dose volume of not more than 5–ml⁄kgbody weight. Methyl mercury chloride (CH3ClHg) 99.9% pure, CAS no.115–09–3; batch size 8151x, Sigma–Aldrich GmbH) was obtained from Sigma Aldrich, U.S.A. The doses were based on data showing that at this exposure level, the Hg concentration in newborn rats was comparable to that found in human infants from populations with high dietary fish consumption [22,23]. Controls were treated with saline solution.
Prenatal methyl mercury exposure
Twenty eight–28; GD0 pregnant females (F0 Generation) were assigned, based on body weight, to each of the following groups using prior to MeHg treatment initiation: Vehicle Control (n=7); 0.5 mg MeHg⁄kg body weight⁄day (n= 8); 1.0 mg MeHg⁄kg body weight⁄ day (n= 7); 1.5 mg MeHg⁄kg body weight⁄day (n= 6). Throughout gestation, all pregnant rats were weighed and examined for signs of toxicity daily. The females were dosed, orally by gavage, form GD5 to till parturition.
Evaluations of F1 Generation
General condition
Beginning on GD21, dams were inspected frequently between 0800 and 2000h for birth until delivery, each presumably pregnant female was checked twice daily for completion of or difficulties in parturition. The day of parturition was defined as postnatal day (PND0), meaning the maximum resolution for gestational length was half a day. The pups were counted, examined for gross malformation and weighed individually. The body weight of pups and maternal behaviors were recorded daily during nursing. The offspring was considered the experimental unit. After parturition, the neonates were observed for mortality and signs of toxicity.
Assessment of the reproduction success
The offspring were evaluated for survival, growth, development and behaviors. When parturition was complete, the numbers of stillborn, implantation, postimplantation, resorption and live pups in each litter were recorded. Following variables were observed: Birth measures: the offspring were examined on PND1 for morphological anomalies (e.g., missing digits, facial malformations), sex by relative anogenital distance and culled pseudo–randomly to twenty animals each and balanced for sex (20 females and 20 males) to the extent possible.
Assessment of the offspring’s morphological development
Gestation length was calculated at birth and the following offspring data were collected on PND1: Pups size, sex ratio (as percent males), body weight for each pup and the number of malformed offspring. On PND1, the pups were identified within each groups of treatment and were assessed for Males Body length (mm); Females Body Length (mm); Males tail Length (mm); Females tail Length (mm); Pups viability at birth; Pups affected per dam; Pups mortality at birth (PND1–4) and the pups from each litter were weighed on PND1, 3,5,7,9,14,15,21,28.
Maternal toxicity & Behaviours
A maternal toxicity and behaviors were observed daily in the home cage of each dam and her litter between gestational day and post–delivery (PND) 1 till 14.
Terminal Evaluations
Females that did not mate were euthanized approximately 3 weeks after the completion of the mating period and were subjected to necropsy. The pregnancy status of animals that did not mate was confirmed and recorded in datasheet. Dams whose whole litters were born dead or died prior to weaning were also recorded. The dams with normal pups were euthanized approximately 3 weeks after weaning of the pups. The method of euthanasia was carbon dioxide asphyxiation followed by exsanguinations from the abdominal aorta. The number of implantation site scars was recorded for the pregnant animals.
Statistical analysis
Data were analysed by one–way analysis of variance (ANOVA) followed by Duncan test. The level of statistical significance was set at p<0.05. All data are expressed as means ± S.E.M.
Results
Pregnant females (dams) were divided into four groups of 30 animals: control (with free access to fresh tap water), 0.5mg⁄kg⁄ day MeHg; 1.0mg⁄kg⁄day MeHg and 1.5mg⁄kg⁄day MeHg by oral gavages. Dam’s body weight was noted every day during gestation. After birth the number of pups for each group was as follows: control (N = 66), MeHg 0.5mg⁄kg⁄day (N = 80); MeHg 1.0mg⁄kg⁄day (N =73) and MeHg 1.5mg⁄kg⁄day (N = 43) per each groups of exposure. We have randomly selected either sex of twenty offspring per each groups of exposure to achieves the developmental,morphological milestonesand reproductive test.
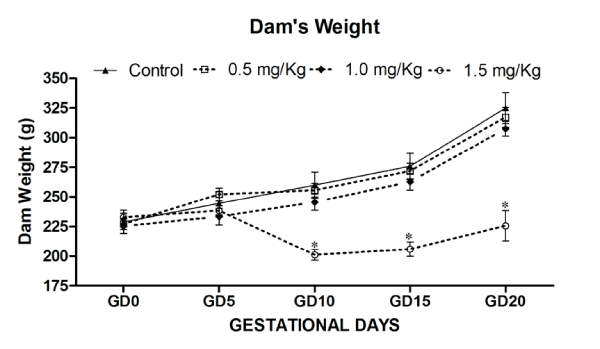
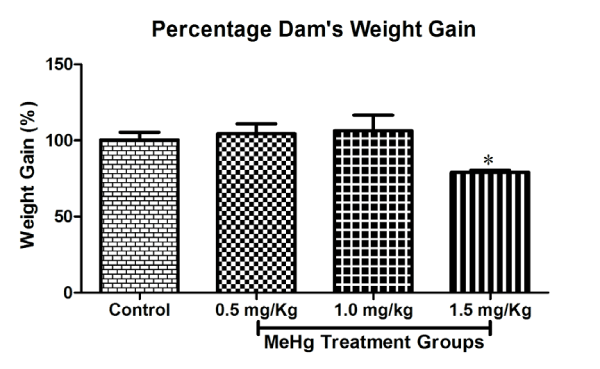
A summary of the distribution and fate of all mated rats of the study is given in Table 1. During pregnancy, the treatment groups did not differ in water and food intake, and in the rate of the body mass increase. The pregnant rats treated with 0.5, 1.0 and 1.5mg⁄kg⁄ day MeHg from GD5 to till parturition produced neither maternal toxicity nor any noticeable signs or symptoms. The behaviour of the treated rats was similar to that of the control rats. On day 4 of gestation, the maternal body weight (g) of control (227.7±16.2) with respect to treatment groups was 0.5mg⁄kg⁄day MeHg (225.2±10.7); 1.0mg⁄kg⁄day MeHg (215.4±10.0) and 1.5mg⁄kg⁄day MeHg (182.8±3.5) remained almost within the range. On day 20 of gestation, the maternal body weight gain (g) of control and three dose levels (0.5, 1.0 and 1.5mg⁄kg⁄day) MeHg exposed dams were 111.2±4.1; 115.4±3.4; 111.0±2.0 and 79.4±2.1. Maternal weight gain of dams during gestation and weight gain during treatment was significantly reduced in high dose (1.5mg⁄kg⁄day) MeHg treatment group [F (3,20) = 3.43, p<0.05] without any sign of anxiety, hind limb ataxia or gait alterations. The 0.5 and 1.0mg⁄kg⁄day MeHg–treatment groups did not differ from the control group in the level of food and water consumption and body weight gain, whereas maternal body weight during gestational period (GD0–20) significantly reduced in 1.5 mg⁄ kg⁄day MeHg–treatment group [F (3,144) = 6.629, p< 0.01] (Figure 1 and 2).
Dose of MeHg
Control
0.5 mg/kg MeHg
1.0 mg/kg MeHg
1.5 mg/kg MeHg
No. of vaginal smear positive females (GD 0)
7
8
7
6
No. of pregnant female
(Day 10)
7
8
7
6
Death
0
0
0
0
Absorption and/or early delivery
0
0
0
0
Evaluated at term
7
8
7
6
Resorbed litters
0
0
0
1
No. of litters
7
8
7
6
Live pups
66
80
73
43
Table 1: The distribution and fate of all mated rats in the study.
Figure 1: Effects on Maternal body weight of rat (Dam) exposed to MeHg on gestational day 5 to till parturition. Body weight (g) during gestation period exposed to MeHg. Data are presented as mean ± S.E.M. Significantly different from the control groups: *p < 0.05.
Figure 2: Effects on Maternal weight Gain (%) of rat (Dam) exposed to MeHg on gestational day 5 to till parturition. Body weight gain (%) during gestation period exposed to MeHg. Data are presented as mean ± S.E.M.
The reproduction success, as measured were unaffected in 0.5 and 1.0 mg⁄kg⁄day MeHg– treatment groups by measuring different gestational parameters (Table 2). There were no deaths, absorption or early deliveries observed in all dose levels MeHg exposures. In contrast, dam treated with 1.5mg⁄kg⁄day MeHg–treatment group alone caused reduction in percentages of resorbed litters (33.33%), dead fetuses, as well as the percentage of post implantation loss were significantly affected in 1.5mg⁄kg⁄day MeHg–treatment group (Table 2). The control and treated females gave birth on the 21st day of pregnancy. The total number of pups per treatment groups was lower in each group but the difference reach significance with higher dose (control: 66; MeHg 0.5mg⁄kg⁄day: 80; MeHg 1.0mg⁄kg⁄day 73; MeHg1.5mg⁄kg⁄day: 43). The sex ratio expressed as the percentage of males among the total number of pups was similar in all groups (respectively 49%, 54%, 49%, and 59%). Abortion or death of whole litter was seen in 1.5mg⁄kg⁄day MeHg–treatment group.
Parameters
Control
0.5 mg/kg MeHg
1.0 mg/kg MeHg
1.5 mg/kg MeHg
No. of dams
7
8
7
6
Implants/litter
9.86 ± 2.54
10.00 ± 2.33
10.43 ± 0.79
9.67 ± 1.67
Live fetuses/Litter
9.43 ± 2.15
9.88 ± 2.30
10.43 ± 0.79
5.33 ± 2.67
Dead fetuses/Litter
0.13 ± 0.79
0.13 ± 0.35
0.14 ± 0.38
2.00 ± 2.00
Total resorbed/Litter (%)
0.00
0.00
0.00
33.28**
Total resorbed and dead fetuses/Litter (%)
0.00
0.00
0.00
33.33**
Postimplantation loss (%)
1.32
1.30
1.34
44.83***
Total male pups/dam
4.71 ± 2.98
5.25 ± 2.87
5.14 ± 1.95
4.33 ± 0.88
Total females/dam
4.71 ± 1.98
4.63 ± 2.39
5.29 ± 2.29
3.00 ± 1.00
Sex ratio (M/M+F)
0.49
0.54
0.49
0.59
Average Sex ratio, male (%)
49
54
49
59
Males fetal body weight
(g; PND 1)
6.16 ± 0.42
6.14 ± 0.35
6.06 ± 0.30
6.30 ± 0.09
Females fetal body weight (g; PND 1)
5.67 ± 1.06
5.88 ± 0.41
5.81 ± 0.42
6.14 ± 0.09
Males body length (mm)
68.17 ± 1.24
69.00 ± 1.23
66.58 ± 1.71
70.44 ± 0.63
Females body length (mm)
67.97 ± 1.23
68.54 ± 1.10
66.25 ± 2.25
69.57 ± 0.57
Males tail length (mm)
18.09 ± 0.69
18.83 ± 0.75
17.22 ± 1.27
17.44 ± 0.24
Females tail length (mm)
18.53 ± 0.87
18.92 ± 0.68
17.53 ± 1.11
17.29 ± 0.29
Table 2: Effects of MeHg on gestational parameters in pregnant rat.
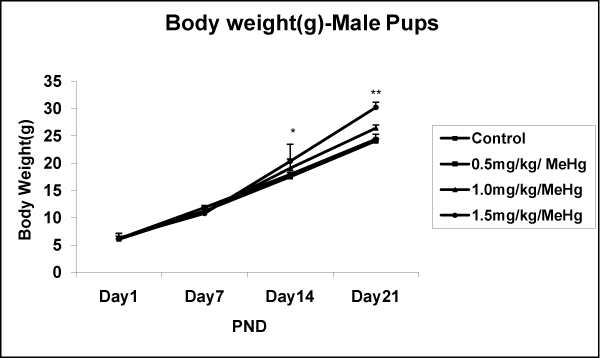
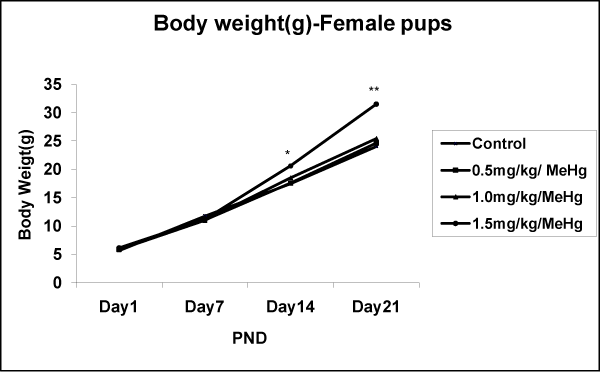
The number of pups delivered varied from 7 to 10 in both control and MeHg–treatment groups. Body weight gain of pups on PND1, 7,14, and 21 did not differ among groups and were not affected in MeHg–treated offspring’s body weight, as compared to control, neither at birth nor at PND14 and PND21 with 0.5 and 1.0mg⁄kg⁄day MeHg–treatment groups. However, results indicate that a significant increase in the body weight of either sex of offspring at all age (PND1– 28) compared to control group (Fig.4A & 4B). The post hoc test indicated an effect dose–dependant p < 0.01. Concerning the effect of day, the statistical test showed a significant increase in weight of male offspring exposed to MeHg [F (24,684) = 8.083, p < 0.001] and female offspring [F (24,684) = 5.121, p < 0.001] (Figure 3A and 3B).
Figure 3A: Effects on body weight in the Male offspring of rat exposed to MeHg on gestational day 5 to till parturition. Body weight (g) of male offspring of rat exposed to MeHg. Data are presented as mean ± S.E.M. Significantly different from the control groups: **p < 0.001.
Figure 3B: Effects on body weight in the Female offspring of rat exposed to MeHg on gestational day 5 to till parturition. Body weight of male offspring of rat exposed to MeHg. Data are presented as mean ± S.E.M. Significantly different from the control groups: **p < 0.01.
There were no significant differences between in the number of pups per litter, male⁄female ratio, or the number of stillbirths in 0.5 and 1.0mg⁄kg⁄day MeHg–treatment groups. The number of dams that delivered percentage of viable pups per dam treated with 1.5 mg⁄ kg⁄day MeHg–treatment group affected significantly as compared to control as well as 0.5 and 1.0mg⁄kg⁄day MeHg treatment groups. The values of the viability index (i.e. percentage of pups surviving beyond PND4) were notably lower in 1.5mg⁄kg⁄day MeHg–treatment group (Table 3). The length of gestation was significantly different between the control and 1.0 and 1.5 mg⁄kg⁄day MeHg– treatment groups. Prenatal administration of MeHg resulted in non–significant change in percentage of live births as well as on viability during the first day of postnatal life with 0.5 and 1.0mg⁄kg⁄day MeHg–treatment groups (Table: 3). The percentages of pups’ viability at birth as well as resorption per litter were significantly affected with 1.5 mg⁄ kg⁄day MeHg–treatment group. As a result, there was a significant effect of MeHg on post–implantation loss; with the 1.5mg⁄kg⁄day MeHg treated dams, showing a significant change in percentage of resorption of pups whereas no statistical difference was noted in the number of pups per litter, in general appearance, stillbirths, pups weight, mean pup weight, litter size, sex ratio, the viability index and mortality with 0.5 and 1.0 mg⁄kg⁄day MeHg–treatment groups (Table 2 and 3).
MeHg- Treatment groups
Control
0.5mg/kg/day
1.0mg/kg/day
1.5mg/kg/day
Percentage of live pups at birth
100
100
100
77.34*
No. of fetuses/litter
9.4 ± 2.1
9.8 ± 2.3
10.4 ± 0.7
5.3 ± 2.6
Viability (%)
100
98.7
98.6
72.7*
Gestational length (day)
21.00 ± 0.00
21.0 ± 0.0
21.1 ± 0.0***
22.0 ± 0.0***
Table 3: Physical and functional assessment in the offspring of rat exposed to methyl mercury on gestational day 5 to till parturition. Data are presented as mean ± S.E.M. Significantly different from the control groups: *p < 0.05; ***p < 0.001.
Discussion
Attention has been given to consequences of exposure to methylmercury (MeHg) during pregnancy and the developing foetus. Methylmercury biomagnifies through the food chain and can reach human populations via the consumption of contaminated fish and seafood. MeHg exposure through maternal transfer can induce neurological damage to the developing fetus [19], and such deficits may not manifest themselves until much later [24]. A variety of exposure regimens, therefore, has been used to identify the adverse effects of MeHg on the developing offspring taking into account the human exposure scenario of chronic ingestion of MeHg through the consumption contaminated fish. However, several experimental studies have confirmed the toxic effect of MeHg on reproduction and offspring neurobehavioral functions, for only a brief period during gestation [3,25–30], perinatal [31] and gestational through postnatal MeHg exposures [32–34]. In addition, the endpoints evaluated were often limited in scope. The selection of dose levels, manner of administration and duration of exposure will directly impact on the out comes being measured.
The fetus is especially susceptible to MeHg–induced embryo⁄ fetal toxicity found in animals exposed during gestation [3]. Earlier studies have reported that methylmercury–induced embryo⁄fetal toxicity (including teratrogenesis) in mice and rats [9,14,35]. Our experimental data also indicate, the number of pups per litter, gender proportion in litters and pup viability were not affected in 0.5 or 1.0mg⁄kg⁄day MeHg exposure; where as a high incidence of prenatal mortality, increasing the percentage of postimplantation loss up to 44.83% or resorption (33.33%) in 1.5mg⁄kg⁄day MeHg–treatment group.
The pregnancy length of the animals remarkably increased in high dose of MeHg (1.5 mg⁄kg⁄day) in the present study. Rats treated with MeHg by gavage at 6mg⁄kg⁄day from GD 6 to 9 and reported a comparable extension of gestation length, reduced embryonic implantations in the uterus and the number of dams bearing live litters was markedly diminished. Failure to deliver or sustain live pups illustrated the extreme toxicity of this dose [36]. In contrast, the present study with MeHg by gavage at 1.0 and 1.5mg⁄kg⁄day from GD5 to till parturition, showed a significantly extension of gestational length, reduced embryonic implantations in the uterus and the number of dams bearing live litters was markedly diminished, failure to deliver or sustain live pups illustrated the extreme toxicity in 1.5mg⁄kg⁄day MeHg–treatment group; a reasonable interpretation of which may be that the exposure in the present study was started after implantation of embryo. The pregnant mother, exposed to MeHg at high doses from contaminated fish in Japan reported miscarriages, or had children stillborn or dying shortly after birth [37]. Dietary exposure via drinking water during gestation and lactation at doses of 0.5 and 2.0mg⁄kg⁄day was used in some studies [37,38]. Some of the data could be compared with those studies, rats received MeHg at two dosing levels: 2.0 or 6.0mg⁄kg b.w. from GD6 to GD9 as well as 0.5 and 2.0 mg⁄kg b.w. from day 7 of pregnancy (GD7) up to day 21 (PND21) after the delivery to MeHg in drinking water [39].
Effects of MeHg on reproduction have been studied in nonhuman primates, showing diminished conception rates and increased incidences of abortions and stillbirths in Macaques treated for 4 months at 70mg⁄kg⁄day [36]. Our earlier study also confirmed that the prenatal administration of MeHg at 2.0 mg⁄kg⁄day from GD5 to till parturition produced adverse effects on developmental outcomes and high–teratogenic potential of MeHg [21]. Harada [37] reported altered physical growth of Japanese children exposed to MeHg in–utero. In the present study, the body weights of the pups were unaffected at birth and continued to be unaffected throughout the pre–weaning period with respective MeHg–treated groups. The body weight of female offspring increased in postnatal day dependent (PDN1–21) whereas bodyweight of male offspring increased only at PND21. In conclusion, prenatal administration of MeHg at dose of 1.5mg⁄kg⁄day produced adverse effects on developmental milestones, thereby confirming the high–teratogenic potential of MeHg.
In the present study, MeHg produced significant effects on the female offspring outnumbered the male offspring only in 1.5mg⁄ kg⁄day MeHg treatment group (Sex ratio: 0.59). However, Tanaka [40,41] reported that the females sometimes outnumbered the males in control mice (sex ratio: <0.75). It seems, therefore, that the effect on sex ratio is not caused by MeHg treatment. There was no significant difference between 0.5 and1.0mg⁄kg⁄day MeHg–treatment groups, as compared with controls in the number of pups per litter, male⁄female ratio, or the number of stillbirths. In the 1.5mg⁄kg⁄day MeHg–treatment group, however, the values of the viability index (i.e. percentage of pups surviving beyond PND4) were notably lower. However, outcomes from the present offspring’s morphological development data indicating the growth was retarded in the progeny of the each exposed groups, possibly due to the poor health of the mothers.
The reproduction success, were unaffected in 0.5 and 1.0 mg⁄ kg⁄day MeHg–treatment groups by measuring different gestational parameters. However, high dose of MeHg–treatment group resulted in 66.56% of resorption of the pups which is one of the most common abnormal signs observed in severe human cases of MeHg poisoning study along with gait abnormalities and ataxia [42]. However, in the present study we could not found gait abnormalities and ataxia in dam treated with 1.5mg⁄kg⁄day MeHg throughout the exposure period.
Alteration in the behavior of the mother is known to affect infant development and several drugs have been shown to disrupt elements of maternal behaviors [43]. Thus, any disturbance to maternal care or the delicate mother–pup relationship may explain different patterns of behaviors in the offspring rather than direct effects of prenatal exposure to a toxicant. The results of the present study suggested that control mothers [dam] spent more time involved in the pup–directed behaviors of nursing and licking and less time in nest–building during the first two postnatal weeks than dams treated with methyl mercury during gestation.
In conclusion, it will be interesting to see how the developmental and neurobehavioral effects of early life MeHg exposure are manifested throughout the life span of rodents. Thus, future studies will examine learning and memory of rats at the different stages of the life span, e.g., from early to young to old adulthood, following embryonic MeHg exposure. The results of the study confirmed the high–teratogenic potential of MeHg suggest to pay increased attention to MeHg concerning its exogenous exposure during pregnancy.
Acknowledgement
The authors acknowledge the Indian Council of Medical Research (ICMR), New Delhi, India for funding the ad–hoc Research Project No.2010–04390 and awarding Senior Research Fellow (SRF) to the author (DKD) under this project.
References
- Takeuchi T. Human effects of methylmercury as an environmental neurotoxicant. Blum K, Manzo L, editors. In: Neurotoxicology New York: Marcel Dekker. 1985; 345-367.
- Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, et al. Cognitive deicit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol. 1997; 19: 417-428.
- Eccles CU, Annau Z. Prenatal methyl mercury exposure: I. Alterations in neonatal activity. Neurobehav Toxicol Teratol. 1982; 4: 371-376.
- Eccles CU, Annau Z. Prenatal methyl mercury exposure: II. Alterations in learning and psychotropic drug sensitivity in adult offspring. Neurobehav Toxicol Teratol. 1982; 4: 377-382.
- Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006; 368: 2167-2178.
- Harris SB, Wilson JG, Printz RH. Embryotoxicity of methyl mercuric chloride in golden hamsters. Teratology. 1972; 6: 139-142.
- Hoskins BB, Hupp EW. Methylmercury effects in rat, hamster, and squirrel monkey. Environ Res. 1978; 15: 5-19.
- Khera KS. Teratogenic effects of methylmercury in the cat: note on the use of this species as a model for teratogenicity studies. Teratology. 1973; 8: 293-303.
- Fuyuta M, Fujimoto T, Hirata S. Embryotoxic effects of methylmercuric chloride administered to mice and rats during orangogenesis. Teratology. 1978; 18: 353-366.
- Yasuda Y, Datu AR, Hirata S, Fujimoto T. Characteristics of growth and palatal shelf development in ICR mice after exposure to methylmercury. Teratology. 1985; 32: 273-286.
- Ornaghi F, Ferrini S, Prati M, Giavini E. The protective effects of N-acetyl- L-cysteine against methyl mercury embryotoxicity in mice. Fundam Appl Toxicol. 1993; 20: 437-445.
- Sanchez DJ, Gomez M, Llobet JM, Domingo JL. Effects of meso-2,3- dimercaptosuccinic acid (DMSA) on methyl mercury-induced teratogenesis in mice. Ecotoxicol Environ Saf. 1993; 26: 33-39.
- Gomez M, Sanchez DJ, Colomina MT, Domingo JL, Corbella J. Evaluation of the protective activity of 2,3-dimercaptopropanol and sodium 2,3-dimercaptopropane-1-sulfonate on methylmercury-induced developmental toxicity in mice. Arch Environ Contam Toxicol. 1994; 26: 64- 68.
- Baker JT. Interactive effects of ish oil and methylmercury on the fatty acid proile of adult rat forebrain phospholipids. A Thesis Submitted to the Graduate Faculty of Auburn University in Partial Fulillment of the Requirements for the Degree of Master of Science Auburn, Alabama. 2007.
- Domingo JL. Metal-induced developmental toxicity in mammals: a review. J Toxicol Environ Health. 1994; 42: 123-141.
- Golub MS. Maternal toxicity and the identiication of inorganic arsenic as a developmental toxicant. Reprod Toxicol. 1994; 8: 283-295.
- Gandhi DN, Panchal GM. Effects of low-level arsenic exposure on the development of neurobehavioral toxicity in rats. Res J Environ Toxicol. 2011; 5: 348-358.
- Gandhi DN, Panchal GM, Patel KG. Developmental and neurobehavioural toxicity study of arsenic on rats following gestational exposure. Indian J Exp Biol. 2012; 50: 147-155.
- Gray DG. A physiologically based pharmacokinetic model for methyl mercury in the pregnant rat and fetus. Toxicol Appl Pharmacol. 1995; 132: 91-102.
- Cagiano R, De Salvia MA, Renna G, Tortella E, Braghiroli D, Parenti C, et al. Evidence that exposure to methyl mercury during gestation induces behavioral and neurochemical changes in offspring of rats. Neurotoxicol Teratol. 1990; 12: 23-28.
- Huot RL, Gonzalez ME, Ladd CO, Thrivikraman KV, Plotsky PM. Foster litters prevent hypothalamic-pituitary-adrenal axis sensitization mediated by neonatal maternal separation. Psychoneuroendocrinology. 2004; 29: 279- 289.
- Gandhi DN, Panchal GM, Dhull DK. Inluence of gestational exposure on the effects of prenatal exposure to methyl mercury on postnatal development in rats. Cent Eur J Public Health. 2013; 21: 30-35.
- Cernichiari E, Brewer R, Myers GJ, Marsh DO, Lapham LW, Cox C, et al. Monitoring methylmercury during pregnancy: maternal hair predicts fetal brain exposure. Neurotoxicology. 1995; 16: 705-710.
- Rossi AD, Ahlbom E, Ogren SO, Nicotera P, Ceccatelli S. Prenatal exposure to methylmercury alters locomotor activity of male but not female rats. Exp Brain Res. 1997; 117: 428-436.
- Rice DC. Evidence for delayed neurotoxicity produced by methylmercury. Neurotoxicology. 1996; 17: 583-596.
- Cuomo V, Ambrosi L, Annau Z, Cagiano R, Brunello N, Racagni G. Behavioural and neurochemical changes in offspring of rats exposed to methyl mercury during gestation. Neurobehav Toxicol Teratol. 1984; 6: 249-254.
- Gilbert SG, Burbacher TM, Rice DC. Effects of in utero methylmercury exposure on a spatial delayed alternation task in monkeys. Toxicol Appl Pharmacol. 1993; 123: 130-136.
- Gilbert SG, Rice DC, Burbacher TM. Fixed interval/ixed ratio performance in adult monkeys exposed in utero to methylmercury. Neurotoxicol Teratol. 1996; 18: 539-546.
- Fredriksson A, Dencker L, Archer T, Danielsson BR. Prenatal coexposure to metallic mercury vapour and methylmercury produce interactive behavioural changes in adult rats. Neurotoxicol Teratol. 1996; 18: 129-134.
- Reed MN, Newland MC. Prenatal methylmercury exposure increases responding under clocked and unclocked ixed interval schedules of reinforcement. Neurotoxicol Teratol. 2007; 29: 492-402.
- Reed MN, Banna KM, Donlin WD, Newland MC. Effects of gestational exposure to methylmercury and dietary selenium on reinforcement eficacy in adulthood. Neurotoxicol Teratol. 2008; 30: 29-37.
- Falluel-Morel A, Sokolowski K, Sisti HM, Zhou X, Shors TJ, Dicicco-Bloom E. Developmental mercury exposure elicits acute hippocampal cell death, reductions in neurogenesis, and severe learning deicits during puberty. J Neurochem. 2007; 103: 1968-1981.
- Rice DC. Effects of pre- plus postnatal exposure to methylmercury in the monkey on ixed interval and discrimination reversal performance. Neurotoxicology. 1992; 13: 443-452.
- Goulet S, Doré FY, Mirault ME. Neurobehavioral changes in mice chronically exposed to methylmercury during fetal and early postnatal development. Neurotoxicol Teratol. 2003; 25: 335-347.
- Onishchenko N, Tamm C, Vahter M, Hökfelt T, Johnson JA, Johnson DA, et al. Developmental exposure to methylmercury alters learning and induces depression-like behavior in male mice. Toxicol Sci. 2007; 97: 428-437.
- Burbacher TM, Mohamed MK, Mottett NK. Methylmercury effects on reproduction and offspring size at birth. Reprod Toxicol. 1987; 1: 267-278.
- Vorhees CV. Behavioral effects of prenatal methylmercury in rats: a parallel trial to the Collaborative Behavioral Teratology Study. Neurobehav Toxicol Teratol. 1985; 7: 717-725.
- Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995; 25: 1-24.
- Piedraita B, Erceg S, Cauli O, Felipo V. Developmental exposure to polychlorinated biphenyls or methylmercury, but not to its combination, impairs the glutamate-nitric oxidecyclic gmp pathways and learning in 3-months-old rats. Neuroscience. 2008; 154: 1408-1416.
- Sitarek K, Gralewicz S. Early developmental effects of separate or combined perinatal exposure to methylmercury (MeHg) and 2,2’,4,4’,5,5’-hexachlorobiphenyl (PCB 153) in the rat. Int J Occup Med Environ Health. 2009; 22: 89-105.
- Tanaka T. Reproductive and neurobehavioural effects of bis(2-ethylhexyl) phthalate (DEHP) in a cross-mating toxicity study of mice. Food Chem Toxicol. 2005; 43: 581-589.
- Tanaka T. Reproductive and neurobehavioural effects of thiabendazole administered to mice in the diet. Food Addit Contam. 2001; 18: 375-383.
- World Health Organization: Environmental Health Criteria 101: Methylmercury. Published under the joint sponsorship of the United Nations Environmental Programme, the International Labour Organization, and the World Health Organisation. World Health Organisation, Geneva. 1990; 140.
- Laviola G, Sedowoia K, Innes J, Clayton R, Manning A. Genetic differences in maternal behaviour patterns in mice administered phenobarbital during pregnancy. Psychopharmacology (Berl). 1990; 102: 383-390.