Research Article
Austin J Pharmacol Ther. 2014; 2 (5). 1028
Effect of Nobiletin on Diabetic Neuropathy in Experimental Rats
Parkar N1,2 and Addepalli V2*
1Department of Pharmacology, Bhanuben Nanavati College of Pharmacy, India
2Department of Pharmacology, NMIMS University, India
*Corresponding author: : Addepalli V, Department of Pharmacology, SPP School of Pharmacy and Technology Management, NMIMS University, Vile Parle (W), Mumbai, 400 056, India
Received: June 11, 2014; Accepted: July 31, 2014; Published: Aug 04, 2014
Abstract
Diabetic neuropathy (DN) is a microvascular complication of diabetes that leads to allodynia, nerve conduction slowing and progressive sensory loss. Despite the prevalence and severity of DN, currently there is no treatment available for DN. Objective of present study was to evaluate efficacy of nobiletin in the treatment of diabetic neuropathy in rats. Diabetes was induced in rats using a single dose of streptozotocin (50mg/kg i.p.). Four weeks after the induction of diabetes, treatment with nobiletin (10mg/kg and 25mg/kg) was given for further four weeks. At the end of eight weeks, the nociception latency was measured using hot plate and tail flick test. Further the nerve conduction velocity was measured and the histopathology of the sciatic nerve was studied. The results indicated that nobiletin caused improvement in nerve conduction velocity at a dose of 25mg/kg (42.58 ± 2.02** vs. control 30.00 ± 1.51) and sciatic nerve histology. The nociception latency also was improved. Thus, the study showed efficacy of nobiletin in the treatment of diabetic neuropathy in rats.
Keywords: Diabetic neuropathy; Nobiletin; Streptozotocin
Abbreviations
DM: Diabetes Mellitus; DN: Diabetic Neuropathy; STZ: Streptozotocin; BSL: Blood Sugar Levels; PKC: Protein Kinase C; PARP: Poly ADP Ribose Polymerase; MMP-2: Matrix Metalloproteinase–2; MMP-9: Matrix Metalloproteinase–9; GOD/POD: Glucose Oxidase-peroxidase; NORMO: Normal; NOB: Nobiletin; MINO: Minocycline; MNCV: Motor Nerve Conduction Velocity; SD: Standard Deviation; ANOVA: Analysis of Variance; CMC: Carboxy Methyl Cellulose
Introduction
Diabetes mellitus (DM) is a chronic metabolic condition affecting a large majority of population worldwide. Several factors like changing patterns of diet and physical activity, sedentary lifestyles and increase in obesity are responsible for the increasing incidence of diabetes. People with prolonged hyperglycemia develop several vascular complications that are responsible for increase in the ratio of morbidity and mortality of affected individuals. Diabetic peripheral neuropathy (DN) is a multifaceted and potentially severe complication of diabetes affecting more than 50% of diabetic individuals and is the leading cause of non-traumatic amputation and anatomic failure [1,2]. Hyperglycemia triggers various alternate pathogenetic pathways for the circulating glucose like aldol reductase [3], non-enzymatic glycation [4], protein kinase C (PKC) [5], mitogen activated protein kinases [6] and poly ADP ribose polymerase (PARP) [7] to name a few. The activation of these alternate pathways leads to the production of several toxic metabolites that cause deleterious effects on different biological systems of the affected individuals. Early disorders of nerve function include slowing in nerve conduction velocity followed by axonal degeneration, paranodal demyelination and loss of myelinated fibers [8]. Long term neuropathy leads to more severe effects like severe pain, loss of sensation, foot ulceration and amputation, burns, infection, cellulites, sleep disorder, impaired daily functioning, mood disorders, gangrene, involvement of different systems such as cardiovascular, gastrointestinal and reproductive systems [9,10].
Despite efforts to make an early diagnosis and to stop the progression of DN, currently very few drugs are available to cure this disease and the others only provide symptomatic relief [11]. Several combination strategies of drugs with natural molecules like vitamin E have been tried to reduce the neuropathic pain [12]. Report of ethnobotany suggested that about 800 medicinal plants possess antidiabetic potential and the bioactive compounds such as glycosides, alkaloids, terpenoids and flavonoids (phenols) are effective drugs both in preclinical and clinical studies [13,14]. Flavonoids are a class of secondary metabolites from natural sources that studied for their various activities [15]. Nobiletin is a flavonoid present in peels of citrus fruits and is found to be a potential molecule possessing several biological activities including inhibition of MMP-2 and MMP-9 in cancer cells [16]. Since MMPs are involved in the pathogenesis of diabetic vascular complications, we hypothesize that nobiletin can be a potential molecule in ameliorating the diabetic complications. The present study aimed at evaluating the effect of nobiletin in diabetic neuropathy using STZ diabetic rats.
Materials and Methods
Chemicals and drug solutions
All the reagents and chemicals used for the study were of analytical grade. Nobiletin was purchased from Baoji Hongyuan Biotechnology Co. Ltd. (Baoji City, China). Streptozotocin (STZ) was purchased from Sigma Aldrich (USA). Minocycline gift sample was available from US Vitamins (Mumbai, India). All the biochemical diagnostic kits were procured from Erba Diagnostics, Mumbai, India. Nobiletin and Minocycline were suspended in freshly prepared 0.5% CMC (Carboxy methyl cellulose) solution before use. STZ was dissolved in freshly prepared ice cold Citrate Buffer (pH 4.5) before use.
Animals and Experimental Protocol
Wistar rats (Male, 190–240g) were used for the study. The animals were purchased from Haffkine Institute, Lower Parel (Mumbai, India). Animals were caged in clean environment and maintained at a temperature of 25 ± 1°C, RH 45-55%. 12 hr light/ dark cycle was maintained in the animal house and the animals had free access to food and water ad libitum.
For induction of diabetes, the animals were fasted overnight for 12 hrs. Diabetes was induced with a single dose of STZ (50mg/kg, i.p.) freshly dissolved in ice cold citrate buffer pH 4.5. After STZ injection, the animals were allowed free access to feed and water. Diabetes was checked after 48 hrs by estimating the blood sugar levels (BSL) using a GPD/POD kit. The animals with a BSL of >300mg/kg were considered diabetic and used further for the study.
The doses of nobiletin were calculated on the basis of results of earlier reported in vitro studies and pharmacokinetic data. The doses calculated were such that they would produce a plasma concentration of nobiletin required to inhibit MMP-2 and MMP-9. In the eight week study period, the treatment phase of the study was the last four weeks daily p.o. Grouping of animals was done randomly and animals were divided into five groups of six animals each. Group I served as normal control (NORMO), Group II served as Vehicle Control and received 0.5% CMC solution (1 ml/kg); Group III and IV were the treatment groups and received nobiletin (NOB) at a dose of 10mg/ kg (NOB10) and 25mg/kg (NOB25) respectively; Group V served as standard and received minocycline (MINO) at a dose of 50mg/kg. The BSL and body weight of the animals was estimated weekly during the experiment.
Thermal nociceptive response
The thermal nociceptive response of the animals was measured by determining the hot plate and tail flick latency of the experimental animals on a weekly basis till the end of eight week study protocol. For hot plate latency , the animals were placed into a glass cylinder on a hot plate (IITC, Inc., Model 35-D) adjusted to 54.5 ± 1°C to induce thermal hyperalgesia. The time in seconds, from placing the rat on the hot plate to either licking of the hind paw or attempting to jump out of the cylinder cage was recorded for each rat. The cut off time set for the test was 30 s. The tail flick latency was measured using a 20 amp analgesiometer (INCO, India). A 6 amp current passed through the naked nichrome wire. The reaction cut off time was set at 10s. The heat source and the tail skin were placed 1 cm apart and the tail flick latency was measured in seconds.
Motor Nerve Conduction Velocity (MNCV)
At the end of eight week study period, the motor nerve conduction velocity of the animals was measured in the sciatic-posterior tibial conducting system using a data acquisition system (Iworx data acquisition system, USA) as reported previously. The rats were anesthetized using pentobarbital sodium (45mg/kg, i.p.). The body temperature of the animal was measured using a rectal probe and maintained at 37 ± 1°C throughout the procedure. MNCV was measured in anesthetized rats by stimulating the sciatic and the tibial nerve using 26 gauge bipolar needle electrodes with 8 V single stimuli [17,18]. The receiving electrodes were placed on the foot muscle. MNCV was calculated by subtracting the distal latency from the proximal latency, and the result was divided into the distance between the stimulating and recording electrode.
Sciatic nerve histology
The rats were sacrificed and the sciatic nerve was carefully removed from each animal. The nerve tissue was washed with saline and fixed in 10% formalin for histopathological study. Slides were prepared by embedding the nerve in paraffin and staining with hematoxylin and eosin. The histology of sciatic nerves was studied using a bright field microscope and images were taken for reference.
Statistics
All data were expressed as mean ± S.D. Statistical analysis was performed using Graph Pad Prism (version 4.0, Gra.ph Pad Inc., San Diego, (CA) software. For multiple comparisons, one-way analysis of variance (ANOVA) was used. In case ANOVA shows significant differences, post-hoc analysis was performed with Dunnet test, p<0.05 was considered statistically significant.
Results
The experimental animals showed hyperglycemia and marked reduction in bodyweights on induction of diabetes using STZ (Table 1). Diabetes produced four fold increases in blood sugar levels of the animals. The increase in BSL was consistent throughout the study period. Four week treatment with nobiletin or minocycline did not produce any effect on the body weight and blood sugar levels of the animals (Table 1).
Groups
BSL (mg/dL)
Body weights (g)
NORMO
114.33 ± 10.10**
233.33 ± 8.45*
DB + 0.5% CMC
442.03 ± 30.98
168.33 ± 11.74
NOB10
439.08 ± 19.23
175 ± 11.24
NOB25
453.63 ± 27.41
172 ± 14.99
MINO
465.67 ± 28.52
178.17 ± 13.83
Table 1: Effect of four week treatment with NOB10, NOB25 and MINO on body weights and blood sugar levels of experimental rats. Values are expressed as mean ± SD (n = 6 for all groups).
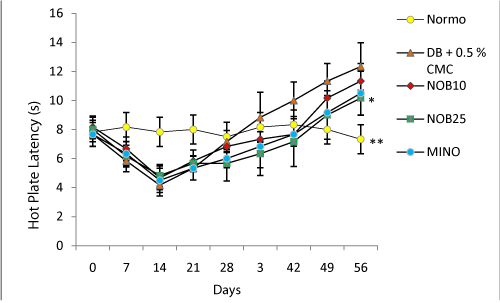
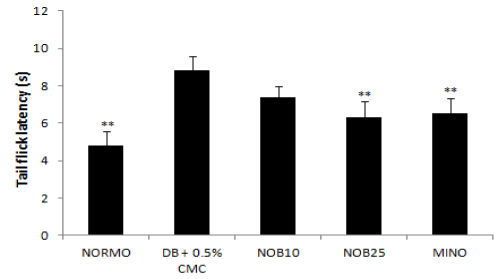
The hot plate latency of STZ diabetic rats was higher compared to normal group. Weekly analysis of hot plate latency indicated that there was a dip in the latency around 14 days from the induction of diabetes which normalised and increased further till the eighth week on progression of diabetes. Four week treatment with nobiletin (NOB25) significantly reduced the latency compared to the control group (Figure 1). Similarly, in tail flick latency, an early phase of thermal hyperalgesia was seen 1–3 weeks after STZ treatment. It was observed that hyperalgesia was followed by a second phase of apparently normal tail flick latency and then a final phase of hypoalgesia at the eighth week. Treatment with nobiletin (NOB25) reversed tail flick latency significantly at the end of eight weeks when compared to diabetic control (Figure 2).
Figure 1: Effect of four week treatment with NOB10, NOB25 and MINO on hot plate latency in experimental animals. Values are expressed as mean ± SD (n=6 for all groups). *P < 0.05, **P<0.01 versus vehicle-treated diabetic rats (n = 6 for all groups)
Figure 2: Effect of four week treatment with NOB10, NOB25 and MINO on tail flick latency in experimental animals. Values are expressed as mean ± SD (n=6 for all groups). **P<0.01 versus vehicle-treated diabetic rats (n = 6 for all groups).
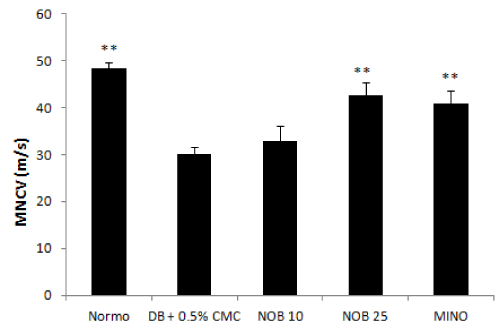
The MNCV values of normal and diabetic group indicated that diabetes compromised the motor nerve conduction velocity of the experimental animals. A significant decrease in MNCV was observed after eight weeks of diabetes induction. MNCV in diabetic rats was 30 ± 1.51 m/s as compared to control group (48.28 ± 1.34/s m/s). Four week treatment with nobiletin (NOB25) produced a significant reversal of the motor nerve conduction velocity when compared to diabetic control. NOB10 improved the MNCV but the change was not significant (Figure 3).
Figure 3: Effect of 4 week treatment with NOB10, NOB25 and MINO on motor nerve conduction velocity. Values are expressed as mean ± SD (n=6 for all groups). **P<0.01 versus vehicle treated diabetic rats (n = 6 for all groups)
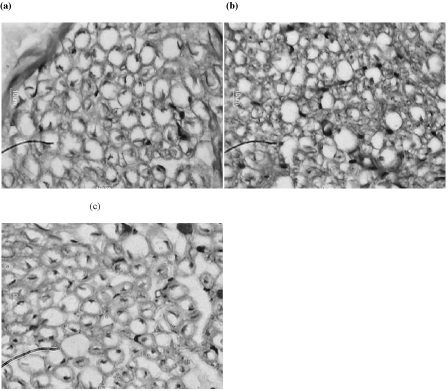
The microscopic preparations of sciatic nerves indicated normal histology in normoglycemic group. In diabetic rats, there was an increase in the connective tissue around the epineurium with slight disruption of perineurium. An endoneurial edema with dissociation of nerve fibers was observed. Some of myelinated fibers exhibited axonal degeneration and some of them were demyelinated. The NOB25 treated group showed reduction in connective tissue layer, composed fascicles and all other abnormalities compared to control. Treatment with NOB10 did not produce a major difference in the sciatic nerve histology when compared to control (Figure 4).
Figure 4: Representative images of histology of sciatic nerve with Hematoxylin and Eosin staining from different groups. (a) Normal (b) DB + 0.5% CMC and (c) NOB25.
Discussion
Peripheral neuropathy is a frequent complication of diabetes that ultimately leads to increase in mortality. The present study aimed at studying the effect of long term administration of nobiletin on diabetic neuropathy in STZ-diabetic rats. STZ induced diabetes induced hyperglycemia in 48 hrs which prolonged till the end of the study. There was significant reduction in body weight of the animals. Treatment with nobiletin did not alter the blood sugar levels or bodyweights of the animals over the eight week study period. This study demonstrates protective effect of nobiletin, a MMP-2 and MMP-9 inhibitor in experimental diabetic neuropathy.
Experimental diabetic neuropathy is usually marked by impaired nerve conduction and nerve blood flow deficits along with neuropathic pain and abnormal sensory perceptions [19,20]. We observed significant reduction in MNCV, hyperalgesia and allodynia in diabetic Rats on treatment with nobiletin. Neuropathic pain and abnormal sensory perceptions are a phenomenon related to DN. Assessment of behavioral responses to external stimuli in diabetic animal provides valuable information regarding the mechanisms of abnormal sensation and pain associated with diabetes [21]. In the present study, we assessed sensory responses to thermal stimuli using tail flick and hot plate test. Altered nociception was seen in STZ induced diabetes. STZ-induced hyperalgesia causes a variety of pathophysiological symptoms that can lead to altered nociceptive responses tested in various animal models [22-24]. Eight week diabetic rats demonstrated reduced latencies in both hot plate and tail flick tests. As evident from the results of the present study, four week treatment with nobiletin improved thermal hyperalgesia in experimental animals. Reduced tail-flick latencies were partially corrected by nobiletin treatments. Nobiletin showed a significant antinociceptive effect in diabetic rats with degree of antinociception being more evident in the higher dose group (NOB25).
Our morphological study of the sciatic nerve indicated that diabetes induced histological damage of the sciatic nerve fibers, endoneurial edema and axonal degeneration with occasional secondary segmental demyelination. These results are in accordance with the earlier studies [25-27]. MMP-2 and MMP-9 are known to be involved in the degradation of the ECM components of the basement membrane. They act on different substrates including type IV collagen, fibronectin, and elastin and denatured interstitial collagen [28]. Increased level of MMP-2 and MMP-9 causes degradation of ECM and thickening of basement membrane [29]. ECM degradation may alter the structure of arteries and lead to their constriction. This may further lead to nerve injury causing ischemia of nerve tissue and ultimately neural cell death. Nobiletin is well known for its inhibitory action on MMP-2 to MMP-9. In our study, this inhibition of MMP- 2 and MMP-9 may have resulted in amelioration of condition of neurons which is evident from histology. This amelioration in nerve histology may further have resulted in attenuation of other parameters like MNCV, hot plate latency and tail flick latency.
In the present study, it was evident that streptozotocin induced diabetes in rats developed decrease in sciatic motor nerve conduction velocity and nociception. Treatment with nobiletin improved the MNCV in four week treated animals. Histology of the sciatic nerve showed improved nerve structure in the treatment group compared to control. These effects occurred in the presence of unaltered hyperglycemia. Thus it can be stated that nobiletin acts in diabetic neuropathy by other than glucose lowering mechanism. Most possible mechanism for this amelioration may be the MMP-2 and MMP-9 inhibitory action of nobiletin.
Conclusion
In conclusion, treatment with nobiletin ameliorated diabetic neuropathy in STZ rats which was evident by improved nociceptive latency and nerve conduction velocity. Thus, the results of the present study lead to suggest the protective role of nobiletin in STZ induced diabetic neuropathy.
Acknowledgement
The authors thank SPP School of Pharmacy and Technology Management, NMIMS for their support and providing the necessary facilities.
References
- Low PA, Dotson RM. Symptomatic treatment of painful neuropathy. See comment in PubMed Commons below JAMA. 1998; 280: 1863-1864.
- Vinik AI, Park TS, Stansberry KB, Pittenger GL. Diabetic neuropathies. See comment in PubMed Commons below Diabetologia. 2000; 43: 957-973.
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. See comment in PubMed Commons below Nature. 2001; 414: 813-820.
- Wada R, Yagihashi S. Role of advanced glycation end products and their receptors in development of diabetic neuropathy. See comment in PubMed Commons below Ann N Y Acad Sci. 2005; 1043: 598-604.
- Sima AA. New insights into the metabolic and molecular basis for diabetic neuropathy. See comment in PubMed Commons below Cell Mol Life Sci. 2003; 60: 2445-2464.
- Purves T, Middlemas A, Agthong S, Jude EB, Boulton AJ, Fernyhough P, et al. A role for mitogen-activated protein kinases in the etiology of diabetic neuropathy. See comment in PubMed Commons below FASEB J. 2001; 15: 2508-2514.
- Soriano GF, Pacher P, Mabley J, Liaudet L, Szabo C. Rapid reversal of the diabetic endothelial dysfunction by pharmacological inhibition of poly (ADP-ribose) polymerase. Circulation Research. 2001a; 89: 684–691.
- Sugimoto K, Murakawa Y, Sima AA. Diabetic neuropathy--a continuing enigma. See comment in PubMed Commons below Diabetes Metab Res Rev. 2000; 16: 408-433.
- Booya F, Bandarian F, Larijani B, Pajouhi M, Nooraei M, Lotfi J, et al. Potential risk factors for diabetic neuropathy: a case control study. See comment in PubMed Commons below BMC Neurol. 2005; 5: 24.
- Pajouhi M, Shaban Nejad Khas Z, Mohajeri Tehrani M. Evaluation and prevention of diabetic neuropathy. TUMJ. 2007; 65: 1–6.
- Hosseini A, Mohammad A. Diabetic Neuropathy and Oxidative Stress: Therapeutic Perspectives. Oxd Med Cell Longevity. 2013; 15.
- M.G. Rajanandh, Sourabh Kossy, G Prathiksha. Assessment of antioxidant supplementation on the neuropathic pain score and quality of life in diabetic neuropathy patients - A Randomized controlled study. “Pharmacological Reports”. 2014; 66: 44-48.
- Alarcon-Aguilara FJ, Roman-Ramos R, Perez-Gutierrez S, Aguilar-Contreras A, Contreras-Weber CC, Flores-Saenz JL, et al. Study of the anti-hyperglycemic effect of plants used as antidiabetics. See comment in PubMed Commons below J Ethnopharmacol. 1998; 61: 101-110.
- Loew D, Kaszkin M. Approaching the problem of bioequivalence of herbal medicinal products. See comment in PubMed Commons below Phytother Res. 2002; 16: 705-711.
- Shashank K, Abhay KP. Chemistry and Biological Activities of Flavonoids: An Overview. The Scientific World Journal. 2013; 16.
- Yi-Chieh L, Tsan-Hwang C, Jung-Shin L, Jiun-Hwan C, Yi-Chen L, Yao F, et al. Nobiletin, a citrus flavonoid, suppresses invasion and migration involving FAK/PI3K/Akt and small GTPase signals in human gastric adenocarcinoma AGS cells. Mol Cell Biochem. 2011; 347:103–115.
- Sharma SS, Sayyed SG. Effects of trolox on nerve dysfunction, thermal hyperalgesia and oxidative stress in experimental diabetic neuropathy. See comment in PubMed Commons below Clin Exp Pharmacol Physiol. 2006; 33: 1022-1028.
- Bhatt LK, Veeranjaneyulu A. Minocycline with aspirin: a therapeutic approach in the treatment of diabetic neuropathy. See comment in PubMed Commons below Neurol Sci. 2010; 31: 705-716.
- Sayyed SG, Kumar A, Sharma SS. Effects of U83836E on nerve functions, hyperalgesia and oxidative stress in experimental diabetic neuropathy. See comment in PubMed Commons below Life Sci. 2006; 79: 777-783.
- Stevens MJ, Li F, Drel VR, Abatan OI, Kim H, Burnett D, et al. Nicotinamide reverses neurological and neurovascular deficits in streptozotocin diabetic rats. See comment in PubMed Commons below J Pharmacol Exp Ther. 2007; 320: 458-464.
- Calcutt NA. Modeling diabetic sensory neuropathy in rats. See comment in PubMed Commons below Methods Mol Med. 2004; 99: 55-65.
- Courteix C, Bardin M, Chantelauze C, Lavarenne J, Eschalier A. Study of the sensitivity of the diabetes-induced pain model in rats to a range of analgesics. See comment in PubMed Commons below Pain. 1994; 57: 153-160.
- Hounsom L, Tomlinson DR. Does neuropathy develop in animal models? See comment in PubMed Commons below Clin Neurosci. 1997; 4: 380-389.
- Kamei J, Zushida K, Morita K, Sasaki M, Tanaka S. Role of vanilloid VR1 receptor in thermal allodynia and hyperalgesia in diabetic mice. See comment in PubMed Commons below Eur J Pharmacol. 2001; 422: 83-86.
- Dick PJ, Karnes JL, Lais A, Lofgren EP, Stevens JC. Pathologic alterations of the peripheral nervous system of humans in Peripheral Neuropathy (Dick PJ, Thomas PK, Lambert EH, Bunge R, editors.). W.B. Saunders, Philadelphia. 1984; 760–870.
- Sima AAF, Nathaniel V, McEwen TAJ, Greene DA. Histopathological heterogeneity of neuropathy in insulin-dependent and noninsulin dependent diabetes, and demonstration of axoglial dysjunction in human diabetic neuropathy. J. Clin. Invest. 1988; 81: 349–364.
- Sima AAF, Prashar A, Nathaniel V, Bril V, Werb MR, Greene DA. Overt diabetic neuropathy: Repair of axo-glial dysjunction and axonal atrophy by aldose reductase inhibiton and its correlation to improvment in nerve conducton velocity. Diabetic Med. 1993; 10: 115–121.
- Rundhaug JE. Matrix metalloproteinases, angiogenesis, and cancer: commentary re: A. C. Lockhart et al., Reduction of wound angiogenesis in patients treated with BMS-275291, a broad spectrum matrix metalloproteinase inhibitor. Clin. Cancer Res., 9: 00-00, 2003. See comment in PubMed Commons below Clin Cancer Res. 2003; 9: 551-554.
- Martin A, Komada MR, Sane DC. Abnormal angiogenesis in diabetes mellitus. See comment in PubMed Commons below Med Res Rev. 2003; 23: 117-145.