Review Article
Austin J Pharmacol Ther. 2014; 2 (6).1036
Pharmaceutical Strategies for the Topical Dermal Delivery of Peptides/Proteins for Cosmetic and Therapeutic Applications
Badenhorst Travis, Svirskis Darren and Wu Zimei*
Department of Pharmaceutics, University of Auckland, New Zealand
*Corresponding author: : Zimei Wu, Department of Pharmaceutics, School of Pharmacy, University of Auckland, Private Bag 92019, Auckland 1142, New Zealand
Received: July 10, 2014; Accepted: August 22, 2014; Published: August 27, 2014
Abstract
Bioactive peptides and proteins have attracted great attention in the cosmetic industry for the development of new cosmetic products to reverse the signs of ageing (anti-ageing treatment). These have also led to new therapeutic agents for clinical applications associated with wound healing; fibrosis, excessive scarring, and inflammation. One of the mostly used administration routes is by topical delivery. However, the skin is an intrinsic barrier to peptide/ protein transportation across the stratum corneum. Therefore the key function of a topical delivery system is to enhance the dermal permeability of the peptide/protein enabling it to cross the epidermis and retain in the dermis. The development of pharmaceutical strategies that employ sophisticated carrier systems such as elastic vesicles (such as liposome’s, and noisome), nano fibers, micro-emulsions and conjugation with cell-penetrating peptides may hold the key to enhancing peptide/protein dermal delivery. The aim of this paper is to review noninvasive topical delivery systems that promote dermal delivery of peptides/proteins for either cosmetic purposes or clinical therapeutic applications.
Keywords: Dermal delivery; Anti-ageing peptide/protein; Cosmetics; Carrier systems; Permeability; Wound healing
Introduction
General ageing is characterized by accumulation of molecular damage and progressive failure of maintenance and repair. Skin ageing has many visible and measurable characteristics, including loss of elasticity, dermo-epidermal junction flattening, thinning, decreased barrier function, irregular keratinization leading to a yellow skin tone and decreased skin lipids [1]. In women, skin ageing predominantly takes place after the age of 40 and will be noticed as a reduction in thickness and a decrease of collagen and elastin of between 1 and 2% per year [2]. The loss of elasticity in the dermis is associated with increased collagen cross-linking with age [3]. To slow these ageing processes, anti-ageing cosmetic peptides/ proteins have been produced and have attracted great attention in the cosmetic industry [4]. The targets of these peptides often involve the modulation of collagen production, cell proliferation, inflammation, cell migration, angiogenesis and melanogenesis [5]. Whilst these cosmetic targets are valid, clinical applications of peptides and proteins including treatment of burns [6], reducing inflammation [7] and wound healing [8] have an increasing significant place.
A peptide is an organic compound consisting of two or more amino acids in which the carboxyl group of one is linked to the amino group of another [9]. The distinction between peptides and proteins lies in the size and length of the amino acid chain(s). Compounds with sequences exceeding 50 amino acids are termed proteins. Proteins can be categorized by their function; structural proteins are the building blocks of tissue, such as collagen and elastin. Peptides, similarly to proteins, are biologically active and are often components or precursors of larger proteins such as collagen. Topically administered peptides, such as glycyl-L-histidyl-L-lysine–copper [10], can reverse the signs of ageing [11,12]. These are classified as an enzyme-inhibiting, signaling, neurotransmitter-inhibiting or carrier peptides according to their mechanism of action (Table 1) [5].
Class
Mechanism of anti-ageing action
Example
Carrier peptides
To facilitate the dermal delivery of trace elements, such as copper.
Necessary for enzymatic processes, wound healing, and stabilization of these molecules [5,114,115].
Copper Glycyl-L-Histidyl-L-Lysinetripeptide (GHK) [114]
Signaling peptides
To elicit a function directly though binding to a receptor [116].
To stimulate fibroblast production of collagen, proliferation of elastin, glycosaminoglycans, proteoglycan and fibronectin [114].
Syn®-coll (Palmitoyl Tripeptide-5)
Decorinyl™ (a tetrapeptide) [5]
Neurotransmitter-inhibiting peptides
To decrease facial muscle contraction, and consequently lines, by raising the minimum threshold for muscle activity.
Argireline®,Vialox® and Syn®-ake [5]
Enzyme-inhibiting
Peptides
To directly or indirectly inhibit an enzyme related to ageing process.
Glycine soya protein (Preregen®) [117,118] and Sericin [5]
Table 1: Cosmetic peptides type and function.
The dermis provides nutritional and functional support to the epidermis and contains cells, fibers and amorphous ground substance [13]. In normal human skin, the structural proteins elastin and collagen make up 2-4% and 70–80% respectively of the dry weight of the dermis [14]. Over time these structural proteins deteriorate resulting in cutaneous signs of ageing. This layer has also been identified as being critical in wound healing [15] and maintenance of normal healthy skin [16]. The dermis is therefore the target in both cosmetic and clinical applications where the control of cell differentiation, proliferation and stimulation of collagen and elastin are important [17]. To gain effect for topical cosmetic and clinical applications, delivery to the site of action in the dermis is vital.
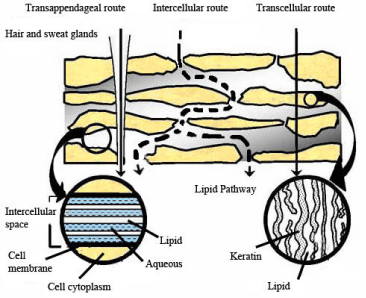
Topical application of cosmetic peptides firstly requires the peptide to diffuse to the site of action through one of three common pathways (Figure 1) [18]. Application also requires there to be no incidence of systemic activity [11]. Systemic activity can be minimized by promoting movement of the bioactive into the skin but not through it. The skin is however, an effective barrier to the transport of compounds that are charged, hydrophilic and have relatively high molecular weights, such as peptides/proteins [19,20]. There are three common approaches that have been employed to aid in the dermal delivery of peptide/proteins: 1) chemical [21] and physical penetration enhancers may be used to transiently modify stratum corneum permeability, 2) chemical modification of the peptide/protein [22] and 3) formulation modification might be performed to render the peptide molecule more permeable [23]. The last two strategies involve chemical derivatisation or encapsulation into a lipophilic core [24]. This paper reviews these pharmaceutical strategies to enhance dermal delivery of peptides/proteins using a topically applied formulation.
Figure 1: A representation of the epidermis showing the three pathways for diffusion of compounds across and into the adjacent dermis, adapted from Fang et al. [18].
Pharmaceutical Strategies to Enhance Dermal Delivery of Peptides/Proteins
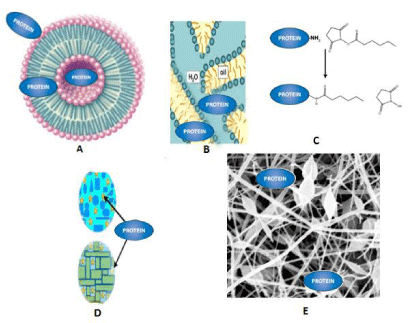
Pharmaceutical strategies are summarized in Table 2. Whilst there is an abundance of data in the literature describing the dermal delivery of small drug molecules, here we focus on the dermal delivery of peptides/proteins to demonstrate the effectiveness of the discussed delivery systems. An illustration is provided (Figure 2) to better the understanding of the concepts in this article.
Figure 2: Pharmaceutical strategies to enhance dermal delivery of peptide/ proteins (A) vesicles-liposome, niosome, ethosome (B) micro-emulsion, (C) conjugation (D) solid lipid and nano-structures carriers, (E) electrospun fiber mats.
Pharmaceutical strategy
Peptide/protein
In vitro/vivo model
Outcome
Reference
Chemical penetration enhancers
ACSSSPSKHCG
Insulin
Abdominal rat skin
Serum levels of Insulin detected
Chen et al. [32]
Human growth hormone
Abdominal rat skin
Elevated serum levels of HGH detected
Chen et al. [32]
Haptide
Recombinant melanoma protein
Mouse ear skin
At least a 2 fold increase in serum levels compared with control
Frankenburg et al. [33]
Trypsin
Bovine insulin
Shaved mouse skin
A 5.2 fold improvement in permeation, dose related
Hou et al. [34]
Ethanol and terpene
Thyrotropin releasing hormone
Human epidermis
Increase of penetration (1.6 + 0.02 µg /cm2/h with penetration enhancer vs 0.92 + 0.03 µg/cm2/h without)
Magnusson et al. [31]
Physical penetration enhancers
Iontophoresis
LHRH
Human skin
Flux increase from 0.006 ± 0.004 µg /cm2/h using AC current to 9.87 ± 4.91 μg /cm2/h using pulsed DC current
Raiman et al.[35]
Electroporation
Cyclosporine A
Rat skin
60 fold increase in permeation compared with passive delivery
Wang et al. [36]
Sonophoresis
Insulin
Porcine skin
Significant reduction in glucose levels over control (-72 ± 5 vs 31 ± 21 mg/dL)
Park et al. [37]
Micro-emulsions
Desmopressin
Human skin
Significant improvement of total amount absorbed into deeper tissues
Getie et al. [63]
anti-TNF monoclonal antibodies
Mouse foot
Reduced inflammation, distal skin penetration, no systemic absorption measurable
Himes et al. [7]
Encapsulation
Liposome
Interferon-2a-b
Guinea pig
Reduced wound contraction and collagen expression
Ghahary et al.[75]
Interferon-a
Viable human skin
2 fold Increase in skin deposition
Foldvari et al.[44]
Niosome
Human tyrosinase plasmid
Abdominal rat skin
Enhancement of transdermal absorption
Manosroi et al [94]
Ethosome
Low molecular weight Heparin
Mouse skin
No skin permeation seen in solution. Ethosomes resulted in skin delivery
Song et al. [119]
Ovalbumin antigen peptide
Piglet skin
Significant improvement in skin permeation over liposomes and solution
Rattanapak et al. [120]
Transfersome
Low molecular weight Heparin
Mouse skin
A 2.5 fold increase in Heparin delivered to skin over ethosomes
Song et al. [119]
Chemical modification
Arginine oligomers
Cyclosporine A
Mouse skin
Delivered into the skin (fluorescence microscopy)
Rothbard et al. [54]
Cyclosporine A
Human skin
Delivered into the skin (fluorescence microscopy)
Rothbard et al. [54]
Low molecular weight protamine
Epidermal Growth Factor
Mouse skin
Acceleration on wound closure, permeation 11 times higher than control
Choi et al. [121]
Electrospun fiber mats
Polycarbonate terpolymers
P12
Porcine wound model
Quick release, no inflammatory response, non-toxic
Macri et al. [6]
Polyethylene glycol polylactide
fibroblast growth factor
Rat wound model
Burst release, sustained release over 4 weeks, enhanced proliferation of fibroblasts
Yang et al. [122]
Solid Lipid Particle
SLN
Cyclosporine A
Abdominal rat skin
A 7.4 fold increase in skin permeation was seen over an oil control
Kim et al. [123]
NLC
Human epidermal growth factor
Rat wound model
Wound area improved over control (96.65 vs 82.32)
Gainza et al. (105)
Combination approaches
Biphasic vesicles
Insulin
Abdominal rat skin
Serum levels increased and a 43.7% decrease in blood glucose was observed
King et al. [112]
Interferon alpha
Human skin
Clinically significant levels were delivered with marked therapeutic effects in patients
Foldvari et al. [113]
Table 2: Summary of pharmaceutical strategies for the topical delivery of peptides/proteins including outcomes.
Penetration enhancers
Chemical penetration enhancers: Chemical penetration enhancers (also known as sorption promoters or accelerants) have been traditionally used for the enhancement of skin penetration [25]. The mode of action is complex specific with most interacting with the lipid domain of the stratum corneum, disrupting these, and causing fluidization. Other mechanisms include disruption of the packing motif, intercellular domains, desmosome connections, metabolic activity or altering thermodynamic activity [25]. Chemical enhancers include the use of dimethylsulphoxide [26], azone [27], pyrrolidones [28] and fatty acids [29] or fatty alcohols [30] amongst others. When co-administered with a peptide/protein their action on the skin is to improve the peptide/protein penetration. For example, Magnusson and Runn [31] reported a significant increase in the flux across human epidermis in vitro of thyrotrophic releasing hormone, from 0.92 + 0.03 to 1.6 + 0.02 μg/cm2/h, with the use of ethanol and cineole. Unfortunately, most peptides/proteins are hydrophilic with high molecular weights requiring substantial action by the penetration enhancer to have an effect [31]. This approach is therefore limited in value for larger peptides and special care needs to be taken to ensure that the enhancer chemicals do not denature the peptides. Whilst there have been a number of successful permeation enhancers employed for peptide delivery to the skin, their use is hampered at high concentrations by irritation [30].
On the other hand, peptides themselves can act as penetration enhancers to enhance dermal delivery of other proteins [32,33]. The mechanism of which is unclear apart from time-lapse studies suggesting transient openings forming in the skin barrier. Chen et al. [32] observed elevated levels of insulin and human growth hormone, following transdermal absorption through rat abdominal skin, after co-administration of a short synthetic peptide ACSSSPSKHCG. Further investigations of this effect are required to extend findings to human skin. A similar study performed by Franken burg et al. [33] observed increased antibody production, in mice, to a topically administered recombinant melanoma protein co-administered with a peptide penetration enhancer. As peptide penetration enhancers are less irritant than chemical enhancers they may find wider use in the future.
An interesting concept has been studied using trypsin, a proteolytic enzyme, to alter the stratum corneum structure enabling the delivery of peptide/proteins [34]. For instance, the permeation flux of bovine insulin, through rat skin, increased 5.2 fold with improvements being dose related. This treatment found that plasma levels of glucose dropped to less than 60% leading this technique to transdermal delivery more than dermal. Fluorescent microscopy indicated penetration was through hair follicles and via the intercellular pathway. This does show however, that this method might be a new viable way of delivering peptides and larger proteins into the skin with very little effort.
Physical penetration enhancers: Physical penetration enhancers such as iontophoresis [35], electro oration [36] and sonophoresis [37] have been used to non-invasively enhance the penetration of peptide/ proteins into the skin. Iontophoresis uses an electrical potential difference to deliver hydro soluble, ionized molecules. The success of this technique, as shown by Raiman et al. [35] when delivering a luteinizing hormone releasing hormone (LHRH) and Nafarelin into human skin. The amount delivered is determined by the quantity of charge, intensity, duration and surface area in contact with the electrode. It was of importance to note that alternating current (AC) could not transport either LHRH or Nafarelin across the skin. Direct current (DC) is therefore preferred for this technique (75% pulsed DC resulted in 9.87 ± 4.91 μg /cm2/h LHRH passing across the epidermis as opposed to AC which delivered 0.006 ± 0.004 μg /cm2/h).
Similarly to iontophoresis, electro oration uses electricity to enhance permeation. Electroporation differs in that higher voltage is used to cause structural perturbation of the lipid bilayer in the stratum corneum. This reversible rearrangement has been shown by Wang et al. [36] using rat skin in a Franz cell experiment, to improve the skin delivery of cyclosporine A (60 fold increase compared to passive delivery). This technique has been shown to be more effective than iontophoresis in delivering heparin across human skin [38], however, has drawback to patient tolerance due to muscle spasms and pain [39].
Sonophoresis uses ultrasound to hypothetically cause micro cavitation in the skin, disordering the stratum corneum lipid bilayers and aiding diffusion. Therefore, sonophoresis doesn’t have the same side-effects of electro oration. Insulin has been successfully delivered to pigs in vivo with significant (P<0.05) reduction in glucose levels compared with the control group [37]. Other proteins delivered using this method include heparin [40] and erythropoietin [41]. The results from all these physical penetration enhancing techniques indicate the feasibility for their use in delivering peptide/proteins into the skin. Their use however is restricted due to the machinery and cost involved. For further reading into other non-invasive techniques including, laser ablation, thermal ablation and radiofrequency ablation readers are referred to a recent review paper by Kalluri et al. [42].
Chemical modification of the peptide/protein
Coupling or conjugation of a peptide/protein to a lipophilic moiety [22] such as lauric [43], palmitic [43] myristic, stearic or oleic [44] acid produce a derivative of the protein/peptide that is more skin permeable. Using this technique the cutaneous absorption into human skin of a parent compound , such as interferon alpha (ITF-α), increased from 0.4 ± 0.1 to 2.1 ± 1.2 μg/cm2, a 5 fold increase [44].
Using conjugates with cell penetrating peptides (CPPs) has also been studied as an attractive approach to translocate the payload into the deeper skin layers. These CPPs including Tat [45], transportan [46], Poly-Arg [47] and penetratin [48] are covalently linked to their cargoes and aid internalization into cells. The first step, according to the currently proposed mechanism, is adsorption via the CPP at the cell surface due to the negative charged membrane components sialic acid [49], phospholipidic acid [50] or heparin sulphate [51], followed by cellular internalization. Among several CPPs, TAT peptide is widely studied and extensively used for drug delivery. Lopes et al. showed that TAT was able to Trans locate a hydrophilic peptide, P20, across the human skin [52].
A study by Nasrollahi et al. [53] illustrated CPPs as a potential approach to successfully deliver topically applied cosmetic proteins. Pep-1, a CPP, showed fast and effective transport of elastin into cultured NIH-3T3 fibroblast cells (ratio of 10:1) without toxic effects. Cyclosporine A (protein for treatment of autoimmune diseases) has been conjugated with arginine oligomers and delivered across mouse and human skin, without detectable levels in circulation [54]. The penetration results indicated that the complex could not only penetrate deep into the skin, but also enter cells. The release from this conjugate was biologically active in both in vivo and in vitro tests resulting in inhibition of inflammation. The dermal deposition and cellular localization depending on concentration applied. Other examples of peptide delivery with CPPs include arginine-rich intracellular delivery with green fluorescent protein [34] and YARA (also a CPP) with a model peptide P20 [55]. In vitro studies across porcine skin indicated that covalently binding the peptide P20 to YARA (a protein transduction domain) increased penetration flux from 0.15 ± 0.03 to 0.35 ± 0.09 nmol/cm2 [55].
Recently a botulinum toxin type A (Bo NTA) was conjugated with a unique CPP (unspecified) [56]. This conjugate was incorporated into a gel for the topical treatment of lateral canthal lines (crow’s feet) and provided to 36 subjects, male and females between the age of 30- 60, to evaluate safety and efficacy. The results collected after 4 weeks after the single application saw 81.6% of participants displaying improvements in canthal lateral lines over a placebo. These results correlated to a 53–84% improvement, with no increase in severity or duration of adverse effects.
These successful studies demonstrate the potential of CPPs to effectively deliver proteins and peptides into the skin for therapeutic applications. Along with the successes of this technique, there is increasing awareness of the side-effects that may result from non-selective cell penetration effects [57]. Uncontrolled CPP-linked cargoes might become life threatening if cytotoxics are used as therapeutic agents. Any systemic administration resulting from topical application is therefore a concern. Future investigation and use of this technique does need to restrict undesired action to a minimum.
Formulation approaches
Micro emulsions: Micro emulsions are of low viscosity, thermodynamically stable systems consisting of two phases of oil and water stabilized by an interfacial film of surfactant molecules, frequently in conjunction with a co-surfactant [58]. Depending on the formulation components and ratio they can be oil in water (o/w), water in oil (w/o) or bi-continuous with the dispersed phase globules ranging from 5 to 100 nm. Micro emulsions increase dermal penetration due to the presence of surfactants, which are widely used as penetration enhancers [59]. Total penetration is also enhanced due to their solubilisation effects allowing incorporation of large amounts of active as well as the hydration effects on the stratum corneum [60,61]. Micro emulsion formulations compared to conventional vehicles, like hydrogels, emulsions and liposomes, have been shown to be superior for both transdermal and dermal delivery. This is particularly true for lipophilic compounds, but hydrophilic compounds also appear to benefit from formulation in micro emulsions [62].
There are a number of reported studies using micro emulsions for dermal peptide delivery in human skin. Water-in-oil micro emulsions were used as they are particularly suitable to entrap protein/peptides in the aqueous droplets and deliver the peptides effectively into the dermal layer [63,64]. A micro emulsion formulation of desmopressin [63] was able to significantly increase (p<0.05) the amount of active absorbed into deeper tissue layers of human skin compared to a conventional emulsion. Other studies using animal models [7] demonstrated that the topical administration of the high molecular weight proteins, anti-TNF monoclonal antibodies Remicade™ and Humira™, in water-in-oil micro emulsions reduced inflammation in the feet of mice. Bio distribution studies found that the molecule rapidly penetrated into the skin strata and also penetrated laterally into the distal regions of the skin with approximately 70% of the protein found in the skin.
A drawback to micro emulsion use, however, is the large amount of surfactant required to reduce the interfacial tension, which increases the probability of skin irritation or toxicity [65]. As a direct result, biocompatible surfactants have been sought to provide the cosmetic industry with efficient micro emulsion delivery systems with low toxicity. One such example is lecithin, a naturally occurring non-toxic phospholipid that acts as a surfactant (Hydrophilic Lipophilic Balance = 8) [66].
In the cosmetic industry, an increasing trend towards cheaper formulations is required for both product development and customer satisfaction. Micro emulsions provide a number of benefits to dermal peptide delivery, without higher costs associated with some other delivery methods, and can be easily prepared and scaled up for larger manufacture.
Encapsulation
Carrier systems that possess an aqueous core surrounded by a lipid or surfactant bilayer and have been extensively studied in literature [67,68] to deliver peptides and proteins to the dermis [69,70]. For the purposes of this review a representative summary is presented examining liposome, transfersomes, ethosomes and niosomes. Each can be further classified as unilamellar (single), oligolamellar (few) or multilamellar (many) according to the number of concentric bilayers. These vesicles possess a hydrophilic core making them ideal carrier systems to encapsulate and protect peptides/proteins within the vesicles.
The use of liposomes has distinct advantages for dermal delivery, they are; biocompatible and biodegradable, able to provide sustained release of the encapsulated active compounds, favor active deposition in the skin, improve stability and provide penetration enhancement to compounds [71-73]. Different mechanisms for liposomal delivery of actives into the skin have been proposed. One of them is the free drug mechanism whereby drug permeates independently (liposome does not cross into the epidermis but act as a depot) after leaving the vesicles [74]. However, this mechanism fails to explain the enhanced dermal penetration which has been reported [71]. A contrasting mechanism suggests that changes take place in the intercellular lipids upon topical application of liposomes, allowing penetration of the vesicles themselves. The vesicles then fuse with skin lipids thereby increasing drug permeation [72]. Of particular importance for absorption into the skin is the size of the elastic vesicle. Carriers larger than 10 μm remain on the surface of the skin, 3-10 μm concentrate in hair follicles and only particles below 3 μm are able to penetrate the stratum corneum [72]. These values should only be used as a guideline as the deformability of the elastic vesicles will clearly have an influence as discussed below.
A study by Ghahary et al.[75] demonstrated that liposome encapsulated interferon-2α-b (IFN-2α-B), an anti-fibrotic factor, significantly reduced wound contraction, tissue cellularity, and collagen expression in dermal wounds of guinea pigs (in vivo) compared with placebo controls. Interferon- α was also placed into liposomes and examined for deposition in human skin [44]. Results indicated a 2-fold increase in skin delivery over the control solution. Liposomes have been used in cosmetics to deliver peptides, such as collagen and UV-absorbers [76]. The first topical product to use liposomes was “Capture” by Dior in 1986. Now several hundred products utilize liposomes [77]. Typical liposomes are phospholipid bilayered vesicles with an aqueous core [72]. A good example if this technology is β-White™, which is a TGF- β biomimetic peptide encapsulated in a liposome (Lucas Meyer).
It has become evident that conventional liposomes, produced from phospholipids alone, often fail to penetrate deeply into the skin but remain confined to the upper layers of the stratum corneum due to their lower potential to deform [78]. To this effect, a newer class of ultra-flexible deformable liposome, transfersomes, has been developed capable of transport through skin [79]. Transfersomes have been defined as vesicular particles consisting of at least one inner aqueous compartment surrounded by a lipid bilayer with appropriately tailored properties attained by addition of surfactants [80]. Compared to conventional liposomes, transfersomes are highly deformable and elastic due to the presence of surfactants acting as ‘edge activators’, which accumulate at high stress zones. Edge activators are often single chain surfactants that can destabilize the lipid bilayer and increase deformability by lowering interfacial tension [71]. These edge activators aid the vesicle to deform, allowing penetration through pores in the stratum corneum 10 times smaller than the conventional liposomes [81], making this carrier system more attractive. Topical delivery by transfersomes and conventional liposomes was compared by Barry et al. [79] who reported the maximum flux (mass•cm-2•s-1) of the female hormone oestradiol was increased by 14-17 fold and 8.3 fold, respectively, compared to an aqueous formulation control. Other studies using cyclosporine A, melatonin and methotrexate, saw increased permeation and dermal deposition (up to 50% of the administered dose in the case of methotrexate) using transfersomes when compared with conventional liposomes [82,83]. These compelling results indicate that certain deformable liposomes are well suited to intradermal delivery and have clinical and cosmetic applications for peptide/proteins [84].
Another lipid carrier, ethosomes, contains a high content of ethanol (20-50%) in addition to phospholipids [85]. Ethanol interacts with the polar head group of lipids in the stratum corneum, resulting in a reduction in the melting point of the lipids, increasing the fluidity, and consequently the permeability. It is worthy of note that this increase in permeability can be achieved following the fusion of ethosomes with cell membranes. The additional benefit of ethosomes is their prolonged physical stability in comparison to conventional liposomes [86]. Data has been published showing the ability of ethosomes for transdermal delivery of testosterone [87] and the deep dermal delivery of bacitracin, a polypeptide antibiotic [88]. This may have implications for skin conditions where non-invasive clinical intervention is required to treat infection. However, ethosomes are so effective at promoting skin permeation that this delivery system is more suited to transdermal applications than dermal delivery as required by anti-ageing cosmetics [89-91].
A second-generation elastic vesicular delivery system, niosomes, was later developed using nonionic surfactants which function as penetration enhancers. These nonionic surfactant based vesicles are formed by the self-assembly of the surfactant into bilayers with or without addition of cholesterol and other lipids. Apart from increasing entrapment efficiency, niosomes are cheaper to produce than liposomes with higher solubilisation capacity, making these elastic vesicles a favorable dermal delivery system for peptides [92]. The mechanism of delivery may be attributed to a combination of the ability of the surfactant molecules to disrupt skin lipids and the flexibility they impart to the vesicles. Bouwstra et al. [93] suggested that niosomal vesicles can be used not only to transport the encapsulated molecules across human epidermis but also to form drug reservoirs in the upper layers of the skin [24]. Manosroi et al. [94] demonstrated the transport of a human tyrosinase plasmid through abdominal rat skin by incorporated it in the cores of cationic niosomes. Results indicated that the flux increased 6 times compared with a solution. The cationic charge adsorbs onto the negatively charged membrane and promotes internalization [49]. In a further development Manosroi et al. [92] reported an additional benefit with the entrapment efficiency can be improved through charge modification of niosomes. The entrapment of insulin in niosomes can be increased from 10.26% in neutral niosomes to 87.15% in charged niosomes through peptide-bilayer interactions. However, consideration must be given to the toxicity implication of introducing charge to the niosomes.
In summary, the use of elastic vesicles manages to overcome the barriers in delivery of peptides/proteins to or through the skin. With increasing study and the advent of technology we will likely see rapid advancement in the topical use of elastic vesicles.
Electrospun fiber mats: Synthetic polymers are often conjugated to peptides/proteins forming new materials to enhance stability and promote absorption into the skin [95]. A number of synthetic polymers are well established, biocompatible [96] and once conjugated to peptides/proteins can be electro spun into biodegradable fiber mats, composed of fibers less than 2μm thick, that control the rate of drug delivery [6]. Polymer selection is crucial. Rapid degradation/ erosion of some synthetic polymers, such as poly lactic acid or poly (lactic-co-glycolic acid), results in a decrease in the local pH that causes inflammation. The drop in pH occurs due to hydrolysis of the polymer chains increasing the number of carboxylic end-groups and total hydronium ion concentration. Hence tyrosine-derived polycarbonates, which can also be electro spun, are used to deliver peptides/proteins to overcome this problem, whilst maintaining the desirable function of controlled drug delivery [6].
A study by Macri et al. [6] used electrospun tyrosine-derived polycarbonate terpolymersto encapsulate P12, a highly cationic, hydrophilic, 14 amino acid peptide, with a molecular weight 1780Da to treat burns in a porcine wound model. It was evident that the fiber mats that were applied topically have the potential to be drug delivery matrices. In addition, in vivo toxicity assessment of these fibers in a porcine excision wound model showed that they lacked inflammatory response and may be suitable for burn and cutaneous wound healing applications in humans [6]. A further study by Yang et al. [97] saw the application of basic fibroblast growth factor (bFGF) embedded in electro spun fibers, polylactide-polyethylene glycol, applied to wounds in diabetic rats. Burst release (14.0 ± 2.2%) from the bFGF-loaded scaffolds was seen followed by gradual release until complete degradation of fibers after 4 weeks. Enhanced proliferation of fibroblasts, cell adhesion and secretion of extracellular matrix are all positive in vitro attributes for the eventual use of this technology in vivo for therapeutic purpose. Other animal studies with supportive evidence for this method include plasmid bFGF [98], soy protein [99] and synthetic human elastin [100].
Most studies using electro spun fibers as a delivery platform have been performed in wounded skin. Drug release into intact skin, as seen in a paper by Azarbayjani et al., is possible using electrospun fibers [101]. In this study levothyroxine (T4) was incorporated into PVA and poly-N-isopropylacrylamide producing a sustained topical delivery of T4 across excised human skin. The flux was slower than the aqueous control (0.59 ± 00.2 vs 0.84 ±0.07 μg/cm2/h) as the polymers retarded release of T4 into the skin, producing the sustained release profile. Drug release was speculated to be a combination of diffusion and polymer erosion. Currently no topical peptide/protein publications exist for intact skin, but from the available evidence of animal models and drug release studies, there is certainly scope.
This is still a new mechanism of erodible, degradable delivery of therapeutic proteins/peptidesto the dermal layer. While current data only describes topical application of peptides/proteins to wounded skin, with advancement this may become a viable way of delivering through intact skin.
Solid Lipid Particle-based Drug Delivery Systems: Solid lipid particle-based drug delivery systems include solid lipid nano particles (SLN) and nano-structured lipid carriers (NLC). First generation SLNs were developed in the early 1990’s as an alternative to liposomes and emulsions [102]. SLNs are produced from solid lipids and particles range in size from 50 to 1000 nm [72]. However there is a potential problem since SLNs form a ‘perfect crystal’ limiting drug loading. After preparation by hot homogenizing techniques, the particles have a higher energy of crystallization, which reduces to low energy upon storage resulting in drug expulsion. To overcome this problem, second generation NLCs were developed by mixing solid-lipids and liquid-lipids. NLCs overcome drug expulsion on storage of SLNs by having a permanently less ordered matrix through the blending of liquid and solid lipids. In addition NLCs tend to have lower water content and a higher loading capacity [103]. Surfactants, which are commonly used to stabilize SLNs and NLCs, can act as penetration enhancers, disrupting skin integrity and promoting penetration through the stratum corneum [104].
Topical application of SLN containing recombinant human epidermal growth factor has been examined for wound healing potential in rats [105]. Whilst the entrapment in NLCs was greater than that of SLNs (95.7 ± 4.7% vs 73.9 ± 2.2%) there was no significant difference in biological response. In both applied formulations the wound area healed was significantly improved (96.6 ± 3.2 vs 82.3 ± 18.4% in the control) over 15 days. For a detailed review on SLN use for peptide delivery readers are directed to an excellent paper by Almeida and Souto [106].
Although these studies are performed in in vitro and animal models they show promising results for their application in clinical and cosmetic therapeutics. Furthermore, these lipid particles are well tolerated, suited for damaged skin and possess good scale-up feasibility [107] making this an attractive strategy for the cosmetic industry to formulate anti-ageing products that contain peptide/ proteins. Therefore SLNs and NLCs are promising carrier systems for dermal delivery of protein/peptides.
Combination delivery approaches
To enhance dermal delivery a synergistic combination of approaches may be used. The strengths of one approach can be used to overcome the drawbacks of another. It has been reported that stability, both chemical and microbial, of the peptides are significantly improved by coating liposomes with a polymer, such as polyethylene glycol (PEG, known as PEGylated liposomes) [108]. The surface coating of polymer improved the physical stability by creating a stable static layer around the liposomes to prevent aggregation. In addition to enhancing the liposomal and formulation stability, polymer coated carrier systems have the potential to improve the delivery of proteins and peptides to the skin by increasing dermal permeation [108]. The results indicated that a carrageen an coating of liposomes increased the permeation rate of finasteride and progesterone (steroid hormones) over conventional liposomes (3.4 vs 2.9 μg/cm2/h and 0.74 vs 0.52 μg/cm2/h respectively). This has been attributed to the bio adhesive nature of the carrageen an which maintains the period of contact between the liposome and skin [109].
A similar percutaneous penetration enhancement effect was seen with the addition of polymers to micro emulsion systems with incorporated progesterone [58]. The addition of silicon dioxide and a polymeric emulsifier (Pemulen TR 1) produced an increase in chemical stability of the peptide and caused an increase in skin permeability in relation to the pure micro emulsion. The increase in skin permeation is thought to be due to the opening effect on tight junctions in the skin [110]. These examples demonstrate the advantages that can arise for including polymers in formulations containing peptides/proteins.
Biphasic systems, such as liposomes in a sub micrometer emulsion phase, forms a multi compartmental delivery system that contains aqueous compartments, micellar domains, phospholipid bilayers and oil droplets. The structure formed and permeation-enhancing excipients used can be carefully selected to facilitate encapsulation and transport of larger molecules into the skin. Interferon alpha (IFN-α) has been used as a model protein to better understand the transport of a biphasic vesicle through human stratum corneum [111]. Full thickness human skin was used in flow-through diffusion cells to image the distribution of IFN-α using confocal microscopy. The results showed the biphasic vesicles crossing the epidermis and moving into the deeper tissue layers.
Biphasic vesicles interact with lipids in the skin, re-ordering them as opposed to disordering them [111]. This allows peptides/ proteins to be delivered into the skin and sometimes through the skin as evidenced by King et al. in a study with rats and insulin [112]. The decrease in blood glucose of 43.7 ± 3.8% (n = 25) was observed, within four hours, compared with initial blood glucose levels. Levels were not statistically different from the insulin levels obtained 2 hours after subcutaneous administration of recombinant human insulin solution. Bioavailability from the biphasic system was 21.5 ± 6.9%. Similarly, transdermal delivery was shown in a clinical trial on 12 patients [113]. Patches placed on the upper inner arm were used to treat genital warts. Twice daily application resulted in a decrease in lesion size; synthesize activity and tissue viral load. This meant that clinically significant levels of Interferon alpha could be delivered across human skin with therapeutic effects in human patients.
Whilst examples of peptide/protein delivery using this method have shown it to be successful, this technique lends itself to transdermal more so than dermal delivery. It might be, as is the case with vesicles, that future formulations could be altered to suit dermal delivery. This would provide an excellent alternative delivery system for other large peptides/proteins.
Conclusion
Peptides and proteins play a significant role in cosmetic anti-ageing as well as therapeutic applications such as wound healing, reducing excessive scarring, fibrosis, inflammation and to treat burns. With the advent of new technologies and new peptide drugs being developed, we are beginning to see promising signs of dermal regeneration, collagen and elastin synthesis, increased glycosaminoglycan, proteoglycan and fibronectin production as well as improved wound healing in vivo. Effective formulation and topical delivery strategies are required to overcome the stratum corneum barrier and to properly release the active from the vehicles at the target site in sufficient quantities to exert an effect. Effective delivery approaches are available to enhance the dermal delivery of peptides including the use of elastic vehicles, solid lipid based drug delivery systems, micro-emulsions and electro spun fibres. Each method has advantages and drawbacks and its value depends on the vehicle, excipients and physicochemical characteristics of the peptideor proteinused. The development of ‘one size fits all’ peptide/ protein-containing formulation is unlikely as each peptide becomes the subject of an individual case study. To gain optimal results a combination of these approaches may be utilized.
Acknowledgement
The authors wish to acknowledge support of a Technology for Industry Fellowship (Grant Number: ENDU1101) for Mr. Travis Badenhorst provided by New Zealand Ministry of Science and Technology and Coselle Ltd, a cosmetic industry company (Auckland, New Zealand).
References
- Kerscher M, Trueb RM. Dermatokosmetik. Steinkopff. 2009.
- Brincat M, Kabalan S, Studd JW, Moniz CF, de Trafford J, Montgomery J. A study of the decrease of skin collagen content, skin thickness, and bone mass in the postmenopausal woman. Obstet Gynecol. 1987; 70: 840-845.
- Szauter KM, Cao T, Boyd CD, Csiszar K. Lysyl oxidase in development, aging and pathologies of the skin. Pathol Biol (Paris). 2005; 53: 448-456.
- Draelos ZD. Cosmetic dermatology: products and procedures: John Wiley & Sons. 2011.
- Lupo MP, Cole AL. Cosmeceutical peptides. Dermatol Ther. 2007; 20: 343-349.
- Macri LK, Sheihet L, Singer AJ, Kohn J, Clark RA. Ultrafast and fast bioerodible electrospun fiber mats for topical delivery of a hydrophilic peptide. J Control Release. 2012; 161: 813-820.
- Himes R, Lee S, McMenigall K, Russell-Jones GJ. Reduction in inflammation in the footpad of carrageenan treated mice following the topical administration of anti-TNF molecules formulated in a micro-emulsion. J Control Release. 2010; 145: 210-213.
- Lopes LB, Furnish EJ, Komalavilas P, Flynn CR, Ashby P, Hansen A, et al. Cell permeant peptide analogues of the small heat shock protein, HSP20, reduce TGF-beta1-induced CTGF expression in keloid fibroblasts. J Invest Dermatol. 2009; 129: 590-598.
- Encyclopedia BC. Peptide nd. 1994-2008.
- Leyden J, Stevens T, Finkey M, Barkovic S. Skin care benefits of copper peptide containing facial cream. American Academy of Dermatology 60th Annual Meeting. 2002.
- Lintner K, Peschard O. Biologically active peptides: from a laboratory bench curiosity to a functional skin care product. Int J Cosmet Sci. 2000; 22: 207-218.
- Katayama K, Armendariz-Borunda J, Raghow R, Kang AH, Seyer JM. A pentapeptide from type I procollagen promotes extracellular matrix production. J Biol Chem. 1993; 268: 9941-9944.
- Weller R. Clinical Dermatology. 4 edn: Blackwell Publishing. 2008.
- Waller JM, Maibach HI. Age and skin structure and function, a quantitative approach (II): protein, glycosaminoglycan, water, and lipid content and structure. Skin Res Technol. 2006; 12: 145-154.
- Ruszczak Z. Effect of collagen matrices on dermal wound healing. Adv Drug Deliv Rev. 2003; 55: 1595-1611.
- Rnjak-Kovacina J, Weiss AS. The Role of Elastin in Wound Healing and Dermal Substitute Design. In: Dermal Replacements in General, Burn, and Plastic Surgery. Springer. 2013; 57-66.
- Rnjak-Kovacina J, Weiss AS. The Role of Elastin in Wound Healing and Dermal Substitute Design. In: Dermal Replacements in General, Burn, and Plastic Surgery. Springer. 2013; 57-66.
- Fang JY, Leu YL. Prodrug strategy for enhancing drug delivery via skin. Curr Drug Discov Technol. 2006; 3: 211-224.
- Kielhorn J. Environmental Health Criteria Series, Number 235: Dermal Absorption. Geneva, CHE: World Health Organization; 2006.
- Magnusson BM, Pugh WJ, Roberts MS. Simple rules defining the potential of compounds for transdermal delivery or toxicity. Pharm Res. 2004; 21: 1047-1054.
- López A, Llinares F, Cortell C, Herráez M. Comparative enhancer effects of Span20 with Tween20 and Azone on the in vitro percutaneous penetration of compounds with different lipophilicities. Int J Pharm. 2000; 202: 133-140.
- Caccetta R, Blanchfield JT, Harrison J, Toth I, Benson HA. Epidermal penetration of a therapeutic peptide by lipid conjugation; Stereo-selective peptide availability of a topical diastereomeric lipopeptide. International Journal of Peptide Research and Therapeutics. 2006; 12: 327-333.
- Alvi IA, Madan J, Kaushik D, Sardana S, Pandey RS, Ali A. Comparative study of transfersomes, liposomes, and niosomes for topical delivery of 5-fluorouracil to skin cancer cells: preparation, characterization, in-vitro release, and cytotoxicity analysis. Anti-cancer drugs. 2011; 22: 774-782.
- Schuetz YB, Naik A, Guy RH, Kalia YN. Emerging strategies for the transdermal delivery of peptide and protein drugs. Expert Opin Drug Deliv. 2005; 2: 533-548.
- Williams AC, Barry BW. Penetration enhancers. Adv Drug Deliv Rev. 2012; 64: 128-137.
- Chan TC, Walters KA, Hui X, Maibach HI. 11 Drug Penetration Enhancement through Human Nail and Skin. Dermatological and Cosmeceutical Development: Absorption Efficacy and Toxicity. 2013; 189.
- Wiechers JW, de Zeeuw RA. Transdermal drug delivery: efficacy and potential applications of the penetration enhancer Azone. Drug Des Deliv. 1990; 6: 87-100.
- Barry BW, Bennett SL. Effect of penetration enhancers on the permeation of mannitol, hydrocortisone and progesterone through human skin. J Pharm Pharmacol. 1987; 39: 535-546.
- Aungst BJ, J Rogers N, Shefter E. Enhancement of naloxone penetration through human skin in vitro using fatty acids, fatty alcohols, surfactants, sulfoxides and amides. Int J Pharm. 1986; 33: 225-234.
- Kanikkannan N, Singh M. Skin permeation enhancement effect and skin irritation of saturated fatty alcohols. Int J Pharm. 2002; 248: 219-228.
- Magnusson BM, Runn P. Effect of penetration enhancers on the permeation of the thyrotropin releasing hormone analogue pGlu-3-methyl-His-Pro amide through human epidermis. Int J Pharm. 1999; 178: 149-159.
- Chen Y, Shen Y, Guo X, Zhang C, Yang W, Ma M, et al. Transdermal protein delivery by a coadministered peptide identified via phage display. Nat Biotechnol. 2006; 24: 455-460.
- Frankenburg S, Grinberg I, Bazak Z, Fingerut L, Pitcovski J, Gorodetsky R, et al. Immunological activation following transcutaneous delivery of HR-gp100 protein. Vaccine. 2007; 25: 4564-4570.
- Hou YW, Chan MH, Hsu HR, Liu BR, Chen CP, Chen HH, et al. Transdermal delivery of proteins mediated by non-covalently associated arginine-rich intracellular delivery peptides. Exp Dermatol. 2007; 16: 999-1006.
- Raiman J, Koljonen M, Huikko K, Kostiainen R, Hirvonen J. Delivery and stability of LHRH and Nafarelin in human skin: the effect of constant/pulsed iontophoresis. Eur J Pharm Sci. 2004; 21: 371-377.
- Wang S, Kara M, Krishnan TR. Transdermal delivery of cyclosporin-A using electroporation. J Control Release. 1998; 50: 61-70.
- Park EJ, Werner J, Smith NB. Ultrasound mediated transdermal insulin delivery in pigs using a lightweight transducer. Pharm Res. 2007; 24: 1396-1401.
- Prausnitz MR, Edelman ER, Gimm JA, Langer R, Weaver JC. Transdermal delivery of heparin by skin electroporation. Biotechnology (N Y). 1995; 13: 1205-1209.
- Gaudy C, Richard MA, Folchetti G, Bonerandi JJ, Grob JJ. Randomized controlled study of electrochemotherapy in the local treatment of skin metastases of melanoma. J Cutan Med Surg. 2006; 10: 115-121.
- Smith NB, Lee S, Shung KK. Ultrasound-mediated transdermal in vivo transport of insulin with low-profile cymbal arrays. Ultrasound Med Biol. 2003; 29: 1205-1210.
- Mitragotri S, Blankschtein D, Langer R. Ultrasound-mediated transdermal protein delivery. Science. 1995; 269: 850-853.
- Kalluri H, Banga AK. Transdermal delivery of proteins. AAPS PharmSciTech. 2011; 12: 431-441.
- Muranishi S, Sakai A, Yamada K, Murakami M, Takada K, Kiso Y. Lipophilic peptides: synthesis of lauroyl thyrotropin-releasing hormone and its biological activity. Pharm Res. 1991; 8: 649-652.
- Foldvari M, Baca-Estrada ME, He Z, Hu J, Attah-Poku S, King M. Dermal and transdermal delivery of protein pharmaceuticals: lipid-based delivery systems for interferon alpha. Biotechnol Appl Biochem. 1999; 30: 129-137.
- Fawell S, Seery J, Daikh Y, Moore C, Chen LL, Pepinsky B, et al. Tat-mediated delivery of heterologous proteins into cells. Proc Natl Acad Sci U S A. 1994; 91: 664-668.
- Pooga M, Kut C, Kihlmark M, Hällbrink M, Fernaeus S, Raid R, et al. Cellular translocation of proteins by transportan. FASEB J. 2001; 15: 1451-1453.
- Wender PA, Mitchell DJ, Pattabiraman K, Pelkey ET, Steinman L, Rothbard JB. The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: peptoid molecular transporters. Proc Natl Acad Sci U S A. 2000; 97: 13003-13008.
- Derossi D, Joliot AH, Chassaing G, Prochiantz A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J Biol Chem. 1994; 269: 10444-10450.
- Nakase I, Tadokoro A, Kawabata N, Takeuchi T, Katoh H, Hiramoto K, et al. Interaction of arginine-rich peptides with membrane-associated proteoglycans is crucial for induction of actin organization and macropinocytosis. Biochemistry. 2007; 46: 492-501.
- Brooks H, Lebleu B, Vivès E. Tat peptide-mediated cellular delivery: back to basics. Adv Drug Deliv Rev. 2005; 57: 559-577.
- Vivès E, Richard JP, Rispal C, Lebleu B. TAT peptide internalization: seeking the mechanism of entry. Curr Protein Pept Sci. 2003; 4: 125-132.
- Lopes LB, Brophy CM, Furnish E, Flynn CR, Sparks O, Komalavilas P, et al. Comparative study of the skin penetration of protein transduction domains and a conjugated peptide. Pharm Res. 2005; 22: 750-757.
- Nasrollahi SA, Fouladdel S, Taghibiglou C, Azizi E, Farboud ES. A peptide carrier for the delivery of elastin into fibroblast cells. Int J Dermatol. 2012; 51: 923-929.
- Rothbard JB, Garlington S, Lin Q, Kirschberg T, Kreider E, McGrane PL, et al. Conjugation of arginine oligomers to cyclosporin A facilitates topical delivery and inhibition of inflammation. Nat Med. 2000; 6: 1253-1257.
- Lopes LB, Furnish E, Komalavilas P, Seal BL, Panitch A, Bentley MV, et al. Enhanced skin penetration of P20 phosphopeptide using protein transduction domains. Eur J Pharm Biopharm. 2008; 68: 441-445.
- Brandt F, O'Connell C, Cazzaniga A, Waugh JM. Efficacy and Safety Evaluation of a Novel Botulinum Toxin Topical Gel for the Treatment of Moderate to Severe Lateral Canthal Lines. Dermatologic Surgery. 2010; 36: 2111-2118.
- Huang Y, Jiang Y, Wang H, Wang J, Shin MC, Byun Y, et al. Curb challenges of the "Trojan Horse" approach: smart strategies in achieving effective yet safe cell-penetrating peptide-based drug delivery. Adv Drug Deliv Rev. 2013; 65: 1299-1315.
- Biruss B, Valenta C. The advantage of polymer addition to a non-ionic oil in water microemulsion for the dermal delivery of progesterone. Int J Pharm. 2008; 349: 269-273.
- Gloor M, Hauth A, Gehring W. O/W emulsions compromise the stratum corneum barrier and improve drug penetration. Pharmazie. 2003; 58: 709-715.
- Delgado-Charro MB, Iglesias-Vilas G, Blanco-Mendez J, Lopez-Quintela MA, Marty J-P, Guy RH. Delivery of a hydrophilic solute through the skin from novel microemulsion systems. Eur J Pharm Biopharm. 1997; 43: 37-42.
- Peltola S, Saarinen-Savolainen P, Kiesvaara J, Suhonen TM, Urtti A. Microemulsions for topical delivery of estradiol. Int J Pharm. 2003; 254: 99-107.
- Kreilgaard M. Influence of microemulsions on cutaneous drug delivery. Adv Drug Deliv Rev. 2002; 54: S77-98.
- Getie M, Wohlrab J, Neubert RH. Dermal delivery of desmopressin acetate using colloidal carrier systems. J Pharm Pharmacol. 2005; 57: 423-427.
- Goebel A, Neubert RH. Dermal peptide delivery using colloidal carrier systems. Skin Pharmacol Physiol. 2008; 21: 3-9.
- Boonme P. Applications of microemulsions in cosmetics. J Cosmet Dermatol. 2007; 6: 223-228.
- Martins S, Sarmento B, Ferreira DC, Souto EB. Lipid-based colloidal carriers for peptide and protein delivery - Liposomes versus lipid nanoparticles. International Journal of Nanomedicine. 2007; 2: 595-607.
- Choi MJ, Maibach HI. Elastic vesicles as topical/transdermal drug delivery systems. Int J Cosmet Sci. 2005; 27: 211-221.
- Choi MJ, Maibach HI. Liposomes and niosomes as topical drug delivery systems. Skin Pharmacol Physiol. 2005; 18: 209-219.
- Manosroi A, Khanrin P, Lohcharoenkal W, Werner RG, Götz F, Manosroi W, Manosroi J. Transdermal absorption enhancement through rat skin of gallidermin loaded in niosomes. Int J Pharm. 2010; 392: 304-310.
- Wolf P, Maier H, Müllegger RR, Chadwick CA, Hofmann-Wellenhof R, Soyer HP, et al. Topical treatment with liposomes containing T4 endonuclease V protects human skin in vivo from ultraviolet-induced upregulation of interleukin-10 and tumor necrosis factor-a. J Invest Dermatol. 2000; 114: 149-156.
- Honeywell-Nguyen PL, Bouwstra JA. Vesicles as a tool for transdermal and dermal delivery. Drug Discov Today Technol. 2005; 2: 67-74.
- Narasimha Murthy S, Shivakumar HN. Topical and Transdermal Drug Delivery. Vitthal SK, editor. Boston: William Andrew Publishing. 2010.
- Pierre MB, Dos Santos Miranda Costa I. Liposomal systems as drug delivery vehicles for dermal and transdermal applications. Arch Dermatol Res. 2011; 303: 607-621.
- El Maghraby GM, Barry BW, Williams AC. Liposomes and skin: from drug delivery to model membranes. Eur J Pharm Sci. 2008; 34: 203-222.
- Ghahary A, Tredget EE, Shen Q, Kilani RT, Scott PG, Takeuchi M. Liposome associated interferon-alpha-2b functions as an anti-fibrogenic factor in dermal wounds in the guinea pig. Mol Cell Biochem. 2000; 208: 129-137.
- Patravale VB, Mandawgade SD. Novel cosmetic delivery systems: an application update. Int J Cosmet Sci. 2008; 30: 19-33.
- Uchegbu IF, Florence AT. Non-ionic surfactant vesicles (niosomes): Physical and pharmaceutical chemistry. Adv Colloid Interface Sci. 1995; 58: 1-55.
- Elsayed MM, Abdallah OY, Naggar VF, Khalafallah NM. Lipid vesicles for skin delivery of drugs: reviewing three decades of research. Int J Pharm. 2007; 332: 1-16.
- E Maghraby GM, Williams AC, Barry BW. Skin delivery of oestradiol from deformable and traditional liposomes: mechanistic studies. J Pharm Pharmacol. 1999; 51: 1123-1134.
- Ozer AY, Hincal AA, Bouwstra JA. A novel drug delivery system: Non-ionic surfactant vesicles. Eur J Pharm Biopharm. 1991; 37: 75-79.
- Cevc G, Gebauer D. Hydration-driven transport of deformable lipid vesicles through fine pores and the skin barrier. Biophys J. 2003; 84: 1010-1024.
- Guo J, Ping Q, Sun G, Jiao C. Lecithin vesicular carriers for transdermal delivery of cyclosporin A. Int J Pharm. 2000; 194: 201-207.
- Trotta M, Peira E, Carlotti ME, Gallarate M. Deformable liposomes for dermal administration of methotrexate. Int J Pharm. 2004; 270: 119-125.
- Elsayed MM, Abdallah OY, Naggar VF, Khalafallah NM. Deformable liposomes and ethosomes as carriers for skin delivery of ketotifen. Pharmazie. 2007; 62: 133-137.
- Dayan N, Touitou E. Carriers for skin delivery of trihexyphenidyl HCl: ethosomes vs. liposomes. Biomaterials. 2000; 21: 1879-1885.
- Wahid AA, Ravouru N, Lakshman SR. Ethosomes: A tool for transdermal drug delivery. Curr Trends Biotechnol Pharm. 2011; 5: 972-981.
- Touitou E, Dayan N, Bergelson L, Godin B, Eliaz M. Ethosomes - novel vesicular carriers for enhanced delivery: characterization and skin penetration properties. J Control Release. 2000; 65: 403-418.
- Godin B, Touitou E. Mechanism of bacitracin permeation enhancement through the skin and cellular membranes from an ethosomal carrier. J Control Release. 2004; 94: 365-379.
- Godin B, Touitou E. Erythromycin ethosomal systems: physicochemical characterization and enhanced antibacterial activity. Curr Drug Deliv. 2005; 2: 269-275.
- Ainbinder D, Touitou E. Testosterone ethosomes for enhanced transdermal delivery. Drug Deliv. 2005; 12: 297-303.
- Nounou MI, El-Khordagui LK, Khalafallah NA, Khalil SA. Liposomal formulation for dermal and transdermal drug delivery: past, present and future. Recent Pat Drug Deliv Formul. 2008; 2: 9-18.
- Manosroi A, Khanrin P, Werner RG, Götz F, Manosroi W, Manosroi J. Entrapment enhancement of peptide drugs in niosomes. J Microencapsul. 2010; 27: 272-280.
- Bouwstra JA, Honeywell-Nguyen PL, Gooris GS, Ponec M. Structure of the skin barrier and its modulation by vesicular formulations. Prog Lipid Res. 2003; 42: 1-36.
- Manosroi J, Khositsuntiwong N, Manosroi W, Götz F, Werner RG, Manosroi A. Potent enhancement of transdermal absorption and stability of human tyrosinase plasmid (pAH7/Tyr) by Tat peptide and an entrapment in elastic cationic niosomes. Drug Deliv. 2013; 20: 10-18.
- Vandermeulen G, Klok H. Peptide/Protein Hybrid Materials: Enhanced Control of Structure and Improved Performance through Conjugation of Biological and Synthetic Polymers. Macromol Biosci. 2004; 4: 383-398.
- Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov. 2003; 2: 347-360.
- Yang Y, Xia T, Zhi W, Wei L, Weng J, Zhang C, et al. Promotion of skin regeneration in diabetic rats by electrospun core-sheath fibers loaded with basic fibroblast growth factor. Biomaterials. 2011; 32: 4243-4254.
- Yang Y, Xia T, Chen F, Wei W, Liu C, He S, et al. Electrospun fibers with plasmid bFGF polyplex loadings promote skin wound healing in diabetic rats. Mol Pharm. 2012; 9: 48-58.
- Har-el Y-e, Gerstenhaber JA, Brodsky R, Huneke RB, Lelkes PI. Electrospun soy protein scaffolds as wound dressings: Enhanced reepithelialization in a porcine model of wound healing. Wound Medicine. 2014; 5: 9-15.
- Rnjak-Kovacina J, Wise SG, Li Z, Maitz PK, Young CJ, Wang Y, et al. Electrospun synthetic human elastin:collagen composite scaffolds for dermal tissue engineering. Acta Biomater. 2012; 8: 3714-3722.
- Azarbayjani AF, Venugopal JR, Ramakrishna S, Lim FC, Chan YW, Chan SY. Smart polymeric nanofibers for topical delivery of levothyroxine. Journal of Pharmacy & Pharmaceutical Sciences. 2010; 13: 400-410.
- Puglia C, Bonina F. Lipid nanoparticles as novel delivery systems for cosmetics and dermal pharmaceuticals. Expert Opin Drug Deliv. 2012; 9: 429-441.
- Pardeike J, Hommoss A, Müller RH. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int J Pharm. 2009; 366: 170-184.
- Souto EB, Doktorovová S. Chapter 6 - Solid Lipid Nanoparticle Formulations: Pharmacokinetic and Biopharmaceutical Aspects in Drug Delivery. Nejat D, editor. Porto, Portugal: Academic Press; 2009; 464: 105-129.
- Gainza G, Pastor M, Aguirre JJ, Villullas S, Pedraz JL, Hernandez RM, et al. A novel strategy for the treatment of chronic wounds based on the topical administration of rhEGF-loaded lipid nanoparticles: In vitro bioactivity and in vivo effectiveness in healing-impaired db/db mice. J Control Release. 2014; 185: 51-61.
- Almeida AJ, Souto E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv Drug Deliv Rev. 2007; 59: 478-490.
- Chen X, Peng L, Gao J. Novel topical drug delivery systems and their potential use in scars treatment. Asian Journal of Pharmaceutical Sciences. 2012; 7: 155-167.
- Biruss B, Valenta C. Skin permeation of different steroid hormones from polymeric coated liposomal formulations. Eur J Pharm Biopharm. 2006; 62: 210-219.
- Song KH, Eddington ND. The influence of stabilizer and bioadhesive polymer on the permeation-enhancing effect of AT1002 in the nasal delivery of a paracellular marker. Arch Pharm Res. 2012; 35: 359-366.
- Valenta C, Auner BG. The use of polymers for dermal and transdermal delivery. Eur J Pharm Biopharm. 2004; 58: 279-289.
- Foldvari M, Badea I, Wettig S, Baboolal D, Kumar P, Creagh AL, et al. Topical Delivery of Interferon Alpha by Biphasic Vesicles: Evidence for a Novel Nanopathway across the Stratum Corneum. Molecular Pharmaceutics. 2010; 7: 751-762.
- King MJ, Badea I, Solomon J, Kumar P, Gaspar KJ, Foldvari M. Transdermal delivery of insulin from a novel biphasic lipid system in diabetic rats. Diabetes Technol Ther. 2002; 4: 479-488.
- Foldvari M, Badea I, Kumar P, Wettig S, Batta R, King MJ, et al. Biphasic vesicles for topical delivery of interferon alpha in human volunteers and treatment of patients with human papillomavirus infections. Curr Drug Deliv. 2011; 8: 307-319.
- Gorouhi F, Maibach HI. Role of topical peptides in preventing or treating aged skin. Int J Cosmet Sci. 2009; 31: 327-345.
- Kang YJ. Copper and homocysteine in cardiovascular diseases. Pharmacol Ther. 2011; 129: 321-331.
- Brzoska T, Böhm M, Lügering A, Loser K, Luger TA. Terminal signal: anti-inflammatory effects of α-melanocyte-stimulating hormone related peptides beyond the pharmacophore. Adv Exp Med Biol. 2010; 681: 107-116.
- Südel KM, Venzke K, Mielke H, Breitenbach U, Mundt C, Jaspers S, et al. Novel aspects of intrinsic and extrinsic aging of human skin: beneficial effects of soy extract. Photochem Photobiol. 2005; 81: 581-587.
- Andre-Frei V, Perrier E, Augustin C, Damour O, Bordat P, Schumann K, et al. A comparison of biological activities of a new soya biopeptide studied in an in vitro skin equivalent model and human volunteers. Int J Cosmet Sci. 1999; 21: 299-311.
- Song YK, Hyun SY, Kim HT, Kim CK, Oh JM. Transdermal delivery of low molecular weight heparin loaded in flexible liposomes with bioavailability enhancement: comparison with ethosomes. J Microencapsul. 2011; 28: 151-158.
- Rattanapak T, Young K, Rades T, Hook S. Comparative study of liposomes, transfersomes, ethosomes and cubosomes for transcutaneous immunisation: characterisation and in vitro skin penetration. J Pharm Pharmacol. 2012; 64: 1560-1569.
- Choi JK, Jang JH, Jang WH, Kim J, Bae IH, Bae J, et al. The effect of epidermal growth factor (EGF) conjugated with low-molecular-weight protamine (LMWP) on wound healing of the skin. Biomaterials. 2012; 33: 8579-8590.
- Yang Y, Xia T, Zhi W, Wei L, Weng J, Zhang C, et al. Promotion of skin regeneration in diabetic rats by electrospun core-sheath fibers loaded with basic fibroblast growth factor. Biomaterials. 2011; 32: 4243-4254.
- Kim ST, Jang DJ, Kim JH, Park JY, Lim JS, Lee SY, et al. Topical administration of cyclosporin A in a solid lipid nanoparticle formulation. Pharmazie. 2009; 64: 510-514.