Research Article
Austin J Pharmacol Ther. 2014; 2 (8).1043
Therapeutic Implications of Punica Granatum Seeds on Doxorubicin-Induced Nephrotoxicity in Wistar Rats
Nirwane AM1*, Gupta PV2 and Patil RA1
1Department of Pharmacology, Bombay College of pharmacy, India
2Department of Pharmacology, Bombay College of Pharmacy, Mumbai, India
*Corresponding author: : Nirwane AM, Department of Pharmacology, Bombay Collage of Pharmacy, India
Received: May 24, 2014; Accepted: September 24, 2014; Published: September 24, 2014
Abstract
Punica granatum Linn (Lythraceae) popularly known as ‘Pomegranate’ is a rich source of flavonoids. Flavonoids have been implicated in nephroprotective activity.
Objectives: To evaluate effect of Punica granatum seeds and its flavonoids on doxorubicin-induced nephrotoxicity in Wistar rats.
Materials and Methods: The anthocyanidins of P. granatum (A-PJ) (100 and 300 mg/kg, p.o., 21 days) and P. granatum juice (PJ) extract (300 mg/kg, p.o., 21 days) was gavaged daily to the rats along with Doxorubicin (DXR) (60 mg/kg, i.p., once 48 h before sacrifice). The levels of blood urea nitrogen (BUN), uric acid, creatinine and Superoxide Dismutase (SOD), catalase (CAT) and ATPase levels in kidney were measured. Morphological and histopathological indices in the kidney of healthy and DXR treated rats were also measured.
Result: In the present study, DXR exhibited increase in the renal biomarkers (BUN, uric acid and creatinine) which were significantly decreased after pretreatment with A-PJ (100, 300 mg/kg) and PJ (300 mg/kg). DXR significantly decreased levels of SOD and CAT which were restored with A-PJ and PJ pre-treatment. The results were similar to that of Limarin (25 mg/kg, p.o., 21 days) which served as a reference standard. Histopathological studies were also in agreement of above.
Conclusion: The study concludes that P. granatum possess potential nephroprotective activity which may be attributive to its flavonoids (anthocyanidins), having preventive potential in treatment of kidney disorders.
Keywords: Blood urea nitrogen; Anthocyanidins; Flavonoids, Pomegranate
Introduction
The introduction of Doxorubicin (DXR) for the treatment of cancer in 1969, this chemical has demonstrated high antitumor efficacy. However, its use in chemotherapy has been limited largely due to its diverse toxicities, including cardiac, renal, hematological and testicular toxicity [1,2], alterations of DNA and the production of free radicals [3-5] mitochondrial damage [6] and iron-dependent oxidative damage to biological macromolecules [7]. DXR is an anthracyclic antibiotic with broad spectrum of anti-neoplastic activity. Oxidative stress has been demonstrated in DXR mediated myocardial, renal [8] and liver [9] damages. DXR injection substantially increased the peroxidative damage in the myocardium, hepatic and renal tissues and markedly increased the serum CK-MB, LDH and the other biochemical variables such as, creatinine, BUN, ALT and AST [10]. DXR treatment also induced peroxidative alterations in various tissues [11,12] which was evident by significant decrease in SOD and CAT. Flavonoids possess a variety of biochemical and pharmacological activities such as antioxidant, antiviral, anticarcinogenic, and anti-inflammatory effects believed to be beneficial for human health [13]. Polyphenols are the major class of pomegranate fruit phytochemicals, including flavonoids (anthocyanins), condensed tannins, (proanthocyanidins) and hydrolysable tannins (ellagitannins and gallotannins) [14-16]. Pomegranate phytochemicals also show potential in chemoprevention of various types of cancers, by exerting antiproliferative effects on tumor cells [17]. The presence of anthocyanins is responsible for the appealing bright red color of juice and other products of pomegranate fruit [18]. So, in the light of above literature, the present study was planned to study the effect of Punica granatum seeds on DXR-induced nephrotoxicity in Wistar rats.
Materials and Methods
Animals
Albino rats (Wistar strain) of either sex weighing between 200- 250 g, were obtained from Bharat Serum and Vaccines Ltd., Thane, India. Animals were housed into groups of five under standard laboratory conditions of temperature 25 ± 1°C with free access to food and water. The experiments were performed during the light portion. The experiments were carried out according to the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), New Delhi, India, and approved by the Institutional Animal Ethical Committee of MGV’s Pharmacy College, Nashik, India.
Plant material and extraction
Fruits of P. granatum (PG) (1Kg) were brought from Malegaon and authenticated by Mr. P. G. Devakar, Botanical Survey of India, Pune, India. Voucher specimen has been retained there (PUGAN12). The seeds were separated and ground to obtain juice. The juice subjected to dryness and concentrated under reduced pressure to obtain juice extract (PJ) (yield: 4% w/w). Anthocyanidins, (A-PJ) (yield: 1.6% w/w), were isolated from PG by slight modification of method described earlier [19]. dissolved in 2-4 drops of methanolic HCl and was subjected to thin layer chromatography using TLC silica gel 60 F254 plates (Merck) and BAW [n-butanol: acetic acid: water (4:1:5)], forestal [Concentrated HCl: acetic acid: water (3:30:10)] and formic acid [Concentrated HCl: formic acid: water (2:5:3)] as mobile phases. Iodine vapors were used for visualizing the spots on the plates and Rf values were calculated (Data not shown). The phytochemical testing of extract was also carried out.
Drugs and Treatment schedule
Doxorubicin (ADRIM 50 mg) was purchased from Dabur Pharma Ltd., India. Limarin was purchased from Serum institute, India). BUN kit (Reckon Diagnostics Pvt. Ltd., India), URIC ACID kit (Crest Biosystems, Goa, India), CREATININE kit (Vital Diagnostics (P) Ltd. India) were used for the study. All drug solutions were freshly prepared in saline before each experiment. PJ and A-PJ was dissolved in distilled water and administered orally. In DXR treated groups, DXR was given once, 48 h before sacrifice. The animals were divided in following experimental groups, 5 animals in each.
Group I: Vehicle (saline) (1 ml/kg, p.o., 21 days), Group II: DXR (60 mg/kg, i.p.), Group III: A-PJ (100 mg/kg, p.o., 21 days), Group IV: PJ (300 mg/kg, p.o., 21 days) + DXR (60 mg/kg, i.p.), Group V: A-PJ (100 mg/kg, p.o., 21 days) + DXR (60 mg/kg, i.p.), Group VI: A-PJ (300 mg/kg, p.o., 21days) + DXR (60 mg/kg, i.p.), Group VII: Limarin (25 mg/kg, p.o., 21 days) + DXR (60 mg/kg, i.p.).
After DXR treatment, animal was sacrificed by cervical dislocation and blood samples were collected by heart puncture method for determination of various biochemical parameters. The both Kidney tissues were immediately dissected out, washed with ice-cold 0.9% saline and weighed. They were then subjected to antioxidant and histopathology studies.
Estimation of body and kidney weight
In each group, body weight of rats was taken before and after DXR treatment. Isolated kidney was weighed after keeping them in ice-cold saline and squeeze out the blood.
Biochemical estimation
Serum was separated from collected blood using centrifuge at 3000 g for 15 min and used for estimation of BUN [20], URIC ACID [21], and CREATININE [22]. The one of the excised kidney was then weighed and homogenized in chilled Tris buffer (10 mM, pH 7.4) at a concentration of 10% w/v. The homogenates were centrifuged at 10,000 g for 20 min. The clear supernatants were used for the assays of Superoxide Dismutase (SOD) [23], catalase (CAT) [24]. The sediment after centrifugation of kidney tissue homogenate were resuspended in ice cold Tris buffer (10 mM, pH 7.4) to get a final concentration of 10% and were used for the estimation of different membrane bound enzymes such as Na+ K+ ATPase [25], Ca2+ ATPase [26], and Mg 2+ ATPase [27], Total proteins [28].
Histopathological studies
The remaining kidney was fixed in 10% formalin. The specimens were then processed for standard procedure and were embedded in paraffin wax. The blocks were then stained according to hematoxylin and eosin method. Five-micrometer thick histological sections were obtained from the paraffin blocks. The sections were examined under the light microscope and photographs were taken under 10X using Motic camera.
Statistics
The mean ± SD values were calculated for each group. One-way ANOVA followed by Dunnett’s multiple comparison tests were used for statistical analysis. Values of p<0.05 were considered statistically significant.
Results
The changes in weights of rats among the experimental group after 21 days were found to be significant. Significant reduction (p<0.05) were observed in the body weight and isolated kidney weight of DXR alone treated group compared to vehicle treated group. This failure to thrive in animals pretreated with PJ (300 mg/kg), A-PJ (100 mg/kg, 300 mg/kg), and Limarin (25 mg/kg) and also treated with DXR and was probably due to DXR. However, pretreatment with PJ, A-PJ and Limarin exhibited significant (p<0.01) elevation in the body weight and isolated kidney weight in these groups compared to DXR treated group (Table 1).
Treatment (mg/kg)
Final body weight
(g)
Isolated kidney weight (g)
Vehicle
242.8 ± 3.58
0.999 ± 0.02
DXR (60)
197.2 ± 4.08*
0.663 ± 0.01*
A-PJ (100)
242.3 ± 1.89#
0.987 ± 0.03#
PJ (300) + DXR (60)
214.6 ± 2.51#
0.706 ± 0.01#
A-PJ (100) + DXR (60)
217.2 ± 1.63#
0.740 ± 0.01#
A-PJ (300) + DXR (60)
234.3 ± 2.97#
0.922 ± 0.01#
Limarin (25) + DXR (60)
237.2 ± 3.09#
0.950 ± 0.01#
Table 1: Effect of Punica granatum on body weight and isolated kidney weight in DXR treated rats.
DXR treated rats showed raised serum activities of BUN, total Protein, uric acid and creatinine when compared to vehicle treated group. A significant reduction (p<0.01) was observed in the serum markers in the animals re-treated with PJ (300 mg/kg), A-PJ (100 mg/ kg, 300 mg/kg), and Limarin (25 mg/kg) compared to DXR treated group (Table 2).
Groups
uric Acid
(mg/dl)
BUN
(mg/dl)
creatinine
(mg/dl)
Control
1.33 ± 0.06
20.3 ± 0.87
0.27 ± 0.02
DXR
11.2 ± 0.07*
46.3 ± 0.97*
1.35 ± 0.10*
A-PJ (100)
1.33 ± 0.08
19.7 ± 0.40
0.29 ± 0.01
PJ (300) + DXR (60)
8.02 ± 0.10#
35.2 ± 1.13#
0.74 ± 0.01#
A-PJ (100) + DXR (60)
6.86 ± 0.08#
34.7 ± 0.21#
0.65 ± 0.01#
A-PJ (300) + DXR (60)
3.02 ± 0.14#
33.0 ± 0.15#
0.50 ± 0.03#
Limarin (25) + DXR (60)
2.12 ± 0.09#
25.0 ± 0.32#
0.44 ± 0.01#
Table 2: Effect of Punica granatum on activity of kidney biomarkers in DXR treated rats.
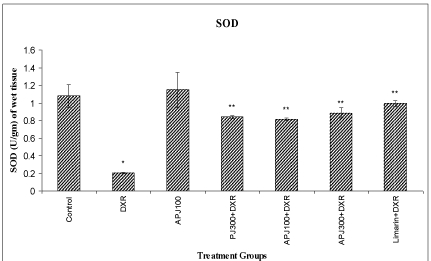
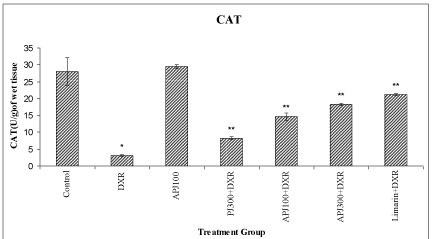
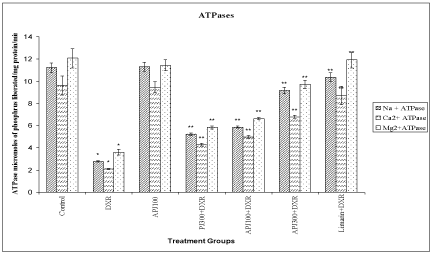
A significant (<0.01) reduction in SOD, CAT and membrane bound ATPases were observed in DXR treated group compared to vehicle treated group. A significant (<0.01) elevation in SOD, CAT and membrane bound ATPases were observed in animals re-treated with PJ (300 mg/kg), A-PJ (100 mg/kg, 300 mg/kg), and Limarin (25 mg/kg) compared to DXR treated group (Figure 1-3).
Effect of P. granatum on levels of kidney Superoxide dismutase in DXR treated rats.
All values are expressed as mean ± SD, N=5. All data are subjected to One Way ANOVA followed by Dunnett’s test. * p<0.01 as compared to control ** p<0.01 when compared to DXR group. Vertical lines represent SD. Abbreviations: A-PJ: Anthocyanidins of P. granatum; PJ: Plain Juice of P. granatum, DXR: DoxorubicinFigure 1:Effect of P. granatum on levels of kidney Superoxide dismutase in DXR treated rats.
All values are expressed as mean ± SD, N=5. All data are subjected to One Way ANOVA followed by Dunnett’s test. * p<0.01 as compared to control ** p<0.01 when compared to DXR group. Vertical lines represent SD. Abbreviations: A-PJ: Anthocyanidins of P. granatum; PJ: Plain Juice of P. granatum, DXR: Doxorubicin
Effect of P. granatum on levels of kidney Catalase in DXR treated rats. All values are expressed as mean ± SD, N=5. All data are subjected to One Way ANOVA followed by Dunnett’s test. * p<0.01 when compared to control and ** p<0.01 when compared to DXR group. Vertical lines represent SD.
Abbreviations: A-PJ: Anthocyanidins of P. granatum; PJ: Plain Juice of P. granatum, DXR: DoxorubicinFigure 2:Effect of P. granatum on levels of kidney Catalase in DXR treated rats. All values are expressed as mean ± SD, N=5. All data are subjected to One Way ANOVA followed by Dunnett’s test. * p<0.01 when compared to control and ** p<0.01 when compared to DXR group. Vertical lines represent SD.
Abbreviations: A-PJ: Anthocyanidins of P. granatum; PJ: Plain Juice of P. granatum, DXR: Doxorubicin
Effect of P. granatum on levels of membrane bound ATPase in DXR treated rats.
All values are expressed as mean ± SD, N=5. All data are subjected to One Way ANOVA followed by Dunnett’s test. * p<0.01 when compared to control and ** p<0.01 when compared to DXR group. Vertical lines represent SD. Abbreviations: A-PJ: Anthocyanidins of P. granatum; PJ: Plain Juice of P. granatum, DXR: DoxorubicinFigure 3:Effect of P. granatum on levels of membrane bound ATPase in DXR treated rats.
All values are expressed as mean ± SD, N=5. All data are subjected to One Way ANOVA followed by Dunnett’s test. * p<0.01 when compared to control and ** p<0.01 when compared to DXR group. Vertical lines represent SD. Abbreviations: A-PJ: Anthocyanidins of P. granatum; PJ: Plain Juice of P. granatum, DXR: Doxorubicin
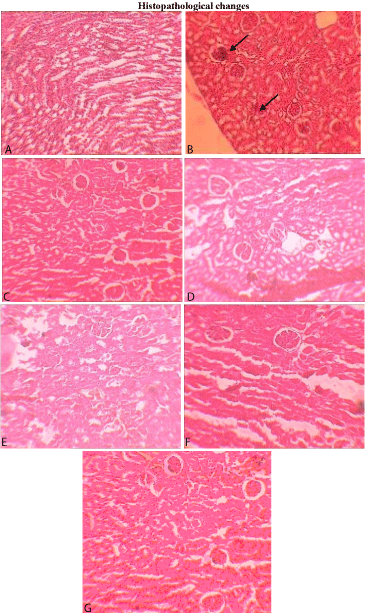
Figure 4: Photomicrographs of histopathological examination (10 X) of kidney tissue. Section A) Control group treated with vehicle shows normal architecture, Section B) group treated with DXR (60 mg/kg) shows significant inflammatory cells, Tubular necrosis, focal area necrosis, cloudy changes in proximal tubules, and glomerular congestion, as indicated by arrows, Section C) group treated with A-PJ (100 mg/kg) shows normal architecture, Section D) group treated with PJ (300 mg/kg) and DXR (60 mg/kg) shows minimum tubular changes and glomerular congestion that may due to increased blood flow to organ, section E) group treated with A-PJ (100 mg/kg) and DXR (60 mg/kg) shows mild congestion, which may due to the vasodilatation, section F) group treated with A-PJ (100 mg/kg) and DXR (60 mg/kg) shows very mild congestion, which may due to the vasodilatation, section G) group treated with Limarin (25 mg/kg) and DXR (60 mg/kg) shows very mild congestion.
Discussion
Many drugs used for cancer chemotherapy are known to produce toxic side effects in multiple organ systems including the kidney. Doxorubicin has long been one of the most extensively used chemotherapeutic agents for treatment of various cancers. The clinical application of this drug is, however, complicated by its potential toxicity to the heart [29], liver and kidney. The DXR is known to generate free radicals either by the enzymatic pathway of redox cycling between a semiquinone form and a quinone form or by the non-enzymatic pathway of forming a DXR-Fe3+ complex [30]. Because of the great importance of DXR in cancer therapy, researchers have expended great efforts trying to prevent or attenuate the side effects of DXR administration. Accordingly, several approaches have been pursued in the 90’s, such as dosage optimization, synthesis, and use of analogues or combined therapy, no hopeful results have been found and the application of different DXR-analogues did not show better antineoplastic value or lower toxicity than DXR. A strategy to diminish the side effects of anticancer drugs with preservation of their chemotherapeutic efficacy is current need of interest.
A great number of plants worldwide showed a strong antioxidant activity [31] and a powerful scavenger activity against free radicals [32]. Flavonoids a group of polyphenolic compounds with known properties which include free radical scavenging, inhibition of hydrolytic and oxidative enzymes and anti-inflammatory action.
Keeping this view, we have attempted to study the effect of Pomegranate juice extract (PJ) and its anthocyanidins fraction (A-PJ) on Doxorubicin induced nephrotoxicity. The preliminary phytochemical screening of Pomegranate juice extract showed the presence of flavonoids, alkaloids, tannins and anthocyanidins.
The phytochemical screening of Anthocyanidins extract of Punica granatum seeds reported for positive tests for flavonoids, saponins, tannins and Phenolic compound [33]. The dose of DXR induced in this study corresponds to the dose that is currently being used in the clinical practice [34]. The overall incidence of cardiotoxicity is about 3% at a total dose of 400 mg/m2 in human subjects [35]. In rats the corresponding dose was found to be 67.75 mg/kg using the dose calculator results. Our pilot study indicated that the dose of DXR 60 mg/kg was effective to induce kidney toxicity in rat.
The results of a present study indicate that the DXR (60 mg/kg, i.p. single dose 48 h before sacrifice) induce pathological changes in serum and biochemical markers, indicative of toxicity of renal and increase in free radical production. In our study, we observed that the isolated kidney weight and body weight was significantly decreased in DXR treated group which was prevented by pre treatment with anthocyanidins extract of P. granatum (100 and 300 mg/kg/day, p.o. for 21 days) at the end of treatment schedule.
A significant rise in kidney biomarkers is an indicator of abnormal functioning of the kidney. This increase suggest that an increased leakage of these enzymes from mitochondria as result of toxicity induced by treatment with DXR. Administration of DXR (60 mg/kg, i.p.) to rats significantly increased serum BUN, Uric acid and Creatinine levels. Our results are in good agreement with those previously reported [36].
In our study, a significant decrease in concentration of SOD and CAT and Membrane bound ATPase levels in DXR treated group was observed. P. granatum extract (100 and 300 mg/kg, p.o. for 21 days) treatment significantly reversed the changes in SOD and CAT enzyme levels induced by DXR treatment.
The histopathology data has revealed that DXR treatment in kidney shows inflammatory cells, tubular necrosis, and congestion. Treatment with P. granatum (100 and 300 mg/kg/day, p.o., for 21 days) has reversed the histopathological features induced by DXR on renal tissues.
Conclusion
In conclusion, this study provides firm evidence that doxorubicin can adversely damage the kidney tissue by inducing significant morphologic, biochemical and histopathological changes.
Our results raise the hope that pre-treatment with PJ and APJ can reduce the damage caused by DXR and can be a potential therapeutic implication in DXR induced nephrotoxicity.
References
- Gillick J, Giles S, Bannigan J, Puri P. Cell death in the early adriamycin rat model. Pediatr Surg Int. 2002; 18: 576-580.
- Yilmaz S, Atessahin A, Sahna E, Karahan I, Ozer S. Protective effect of lycopene on adriamycin-induced cardiotoxicity and nephrotoxicity. Toxicology. 2006; 218: 164-171.
- Quiles JL, Huertas JR, Battino M, Mataix J, Ramírez-Tortosa MC. Antioxidant nutrients and adriamycin toxicity. Toxicology. 2002; 180: 79-95.
- Yin X, Wu H, Chen Y, Kang YJ. Induction of antioxidants by adriamycin in mouse heart. Biochem Pharmacol. 1998; 56: 87-93.
- Hershko C, Link G, Tzahor M, Pinson A. The role of iron and iron chelators in anthracycline cardiotoxicity. Leuk Lymphoma. 1993; 11: 207-214.
- Cini Neri G, Neri B, Bandinelli M, Del Tacca M, Danesi R, Riccardi R. Anthracycline cardiotoxicity: in vivo and in vitro effects on biochemical parameters and heart ultrastructure of the rat. Oncology. 1991; 48: 327-333.
- Thomas CE, Aust SD. Release of iron from ferritin by cardiotoxic anthracycline antibiotics. Arch Biochem Biophys. 1986; 248: 684-689.
- Fadillioglu E, Oztas E, Erdogan H, Yagmurca M, Sogut S, Ucar M, et al. Protective effects of caffeic acid phenethyl ester on doxorubicin-induced cardiotoxicity in rats. J Appl Toxicol. 2004; 24: 47-52.
- Kwiecień I, Michalska M, Włodek L. The selective effect of cystathionine on doxorubicin hepatotoxicity in tumor-bearing mice. Eur J Pharmacol. 2006; 550: 39-46.
- Saad SY, Najjar TA, Al-Rikabi AC. The preventive role of deferoxamine against acute doxorubicin-induced cardiac, renal and hepatic toxicity in rats. Pharmacol Res. 2001; 43: 211-218.
- Cao Y, Kennedy R, Klimberg VS. Glutamine protects against doxorubicin-induced cardiotoxicity. J Surg Res. 1999; 85: 178-182.
- Chen A, Sheu LF, Ho YS, Lin YF, Chou WY, Chou TC, et al. Experimental focal segmental glomerulosclerosis in mice. Nephron. 1998; 78: 440-452.
- Danks MK, Yalowich JC, Beck WT. Atypical multiple drug resistance in a human leukemic cell line selected for resistance to teniposide (VM-26). Cancer Res. 1987; 47: 1297-1301.
- Santagati NA, Duro R, Duro F. Study on pigments present in pomegranate seeds. J Commodity Sci. 1984; 23: 247-254.
- Hernandez F, Melgarejo P, Tomas-Barberan FA, Artes F. Evolution of juice anthocyanins during ripening of new selected pomegranate (Punica granatum) clones. Eur Food Res Technol.1999; 210: 39-42.
- Gil MI, Tomás-Barberán FA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem. 2000; 48: 4581-4589.
- Kim ND, Mehta R, Yu W, Neeman I, Livney T, Amichay A, et al. Chemopreventive and adjuvant therapeutic potential of pomegranate (Punica granatum) for human breast cancer. Breast Cancer Res Treat. 2002; 71: 203-217.
- Jaiswal V, DerMarderosian A, Porter JR. Anthocyanins and polyphenol oxidase from dried arils of pomegranate (Punica granatum L). Food Chemistry. 2010; 118: 11-16.
- Harborne JB. A Guide of Modern Technique of Plant Analysis. 3rd edn. 2007; 66-74.
- Bergmeyer HU, Bowers GN Jr, horder M, Moss DW. Provisional recommendations on IFCC methods for the measurement of catalytic concentrations of enzymes Part 2. IFCC method for aspartate aminotransferase. Clin Chim Acta. 1976; 70: F19–42.
- Schumann G, Bonora R, Ceriotti F, Férard G, Ferrero CA, Franck P. et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 degrees C. Clin Chem Lab Med. 2002; 40: 635-642.
- Henry RJ. “ENZYMES” in Clinical Chemistry Principal and Techniques, Harper & row publishers. New York. 1974; 815.
- Saggu H, Cooksey J, Dexter D, Wells FR, Lees A, Jenner P, et al. A selective increase in particulate superoxide dismutase activity in parkinsonian substantia nigra. J Neurochem. 1989; 53: 692-697.
- Beers RF Jr, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952; 195: 133-140.
- Bonting SL. Presence of enzyme system in mammalian tissues: Membrane and Ion Transport, Wiley Inter Science, London. 1970; 257-263.
- Hjertén S, Pan H. Purification and characterization of two forms of a low-affinity Ca2+-ATPase from erythrocyte membranes. Biochim Biophys Acta. 1983; 728: 281-288.
- Chohen JJ, Kamma DE. The Kidney. 2nd edn. Saunders, Philadelphia. 1981; 141-248.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193: 265-275.
- Singal PK, Li T, Kumar D, Danelisen I, Iliskovic N. Adriamycin-induced heart failure: mechanism and modulation. Mol Cell Biochem. 2000; 207: 77-86.
- Doroshow JH, Locker GY, Myers CE. Enzymatic defenses of the mouse heart against reactive oxygen metabolites: alterations produced by doxorubicin. J Clin Invest. 1980; 65: 128-135.
- Tobe S. Update on calcium antagonists and the kidney. Curr Opin Nephrol Hypertens. 2003; 12: 309–315.
- Kumaran A, Karunakaran RJ. Activity-guided isolation and identification of free radical-scavenging components from an aqueous extract of Coleus aromaticus. Food Chemistry. 2007; 100: 356–361.
- Kokate CK. In: Practical Pharmacognosy, 3rd edn. Vallabh Prakashan, New Delhi. 1994; 07-109.
- Chabner BA, Ryan DP, Paz-Ares L, Garcia-Carbonevo R, Calabresi P. Antineoplastic agents. Hardman JG, Limbird LE, Gilman AG, editors, In: Goodman and Gilman’s the Parmacoll Basis of Thera. McGraw-Hill Companies Inc. USA. 2001; 1389–1459.
- Tallaj JA, Franco V, Rayburn BK, Pinderski L, Benza RL, Pamboukian S, et al. Response of doxorubicin-induced cardiomyopathy to the current management strategy of heart failure. J Heart Lung Transplant. 2005; 24: 2196-2201.
- Injac R, Perse M, Boskovic M, Djordjevic-Milic V, Djordjevic A, Hvala A,et al. Cardioprotective effects of fullerenol C60(OH)24 on a single dose doxorubicin-induced cardiotoxicity in rats with malignant neoplasm. Technol Cancer Res Treat. 2008; 7: 15-25.