
Research Article
Austin J Pharmacol Ther. 2015; 3(1).1066.
Ameliorative Effect of Rutin on Gentamicin-Induced Nephrotoxicity in Murine Model
Ruchi Ramhariya1, Aditya Ganeshpurkar1*, Chitrangada Ayachi1, Piyush Kanojia1, Divya Bansal2 and Nazneen Dubey1
1Drug Discovery Laboratory, Shri Ram Institute of Technology-Pharmacy, India
2Pharmaceutics Research Laboratory, Shri Ram Institute of Technology-Pharmacy, India
*Corresponding author: : Ganeshpurkar Aditya, Shri Ram Institute of Technology- Pharmacy, Jabalpur, M.P., 482002, India
Received: December 15, 2014; Accepted: February 05,2015; Published: February 09, 2015
Abstract
Gentamicin is one of the widely used antibiotics. However, it suffers from drawback of nephrotoxicity, which is predisposed due to oxidative stress. Rutin is one of the major classes of flavonoids and phyto-antioxidant. It has demonstrated cardioprotective, hepatoprotective and neuroprotective effects. However, no studies pertaining to its nephroprotective activity have still performed. Present work was aimed to determine nephroprotective activity of Rutin. Dose of 100 mg/ kg and 200 mg/ kg was taken in present study. Gentamicin (80 mg/ kg) was used to induce nephrotoxicity. At the end of the study body weight, kidney weight, serum creatinine, serum urea, blood urea nitrogen and urine creatinine were determined along with histopathological studies. Administration of Rutin restored the levels of biomarkers. Histopathological studies also demonstrated protective effect on kidney. Thus it could be concluded that rutin demonstrates nephroprotective potential.
Keywords: Rutin; Nephroprotective; Histopathology; Markers
Introduction
Since last 50 years, aminoglycosides are being effectively used as an integral part of antimicrobial chemotherapy [1] and these drugs are still useful to treat various life threatening infections [2]. Low resistance rate, dose dependent effects, along with low cost are some of its merits [3]. However, Gentamicin causes dose dependent nephrotoxicity [4,5].
Gentamicin induced nephrotoxicity is a multifaceted process that is observed by augmentation of plasma urea and creatinine levels along with necrosis of renal tubules that ultimately leads to renal failure [6,7]. One of the reasons behind this manifestation includes increased oxidative stress [9]. Thus anticipation and diminution of oxidative stress seems to be a novel strategy in preventing such situation.
Flavonoids are polyphenol obtained from plants [9]. They are found abundantly in grains, fruits, vegetables, barks, roots, stem, flower, tea and wine [10]. These compounds have benzo-pyran nucleus. Flavonoids are produced by plants as a result of response to preclusion of microbial infection [11]. However, due to high antioxidant capacity, they are believed to have health promoting properties [12,13].
Rutin (3, 3’, 4’, 5, 7-pentahydroxyflavone-3-rhamnoglucoside; Vitamin P) is an important bioflavonoid has demonstrated a number of beneficial pharmacological effects [14]. Rutin is known for various therapeutic effects. It has effectively prevented cognitive impairments by ameliorating oxidative stress and neuro-inflammation in rat model of sporadic dementia of Alzheimer type [15] and established protective effect against cognitive deficits and brain damage in rats with chronic cerebral hypoperfusion [16]. Rutin demonstrated protective effect against reflux oesophagitis by the inhibition of gastric acid secretion, oxidative stress and inflammatory cytokine production [17].
It has reported to be neuroprotective [18], cardioprotective [19], nephroprotective, and protective effect against reproductive toxicity [20] along with anti-inflammatory [21] and antihyperglycemic [22] effects in experimental animals.
In the present context, in vivo protective activity of Rutin against Gentamicin induced nephrotoxicity was evaluated in albino Wistar rats.
Experimental
Drugs and chemicals
Rutin and Gentamicin were purchased from Central Drug House, Mumbai, India. All the other chemicals used were of analytical grade.
Animals
Healthy adult male wistar albino rats (5-6 month; 250-300 g) were used. The animals were housed in polypropylene cages and maintained under standard conditions. They were fed with standard rat pellet diet (Hindustan Lever Ltd, Mumbai India) and water ad libitum. All the animal experimental protocols were approved by Institutional Animal Ethics Committee.
Selection of dose
In accordance to the dose as determined by Motamedshariaty et al., [23], rutin in the dose of 100 and 200 mg/kg orally was used in the present study.
Experimental protocols [24]
Animals were divided into four groups containing five animals each and grouped as
Group I: Control
Group II: Gentamicin (80mg/kg)
Group III: Gentamicin (80mg/kg) + Rutin (100mg/kg)
Group IV: Gentamicin (80mg/kg) + Rutin (200mg/kg)
Except group I animals, other three groups received 100 mg/kg/ day gentamicin by the intraperitoneal (i.p.) route.
After daily dosing, on the day 10, individual rats were placed in separate metabolic cages for 24 h for urine collection to determine urine creatinine content.
Body weight
Body weights of these rats were monitored on 10th day
Kidney function test
Blood urea nitrogen
Blood urea nitrogen was estimated as per manufacturer’s instructions (Span Diagnostic Ltd).
Serum creatinine
Creatinine was estimated using the method described previously by Broad and Sirota, using Jaffe’s reaction. To 1.0mL of serum and kidney homogenates, 8.0 ml of double-distilled water, 0.5mL of 2/3N sulfuric acid and 0.5ml of 10% sodium tungstate were added. This mixture was centrifuged at 4000 rpm for 15 min at 4oC, 5.0ml of the clear supernatant was taken to which 1.5ml of saturated picric acid and 1.5ml of 0.75N sodium hydroxide were added. The absorbance was read at 460 nm after 15 min in a Shimadzu UV- 1700spectrophotometer. Standard and blank were also processed similarly and the creatinine levels were expressed in milligram per deciliter [25,26].
Blood urea nitrogen [25] and serum creatinine [26] were estimated by method reported elsewhere.
Urea estimation
Urea was estimated by method reported by Natelson et al. [25]. Urea was estimated by the reported method. To 0.1ml of serum and kidney homogenates, 3.3ml of double-distilled water, 0.3ml of 10% sodium tungstate and 0.3mL of 0.67N sulfuric acids were added. The samples were centrifuged (4000 rpm, 15 min, 4°C) and to 1ml of the supernatant, 1ml of double-distilled water, 0.4ml of diacetyl monoxime reagent and 0.6ml of sulfuric acid– phosphoric acid mixture were added. The samples were incubated in a boiling water bath for 30 min, cooled to room temperature and the absorbance was read at 480 nm in a Shimadzu UV-1700spectrophotometer (Tokyo, Japan) [27].
Histopathological inspection of kidney
Rats were sacrificed and both kidneys were isolated from each rat. The kidneys were processed for histopathological examination. The kidney were excised quickly and fixed in 10 % formalin and stained with haemotoxylin and eosin and then observed under microscope for degeneration, fatty changes, necrotic changes and evidence of nephrotoxicity if any.
Statistical Analysis
The results were expressed as mean ± SEM. Statistical analysis was carried out by using One way ANOVA followed by Dunnett’s’ test and p<;0.01, p<;0.001 was considered significant.
Results
Body weight
Administration of Gentamicin (80 mg/kg) caused decrement in weight of animals. However, administration of Rutin (200 mg/kg) prevented a significant reduction (P < 0.01) in weight loss as evident from Figure 1. Weight gain in group II animals were observed, which was prevented up to an extent significantly (P < 0.01) by administration of Rutin (200 mg/kg) (Figure 1).
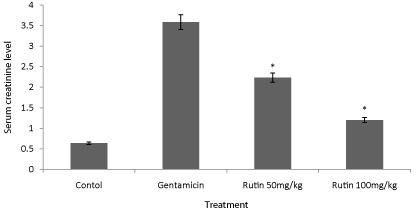
Figure 1: Effect of ethanol extract of Rutin on serum creatinine in gentamicin
treated rats. Results are given as mean ± SEM of five animals in each
group. Drug treated group compared with rest the extract treated groups.
Significance at *p< 0.05.
Kidney function test
Serum creatinine levels were augmented in gentamicin treated animals; however, Rutin at dose 200 mg/kg significantly (P < 0.01) decreased creatinine levels in animals (Figure 2). Blood urea nitrogen was increased in rats treated with only gentamicin; however, treatment with the Rutin at dose 200 mg/kg significantly (P<0.01) reversed the effect of gentamicin signifying nephroprotective activity. Urine creatinine levels were also significantly (P < 0.01) reduced in Rutin-treated group (200 mg/kg) (Figure 3).
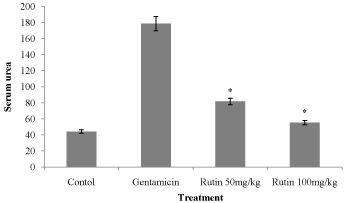
Figure 2: Effect of ethanol extract of Rutin on serum urea in gentamicin
treated rats. Results are given as mean ± SEM of five animals in each
group. Drug treated group compared with rest the extract treated groups.
Significance at *p< 0.05.
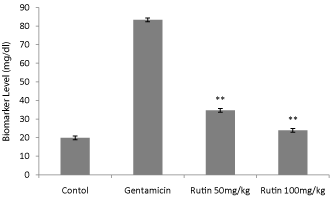
Figure 3: Effect of ethanol extract of Rutin on blood urea nitrogen in
gentamicin treated rats. Results are given as mean ± SEM of five animals
in each group. Drug treated group compared with rest the extract treated
groups. Significance at *p< 0.05.
Urea
Treatment (Group III & IV) with Rutin (200 mg/kg) of demonstrated a significant (P < 0.001) decrease (P<0.001) urea levels as compared to gentamicin treated (Group II) (Figure 3).
Histopathological inspection of kidney
The normal control group of rats illustrated normal histology of rat kidney while Gentamicin treated group (Group II) showed peritubular, cortical glomerular, blood vessel congestion, and interstitial inflammation. Groups treated with Rutin (Group III & IV) were observed to diminish such alterations in kidney histology caused by Gentamicin (Figure 4).
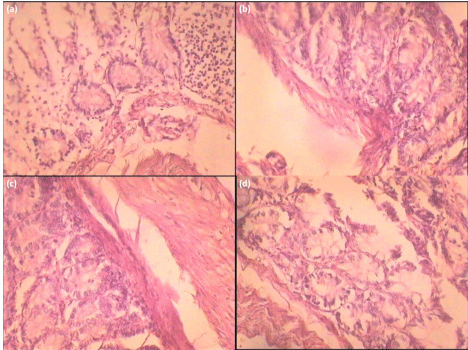
Figure 1: Histopathological evidence of protective effect of Rutin extract
on wistar rats (a) Control (b) Toxicant (c) Extract treated 50 mg/kg, po (d)
Extract treated 100 mg/kg, po.
Discussion
Gentamicin is one of the important antibiotics widely used to treat gram negative bacterial infections. Nephrotoxicity is a major side effect associated with it which is observed in two steps. Transportation and accumulation of Gentamicin at high concentration by ‘renal proximal tubular cells’ is observed in the first step of nephrotoxicity. During the second step, cellular damage due to interaction in between these polycationic drugs is observed [27].The resulting oxidative stress seems to be the major reasons for such pathogenesis.
The present study aimed to evaluate the protective effect of Rutin against Gentamicin induced nephropathy in rats. Administration of Gentamicin to rats caused ‘acute kidney dysfunction’ that is evident by rise in levels of serum urea and creatinine.
Body weight of animals and weight of kidneys were determined to observe nephrotoxic effect of Gentamicin. It is generally observed that ‘toxic kidneys’ put on weight when damage augments [28,29] along with reduction in total body weight [30]. Such effects were also observed in the present study. Rutin aided in prevention of significant reduction in weight loss and weight gain by kidneys.
Damage to kidneys is also observed by alterations in levels of urea, uric acid and creatinine. Administration of rutin in gentamicin treated rats caused significant restoration of these markers. Histopathological assessment of kidney of normal control rat confirmed healthy anatomical features. Gentamicin treated rat’s kidney illustrated presence of inflammatory depositions and cell necrosis. Kidney of rats treated with rutin showed minimal necrosis and least inflammatory accumulations with normal kidney anatomy, which revealed nephroprotective effect.
Rutin is reputed as an effective antioxidant in biological system. In the present work, administration of rutin to rats treated with gentamicin demonstrated nephroprotective activity in murine modelwhich reconfirms its nephroprotective effect [31]. However, chronic studies are necessary to study long term effects.
References
- Swain RA, Kaplan-Machlis B. Therapeutic uses of vitamin E in prevention of atherosclerosis. Altern Med Rev. 1999; 4: 414-423.
- Mingeot-Leclercq MP, Glupczynski Y, Tulkens PM. Aminoglycosides: activity and resistance. Antimicrob Agents Chemother. 1999; 43: 727-737.
- Begg EJ, Barclay ML. Aminoglycosides--50 years on. Br J Clin Pharmacol. 1995; 39: 597-603.
- Khoory BJ, Fanos V, Dall'Agnola A, Cataldi L. Aminoglycosides, risk factors and neonatal kidney. Pediatr Med Chir. 1996; 18: 495-499.
- Rougier F, Claude D, Maurin M, Maire P. Aminoglycoside nephrotoxicity. Curr Drug Targets Infect Disord. 2004; 4: 153-162.
- Al-Majed AA, Mostafa AM, Al-Rikabi AC, Al-Shabanah OA. Protective effects of oral arabic gum administration on gentamicin-induced nephrotoxicity in rats. Pharmacol Res. 2002; 46: 445-451.
- Cuzzocrea S, Mazzon E, Dugo L, Serraino I, Di Paola R, Britti D, et al. A role for superoxide in gentamicin-mediated nephropathy in rats. Eur J Pharmacol. 2002; 450: 67-76.
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford, UK: Clarendon Press. 1999.
- Dufour C, Loonis M. Flavonoids and their oxidation products protect efficiently albumin-bound linoleic acid in a model of plasma oxidation. Biochim Biophys Acta. 2007; 1770: 958-965.
- Middleton E Jr. Effect of plant flavonoids on immune and inflammatory cell function. Adv Exp Med Biol. 1998; 439: 175-182.
- Dixon RA, Dey PM, Lamb CJ. Phytoalexins: enzymology and molecular biology. Adv Enzymol Relat Areas Mol Biol. 1983; 55: 1-136.
- Rice-Evans CA, Miller NJ, Bolwell PG, Bramley PM, Pridham JB. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic Res. 1995; 22: 375-383.
- Cook NC, Samman S. Review: flavonoids-chemistry, metabolism, cardioprotective effects and dietary sources. J Nutr Biochem. 1996; 7: 66-76.
- Sharma S, Ali A, Ali J, Sahni JK, Baboota S. Rutin: therapeutic potential and recent advances in drug delivery. Expert Opin Investig Drugs. 2013; 22: 1063-1079.
- Javed H, Khan MM, Ahmad A, Vaibhav K, Ahmad ME, Khan A, et al. Rutin prevents cognitive impairments by ameliorating oxidative stress and neuroinflammation in rat model of sporadic dementia of Alzheimer type. Neuroscience. 2012; 210: 340-352.
- Qu J, Zhou Q, Du Y, Zhang W, Bai M, Zhang Z, et al. Rutin protects against cognitive deficits and brain damage in rats with chronic cerebral hypoperfusion. Br J Pharmacol. 2014; 171: 3702-3715.
- Shin YK, Sohn UD, Choi MS, Kum C, Sim SS, Lee MY. Effects of rutin and harmaline on rat reflux oesophagitis. Auton Autacoid Pharmacol. 2002; 22: 47-55.
- Magalingam KB, Radhakrishnan A, Haleagrahara N. Rutin, a bioflavonoid antioxidant protects rat pheochromocytoma (PC-12) cells against 6-hydroxydopamine (6-OHDA) - induced neurotoxicity. Int J Mol Med. 2013; 32: 235-240.
- Punithavathi VR, Shanmugapriya K, Prince PS. Protective effects of rutin on mitochondrial damage in isoproterenol-induced cardiotoxic rats: an in vivo and in vitro study. Cardiovasc Toxicol. 2010; 10: 181-189.
- Abarikwu SO, Otuechere CA, Ekor M, Monwuba K, Osobu D. Rutin Ameliorates Cyclophosphamide-induced Reproductive Toxicity in Male Rats. Toxicol Int. 2012; 19: 207-214.
- Selloum L, Bouriche H, Tigrine C, Boudoukha C. Anti-inflammatory effect of rutin on rat paw oedema, and on neutrophils chemotaxis and degranulation. Exp Toxicol Pathol. 2003; 54: 313-318.
- Kamalakkannan N, Prince PS. Antihyperglycaemic and antioxidant effect of rutin, a polyphenolic flavonoid, in streptozotocin-induced diabetic wistar rats. Basic Clin Pharmacol Toxicol. 2006; 98: 97-103.
- Motamedshariaty VS, Amel Farzad S, Nassiri-Asl M, Hosseinzadeh H. Effects of rutin on acrylamide-induced neurotoxicity. Daru. 2014; 22: 27.
- Sonkar N, Ganeshpurkar A, Yadav P, Dubey S, Bansal D2, Dubey N1. An experimetal evaluation of nephroprotective potential of Butea monosperma extract in albino rats. Indian J Pharmacol. 2014; 46: 109-112.
- Brod J, Sirota JH. The renal clearance of endogenous "Creatinine" in man. J Clin Invest. 1948; 27: 645-654.
- Husdan H, Rapoport A. Estimation of creatinine by the Jaffe reaction. A comparison of three methods. Clin Chem. 1968; 14: 222-238.
- NATELSON S, SCOTT ML, BEFFA C. A rapid method for the estimation of urea in biologic fluids. Am J Clin Pathol. 1951; 21: 275-281.
- Shimeda Y, Hirotani Y, Akimoto Y, Shindou K, Ijiri Y, Nishihori T, et al. Protective effects of capsaicin against cisplatin-induced nephrotoxicity in rats. Biol Pharm Bull. 2005; 28: 1635-1638.
- Nematbakhsh M, Ashrafi F, Nasri H, Talebi A, Pezeshki Z, Eshraghi F, et al. A model for prediction of cisplatin induced nephrotoxicity by kidney weight in experimental rats. J Res Med Sci. 2013; 18: 370-373.
- Mazaheri S, Nematbakhsh M, Bahadorani M, Pezeshki Z, Talebi A, Ghannadi AR, et al. Effects of Fennel Essential Oil on Cisplatin-induced Nephrotoxicity in Ovariectomized Rats. Toxicol Int. 2013; 20: 138-145.
- Mazen GMA. The Synergistic Effects of Rutin and Urate Oxidase on Nephrotoxicity in Rats. Arab J Nuclear Sci Appl. 2013: 46; 205-213.