
Research Article
Austin J Pharmacol Ther. 2015; 3(2).1071.
Antioxidant Potential of Hesperidin Protects Gentamicin Induced Nephrotoxicity in Experimental Rats
Jain DP* and Somani RS
Department of Research, Suresh Gyan Vihar University, India
*Corresponding author: : Jain DP, Department of Research, Suresh Gyan Vihar University, Jaipur, 302 001, India
Received: April 07, 2015; Accepted: June 18, 2015; Published: June 23, 2015
Abstract
Acute tubular necrosis is common with aminoglycoside therapy resulting in nephrotoxicity. The bioflavonoid, hesperidin is a specific flavonoid glycoside reported to act as a powerful antioxidant. Therefore, the present study investigates nephroprotective effect of hesperidin against gentamicin induced nephrotoxicity in rats. Thirty rats were randomly divided into five groups (n=6). Group I served as a control, Group II as a gentamicin control and was received vehicle for two days before and then treated with gentamicin (100 mg/kg/day) for eight days. Group III-V were received hesperidin orally at three doses (50, 100 and 200 mg/kg) two days before and eight days along with gentamicin (100 mg/kg/day). Hesperidin at doses of 100 and 200 mg/kg treatment significantly restored the body weight and kidney weight associated with the gentamicin. Moreover, significant changes in the biochemical parameters such as increased levels of BUN, serum creatinine and decreased urinary creatinine and creatinine clearance was observed in the hesperidin treated rats. Further increased MDA levels and decreased SOD and CAT activity as well as GSH levels were attenuated with hesperidin treatment (200 mg/kg). Necrosis and degenerative changes in glomeruli and tubules, observed in gentamicin treated rats were also been restored. Thus it suggests the antioxidant potential of hesperidin protect the gentamicin induced nephrotoxicity in rats.
Keywords: Hesperidin; Gentamicin; Urea nitrogen; Antioxidant; Nephrotoxicity
Introduction
Acute tubular necrosis is a relatively common with aminoglycoside therapy resulting nephrotoxicity [1]. Gentamicin is an aminoglycoside antibiotic used to treat severe gram negative infections [2]. At physiologic pH, aminoglycoside molecules posses a cationic charge due to multiple amine groups, which bind to anionic charge on the phospholipids within the plasma membrane of the proximal tubule [3,4]. The accumulation of gentamicin in the proximal tubule interacts with intracellular metabolic processes, which depressed the renal function. Tubular cell injury due to gentamicin is characterized by cellular necrosis, mitochondrial structural alteration and suppression of free radical defense mechanism. The formations of free ions due to antibiotics results in production of hydrogen peroxide by the renal cortex and also inhibit the synthesis of phospholipase A2 and glutation [5]. Antioxidants have been observed the most consistent, safe and efficacious approaches used to ameliorate or protect gentamicin induced nephrotoxicity. Several reports suggest medicinal plant extracts with antioxidant properties protect nephrotoxicity induced by aminoglycosides [6,7].
Hesperidin is a flavanone glycoside derived from the word “hesperidium”, the kind of fruit produced by citrus trees as it is found abundantly in citrus fruits [8]. Hesperidin is mainly used as antioxidant, as it remarkably prevented indicators of oxidative stress, such as the ROS and lipid peroxidation levels in a dose-dependent manner [9]. Neves et al., proved that hesperidin is effective against sodium arsenite induced acute toxicity in mice, because it exhibits antioxidant activity [10]. It was also reported to protect and prevents embryos from oxidative stress, and may regenerate beta cells of endocrine pancreas in experimental diabetes pregnancy [11]. Moreover, it is reported as anti-inflammatory [12], anticancer [13], antihyperlipidemic [14], antihypertensive [15] and cardioprotective activity in ischemic heart disease in diabetic rats [16]. Therefore the present investigation was carried out to study the antioxidant potential of hesperidin protects gentamicin induced nephrotoxicity.
Materials and Methods
Drugs and chemicals
Gentamicin was purchased from local market of Pune (Genticyn, Piramal Healthcare, India), Hesperidin was obtained as gift sample (NANS Product, Mumbai), malondialdehyde (MDA), tetrabutyl ammonium and superoxide dismutase (Sigma-Aldrich, St. Louis), Catalase (Hi Media Laboratories Pvt. Ltd., Mumbai) and all other reagents and chemical were of analytical grade and purchased from local suppliers of Pune.
Animals
Sprague Dawley (SD) rats (180- 220 gm) were procured from National Institute of Biosciences, Pune. Rats were placed separately in polypropylene cages with paddy husk as bedding. The animals were maintained under standard laboratory conditions at temperature 23 ± 2°C with relative humidity 55 ± 10 % in a 12 h light and 12 h dark cycle throughout the experiment. Animals had free access to water and standard laboratory feed ad libitum (Nutrivet Lab, India). All the experimental procedures and protocols used in this study were reviewed and approved (IAEC/2011-12/33) by the Institutional Animal Ethics Committee (IAEC). Ethical guidelines were strictly followed during all the experimental procedures.
Experimental design
Thirty rats were randomly divided into five equal groups (n=6). Group 1: rats in this group were administered a vehicle 2% (W/V) gum acacia orally and served as a control. Group 2: rats in this group were administered a vehicle two days before and then injected with gentamicin (100 mg/kg/day) intraperitoneally for eight day and served as gentamicin control. Group 3-5: rats in these groups were treated orally with hesperidin at three doses (50, 100 and 200 mg/ kg) two days before and eight days concomitantly with gentamicin intraperitoneally (100 mg/kg). The hesperidin was suspended in 2% (W/V) gum acacia [17]. At the end of the treatment each rat was individually placed in metabolic cage for 24 h urine was collected and centrifuged at 1000 rpm for 10 min to remove cells and debris. Blood was collected from retrorbital plexus under light anesthesia and serum was separated by centrifugation at 3000 rpm for 15 min. The rats were sacrificed by cervical dislocation under ether anesthesia. The abdominal cavity was immediately opened; kidneys were removed and processed for antioxidant as well as histopathological studies.
Body weight and kidney weight change
Body weight of all animals was measured using digital electronic balance. After sacrificing, a kidney was dissected, rinsed in chilled saline, decapsulated blotted on filter paper and quickly weighed.
Relative kidney weight (%) = [Absolute kidney weight/Body weight at sacrifice] × 100
Biochemical estimations
A serum blood urea nitrogen levels was estimated using urea enzymatic colorimetric kit [18]. Serum and urinary levels of creatinine were estimated according to the method of Bartels et al. [19]. Creatinine clearance was calculated as per the following formula;
Ccr (mL/min/kg) = [urinary Cr (mg/dL) × urinary volume (mL)/ serum Cr (mg/dL)] × [1000/body weight (g)] × [1/1440 (min)]
Oxidative stress
Kidney was homogenized in chilled 50mM phosphate buffer saline (pH 7.4) in volume of nine times of its weight to yield 10% (w/v) tissue homogenate. The homogenates were centrifuged at 10500 rpm for 15 min at 4°C. The homogenate was used for the determination of malondialdehyde levels (MDA) [20], reduced glutathione (GSH) [21], and activities of SOD [22] and catalase (CAT) [23]. Protein concentrations of homogenates were determined according to Lowry et al. [24].
Histopathological studies
Kidney of individual rat stored in 10% formalin solution were embedded with paraffin and stained with Hematoxyline-Eosin (HE). HE stained sample was observed under light microscope (100 x).
Statistical analysis
All the data were expressed as the mean ± S.E.M (n=6). Data were subjected to one-way analysis of variance (ANOVA) followed by the Tukey’s multiple comparison test. Whereas, P<0.05 was set minimum levels of significance.
Result
Body weight, kidney weight and relative kidney weight
Intraperitoneal injection of gentamicin produced significant decrease in the body weight and increase in kidney weight compared to control (P<0.01). Whereas, hesperidin (200 mg/kg) produced most significant effect on the body weight and kidney weight which amounted to 8.09% (P<0.05) and 23.96 % (P<0.01) compared to gentamicin control rats. However, hesperidin at doses of 50 and 100 mg/kg body weight could not produce significant changes in the body weight and kidney weight compared to gentamicin control rats (Table 1).
Groups
Body Wt. (g)
Kidney Wt. (g)
% Relative Kidney Wt.
Control
217.67 ± 2.36
0.89 ± 0.01
0.42 0.09
Gentamicin
200.83 ± 3.59a
[8.20 %]
1.21 ± 0.03a
[35.96 %]
0.61 0.02a
199.78 ± 3.21
(1.68 %)
1.10 ± 0.05
(9.09 %)
0.57 0.02
Hesperidin (100)
204.85 ± 2.41
(2.59 %)
1.04 ± 0.04
(14.04 %)
0.50 ± 0.02*
Hesperidin (200)
214.45 ± 1.66*
(8.09 %)
0.92 ± 0.04#
(23.96 %)
0.44 ± 0.01@
aP< 0.01 compared to control. *P< 0.05, #P < 0.01, @P< 0.001 compared to gentamicin.
Numbers in [ ] indicates percentage decrease in body weight as compared to control.
Numbers in ( ) indicates percentage increase in body weight as compared to gentamicin.
Numbers in [ ] indicates percentage increase in kidney weight as compared to control.
Numbers in ( ) indicates percentage decrease in kidney weight as compared to gentamicin.
Table 1: Effect of hesperidin on body weight and kidney weight change.
Biochemical estimations
As shown in Table 2, eight days of gentamicin injection produced significant rise in blood urea nitrogen and serum creatinine levels as well as decrease urinary creatinine levels compared to control rats (P<0.001, P<0.001, P<0.001; respectively). Concomitant administration of gentamicin and hesperidin (100 and 200 mg/ kg) significantly reduced the elevated levels of BUN (P<0.01, P<0.001; respectively) and serum creatinine (P<0.05 and P<0.001; respectively) as well as increase in urinary creatinine levels (P<0.01 and P<0.001; respectively) compared to gentamicin control rats. Further, gentamicin produced decrease in creatinine clearance was significantly increased by hesperidin treatment at a dose of 200 mg/ kg (P<0001) (Figure 1).
Groups
BUN (mg/dl)
Sr. Creatinine (mg/dl)
Ur. Creatinine (mg/dl)
Control
24.50 ± 1.10
1.12 ± 0.08
82.68 ± 2.26
Gentamicin
47.24 ± 2.18b
3.53 ± 0.21b
33.77 ± 2.44b
Hesperidin (50)
39.33 ± 2.29
3.05 ± 0.11
44.28 ± 5.18
Hesperidin (100)
35.18 ± 2.85#
2.74 ± 0.19*
61.05 ± 3.41@
Hesperidin (200)
23.13 ± 1.15@
1.75 ± 0.15@
80.17 ± 3.99@
bP< 0.001 compared to control. *P< 0.05, #P < 0.01, @P< 0.001 compared to gentamicin
Table 2: Effect of hesperidin on biochemical estimations in gentamicin treated rats.
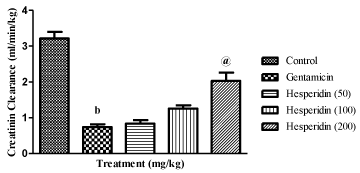
Figure 1: Effect of hesperidin on creatinine clearance in gentamicin treated
rats.
bP< 0.001 compared to control; @P< 0.001 compared to gentamicin.
Oxidative stress
Significant increase in the renal MDA levels (P<0.001) was observed in gentamicin control rats compared to control, this levels was significantly decreased with hesperidin (100 and 200 mg/kg) treatment (P<0.01 and P<0.001; respectively). Further, gentamicin treatment significantly decreased renal SOD (P<0.001) and CAT (P< 0.01) activities as well as GSH (P<0.001) levels compared to control rats. Whereas, hesperidin treatment (200 mg/kg) increased the activity of SOD (P<0.001) and CAT (P<0.01) as well as GSH levels (P<0.01) compared to gentamicin control rats. However hesperidin treatment at doses of 50 and 100 mg did not significant changes on the renal oxidative stress marker, except MDA and GSH levels, observed at dose of 100 mg/kg (Table 3).
Groups
MDA (nmol/mg)
SOD (U/mg)
GSH (nmol/mg)
CAT (U/mg)
Control
7.17 ± 0.48
32.31 ± 1.19
45.64 ± 2.44
18.98 ± 2.28
Gentamicin
11.58 ± 1.08b
23.72 ± 1.47b
30.88 ± 1.27b
13.03 ± 1.27a
Hesperidin(50)
10.98 ± 0.67
23.38 ± 2.17
33.82 ± 2.63
12.96± 0.98
Hesperidin(100)
7.08 ± 0.47#
26.68 ± 1.15
39.80 ± 1.32*
16.01 ± 1.34
Hesperidin(200)
5.84 ± 0.88@
30.88 ± 1.34@
41.48 ± 2.38#
18.75 ± 1.16#
aP< 0.01, bP< 0.001 compared to control. *P< 0.05, #P < 0.01, @P< 0.001 compared to gentamicin
Table 3: Effect of hesperidin on renal oxidative stress in gentamicin treated rats.
Histopathological Studies
Marked tubular necrosis, inflammation, blood vessel congestion and disintegrated nucleus was observed in gentamicin control rats, whereas control rats showed no abnormalities. Rats treated with hesperidin at doses of 100 and 200 mg/kg attenuated these changes. However hesperidin treated rats at a dose of 50 mg/kg showed mild effect on renal abnormalities (Figure 2).
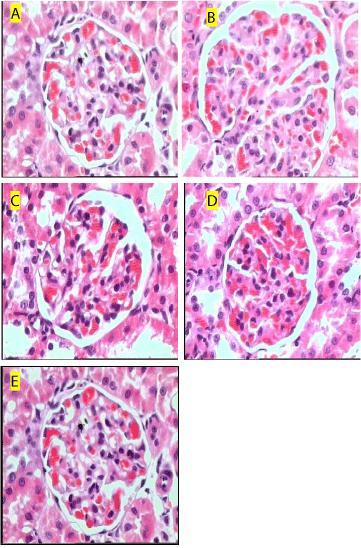
Figure 2: Histological section of kidneys stained with Hematoxyline and
Eosin (100x). control rats showing normal kidney cells (A); Gentamicin
treatment produced marked tubular necrosis, inflammation, and blood vessel
congestion (B); Rats treated with hesperidin (50 mg/kg) showing mild effect
on necrosis (C); Rats treated with hesperidin (100 and 200 mg/kg) showing
normal renal structure with mild congestion (D & E).
Discussion
Drug induced renal failure is the commonest cause of nephrotoxicity in 20-30 % of patient receiving aminoglycoside antibiotics, gentamicin at therapeutic doses. It accumulates in proximal tubules that lead to tubular dysfunction [4]. Various studies suggest high doses of gentamicin in animals; induce necrosis and renal dysfunction due to alteration in structural, metabolic and functional changes at the apical membrane [25,26]. Further, gentamicin forms complexes with mitochondrial Fe2+ to catalyze the formation of free oxygen radicals that alter mitochondrial functions in the renal proximal convoluted tubules. Therefore, antioxidants have been found most consistent approach in preventing or protecting nephrotoxicity associated with the gentamicin use.
In agreement of the previous reports, in the present study intraperitoneal administration of gentamicin (100 mg/kg) produced significant reduction in body weight [27]. According to Ali et al., increased catabolism and anorexia are responsible for decreased food intake and causes body weight loss [28]. Further, subsequent loss of the tubular cells, involved in renal water reabsorption leads to dehydration and decreases body weight [2]. Edema caused by drug induced acute tubular necrosis increase kidney weight [17]. In the present study, hesperidin (200 mg/kg) produced most significant effect on the body weight and kidney weight compared to gentamicin control rats. However, a restoration in the body weight and kidney weight was also observed at hesperidin 50 and 100 mg/kg treated animals, but the results could not found statistically significant.
Elevated levels of serum creatinine, is a potent indicator in the first phases of kidney disease [29]. Previous reports suggests gentamicin produced prominent kidney damage that leads to significantly higher levels of serum creatinine, blood urea nitrogen and decreased urine creatinine as well as marked reduction in creatinine clearance [30,31]. In the present study, we observed the significantly higher levels of serum creatinine, blood urea nitrogen and decreased urine creatinine as well as marked reduction in creatinine clearance following gentamicin treatment. Concomitant administration of gentamicin and hesperidin (100 and 200 mg/kg) significantly reduced the elevated levels of BUN and serum creatinine as well as increase in urinary creatinine levels compared to gentamicin control rats. Further, decrease in creatinine clearance was significantly increased by hesperidin treatment at a dose of 200 mg/kg.
Increase in the generation of Reactive Oxygen Species (ROS) like superoxide anions, hydroxyl radicals and hydrogen peroxides, and Reactive Nitrogen Species (RNS) in the renal cortex lead to renal damage and necrosis via several complex mechanisms including peroxidation of membrane lipids, protein denaturation and DNA damage [3,32]. Free radical scavengers interfere with the production of ROS, and have been used successfully to ameliorate gentamicin nephropathy. Antioxidant enzymes such as Superoxide Dismutase (SOD) and catalase protect the cells against oxidative stress mediated cellular injury by converting the toxic radicals to non-toxic end products. In the present investigation, significantly increased renal MDA levels associated with gentamicin was found decreased with hesperidin treatment (100 and 200 mg/kg). Further, gentamicin induced decreased renal SOD and CAT activities as well as GSH levels were also restored with hesperidin treatment at a dose of 200 mg/ kg. However hesperidin treatment at doses of 50 and 100 mg did not produce statistically significant changes on the renal oxidative stress marker, except MDA and GSH levels, observed at dose of 100 mg/ kg. Moreover, marked tubular necrosis, inflammation, blood vessel congestion and disintegrated nucleus was observed in gentamicin control rats, whereas control rats showed no abnormalities. Rats treated with hesperidin at doses of 100 and 200 mg/kg attenuated these changes. However hesperidin treated rats at a dose of 50 mg/kg showed mild effect on renal abnormalities.
Thus, hesperidin treatment at doses 100 and 200 mg/kg showed renoprotective effect against gentamicin, and this effect may be due to its antioxidant properties.
Conclusion
In conclusion, co-administration gentamicin and hesperidin at doses 100 and 200 mg/kg protect renal dysfunction through inhibiting free-radical formation and restoration of the antioxidant defense systems.
References
- Humes HD. Aminoglycoside nephrotoxicity. Kidney Int. 1988; 33: 900-911.
- Ali BH, Al-Qarawi AA, Haroun EM, Mousa HM. The effect of treatment with gum Arabic on gentamicin nephrotoxicity in rats: a preliminary study. Ren Fail. 2003; 25: 15-20.
- Nagai J, Takano M. Molecular aspects of renal handling of aminoglycosides and strategies for preventing the nephrotoxicity. Drug Metab Pharmacokinet. 2004; 19: 159-170.
- Smith CR, Baughman KL, Edwards CQ, Rogers JF, Lietman PS. Controlled comparison of amikacin and gentamicin. N Engl J Med. 1977; 296: 349-353.
- Soejima A, Ishizuka S, Miyake N, Fukuoka K, Suzuki M, Kamiya Y, et al. Simultaneous inhibition of renal phospholipase A(2) and glutathione synthesis by manoalide and DL-buthionine sulfoximine induces acute tubular dysfunction in rats. Exp Nephrol. 2000; 8: 84-90.
- Pedraza-Chaverrí J, Maldonado PD, Medina-Campos ON, Olivares-Corichi IM, Granados-Silvestre MA, Hernández-Pando R, et al. Garlic ameliorates gentamicin nephrotoxicity: relation to antioxidant enzymes. Free Radic Biol Med. 2000; 29: 602-611.
- Naidu MU, Shifow AA, Kumar KV, Ratnakar KS. Ginkgo biloba extract ameliorates gentamicin-induced nephrotoxicity in rats. Phytomedicine. 2000; 7: 191-197.
- Inderjit, Dakshini KM. Hesperetin 7-rutinoside (hesperidin) and taxifolin 3-arabinoside as germination and growth inhibitors in soils associated with the weed,Pluchea lanceolata (DC) C.B. Clarke (Asteraceae). J Chem Ecol. 1991; 17: 1585-1591.
- das Neves RN, Carvalho F, Carvalho M, Fernandes E, Soares E, de Bastos ML, et al. Protective activity of hesperidin and lipoic acid against sodium arsenite acute toxicity in mice. Toxicol Pathol. 2004; 32: 527-535.
- Chen M, Gu H, Ye Y, Lin B, Sun L, Deng W, et al. Protective effects of hesperidin against oxidative stress of tert-butyl hydroperoxide in human hepatocytes. Food Chem Toxicol. 2010; 48: 2980-2987.
- Ouali K, Trea F, Toumi L, Bairi A, Maurel D, Guellati MA. L’hesperidine, un antioxydant flavonoi de qui diminue le stress oxydatif et previent les malformations fœtales au cours du diabete gestationnel experimental. Phytotherapie. 2007; 5: 204–209.
- Emim JA, Oliveira AB, Lapa AJ. Pharmacological evaluation of the anti-inflammatory activity of a citrus bioflavonoid, hesperidin, and the isoflavonoids, duartin and claussequinone, in rats and mice. J Pharm Pharmacol. 1994; 46: 118-122.
- Lee KH, Yeh MH, Kao ST, Hung CM, Liu CJ, Huang YY, et al. The inhibitory effect of hesperidin on tumor cell invasiveness occurs via suppression of activator protein 1 and nuclear factor-kappaB in human hepatocellular carcinoma cells. Toxicol Lett. 2010; 15: 42-49.
- Monforte MT, Trovato A, Kirjavainen S, Forestieri AM, Galati EM, Lo Curto RB. Biological effects of hesperidin, a Citrus flavonoid. (note II): hypolipidemic activity on experimental hypercholesterolemia in rat. Farmaco. 1995; 50: 595-599.
- Ohtsuki K, Abe A, Mitsuzumi H, Kondo M, Uemura K, Iwasaki Y, et al. Glucosyl hesperidin improves serum cholesterol composition and inhibits hypertrophy in vasculature. J Nutr Sci Vitaminol (Tokyo). 2003; 49: 447-450.
- Agrawal YO, Sharma PK, Shrivastava B, Ojha S, Upadhya HM, Arya DS, et al. Hesperidin Produces Cardioprotective Activity via PPAR-? Pathway in Ischemic Heart Disease Model in Diabetic Rats. PLoS ONE. 2014; 9: e111212.
- Jain DP, Somani RS. Silibinin a bioactive flavanone in milk thistle ameliorate gentamicin induced nephrotoxicity. Pharmacologia. 2015; 9: 38-44.
- Fawcett JK, Scott JE. A rapid and precise method for the determination of urea. J Clin Pathol. 1960; 13: 156-159.
- Bartels H, Böhmer M, Heierli C. [Serum creatinine determination without protein precipitation]. Clin Chim Acta. 1972; 37: 193-197.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissue by thiobarbituric acid reaction. Anal Biochem. 1979; 95: 351-358.
- BEUTLER E, DURON O, KELLY BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963; 61: 882-888.
- Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988; 34: 497-500.
- Luck H. Methods of enzymatic analysis 197, Vol. III, Academic Press, New York, USA.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193: 265-275.
- Hishida A, Nakajima T, Yamada M, Kato A, Honda N. Roles of hemodynamic and tubular factors in gentamicin-mediated nephropathy. Ren Fail. 1994; 16: 109-116.
- Skopicki HA, Zikos D, Sukowski EJ, Fisher KA, Peterson DR. Gentamicin inhibits carrier-mediated dipeptide transport in kidney. Am J Physiol. 1996; 270: F531-538.
- Lakshmi BVS, Sudhakar M. Protective effect of Zingiber officinale on gentamicin induced nephrotoxicity. Int J Pharmacol. 2010; 6: 58–62.
- Ali BH, Abdel Gayoum AA, Bashir AA. Gentamicin nephrotoxicity in rat: some biochemical correlates. Pharmacol Toxicol. 1992; 70: 419-423.
- Gilbert DN, Wood CA, Kohlhepp SJ, Kohnen PW, Houghton DC, Finkbeiner HC, et al. Polyaspartic acid prevents experimental aminoglycoside nephrotoxicity. J Infect Dis. 1989; 159: 945-953.
- Silan C, Uzun O, Comunoğlu NU, Gokçen S, Bedirhan S, Cengiz M. Gentamicin-induced nephrotoxicity in rats ameliorated and healing effects of resveratrol. Biol Pharm Bull. 2007; 30: 79-83.
- Soliman KM, Abdul-Hamid M, Othman AI. Effect of carnosine on gentamicin-induced nephrotoxicity. Med Sci Monit. 2007; 13: BR73-83.
- Nagai J. Molecular mechanisms underlying renal accumulation of aminoglycoside antibiotics and mechanism-based approach for developing nonnephrotoxic aminoglycoside therapy. Yakugaku Zasshi. 2006; 126: 327-335.