
Research Article
Austin J Pharmacol Ther. 2015; 3(3).1073.
Potential Anti-Stress, Anxiolytic and Antidepressant Like Activities of Mono-Hydroxybenzoic Acids and Aspirin in Rodents: A Comparative Study
Saba Anjum Khan¹, Shyam Sunder Chatterjee2,3 and Vikas Kumar¹*
¹Neuropharmacology Research Laboratory, Department of Pharmaceutics, Indian Institute of Technology, Banaras Hindu University, India
²Stettiner Straße, Germany
³Retired Head of Pharmacology Research Laboratories, Dr. Willmar Schwabe GmbH & Co. KG, Germany
*Corresponding author: : Vikas Kumar, Neuropharmacology Research Laboratory, Department of Pharmaceutics, Indian Institute of Technology, Banaras Hindu University, Varanasi, India
Received: July 20, 2015; Accepted: August 17, 2015; Published: August 27, 2015
Abstract
Objective: To compare efficacies of aspirin and mono-hydroxybenzoic acids as anti-stress and potential anxiolytic and antidepressant agents.
Methods: Antidepressant and stress response suppressing effective daily oral doses of aspirin and 3-hydroxybenzoic acid were estimated in tail suspension test using foot shock stressed mice. Efficacies of 20 mg/kg daily oral doses of aspirin and mono-hydroxybenzoic acids in elevated plus maze and forced swimming tests were compared with those of diazepam (5 mg/kg/day) in foot shock stressed rats. Apart from stress triggered alterations in body weights and core temperatures, pretreatment effects on plasma glucose, cortisol and insulin levels and weights of adrenal gland and spleen were also compared in the rat experiment.
Results: Daily 3mg/kg oral doses of aspirin and 3-hydroxybenzoic acid protected mice against stress triggered alterations in body weights and core temperatures, whereas their minimally effective daily doses in tail suspension test were 30 and 3mg/kg respectively. Unlike low dose aspirin, none of the monohydroxybenzoic acids were active in rat forced swimming test for antidepressants, and diazepam like anxiolytic activity in stressed rats was observed for aspirin and 2-hydroxybenzoic acid only. All quantified stress triggered alterations in both mice and rats were either absent or less pronounced in all aspirin or monohydroxybenzoic acid treated groups.
Conclusion: Aspirin and all mono-hydroxybenzoic acids are stress response desensitizers, but only low dose aspirin and salicylic acid possess diazepam like effects in stressed rats. Unlike mono-hydroxybenzoic acids, aspirin possess antidepressants like activities in both stressed mice and rats after its low daily oral doses.
Keywords: Aspirin; Mono-hydroxybenzoic acids; Diazepam; Stresstriggered- hyperthermia; Anxiolytic; Anti-depressant
Introduction
Structurally diverse hydroxybenzoic acid derivatives are common constituents of plant derived food and other products regularly used for health maintenance or for recreational and therapeutic purposes. Amongst them the 2-, 3- and 4-hydroxylated benzoic acids are structurally the simplest ones, and they have been identified as bioactive constituents of numerous edible and other medicinal plants often used in Ayurvedic and other traditionally known systems of medicine and health care, or as ultimate or penultimate metabolites of structurally diverse aromatic phytochemicals, including those of some essential aromatic acids. However, the questions concerning their roles in traditionally known medicinal used herbal remedies still remain unanswered, or at the best can be speculative answered only.
It is now well recognized though, that 2-hydroxybenzoic acid (salicylic acid) possess a broad spectrum of therapeutically interesting bioactivities potentially useful for prevention and cure of diverse metabolic and other chronic diseases, including cancer, and that its bioactivity profile and side effect potentials depend on its daily doses and treatment regimen used [1-4]. Salicylic acid with bactericidal and antiseptic properties has since long been used as a food preservative [5], and such uses of easily hydrolysable esters of 4-hydroxybenzoic acid (Parabens) are still fairly common. Together with other phenolic acids, 3-hydroxybenzoic acid is also encountered in several common edible plants and in a GRAS food additives castoreum. More recent reports indicate also that like salicylic or nicotinic acids, 3-hydroxybenzoic acid could also have therapeutic potentials for treatment diabetes and other metabolic disorders [6-9].
Numerous observations made in our laboratories and elsewhere have consistently reconfirmed that fairly low repeated daily oral doses of herbal extracts and some of their bioactive constituents possess stress response modulating, antidepressant, anxiolytic and diverse other therapeutically interesting bioactivities in rodent models [10]. Recently we have reported also that fairly low daily oral doses of salicylic and 4-hydroxybenzoic acids desensitizes mice against foot shock stress triggered body weight losses and thermoregulatory disturbances, and also posses anxiolytics and antidepressant activities in stressed mice [11]. However, the questions whether aspirin (a pro-drug of salicylic acid) and 3-hydroxybenzoic acid also possess analogous stress response modulating activities after their low oral doses still remained unanswered. Therefore, aim of the two mice experiments reported in this communication was to assess dose ranges and treatment regimen of aspirin and 3-hydroxybenzoic acid in the same mouse bioassay used earlier for the other two mono-hydroxybenzoic acids. Results of these experiments revealed that like salicylic and 4-hydoxybenzoic acids, both aspirin and 3-hydroxybenzozic acid also possess stress response suppressing as well as antidepressant and anxiolytics like activities after their repeated daily fairly low oral doses. Therefore, the third reported experiment was conducted to compare anti-stress, and anxiolytics and antidepressants like effects of aspirin in an analogous bioassay using rats as experimental animals. Results of these experiments are described and discussed in this communication. Implications of the reported observations for better defining bioactive constituents of traditionally known medicinal herbs, or for obtaining novel therapeutic leads from hydroxybenzoic acids and their metabolic precursors are also pointed out.
Materials and Methods
Animals
Adult male Wistar rats (150±50 g) and male Swiss mice (20±5g) were obtained from Central Animal House of Institute of Medical Sciences, Banaras Hindu University, Varanasi (Registration number 542/AB/CPCSEA). They were acclimatized to constant laboratory conditions for at least one week before starting the experiments. They were randomly selected and group-housed (six animals per cage) in poly propylene cages (28×19×12.5 cm) and maintained at an ambient temperature (25±1oC) and relative humidity (50±10%) with a 12:12 h light /dark cycle (light on at 6:00 and off at 18:00). All cages were provided with husk, and except for observation periods all animals have always free access to standard rodent diet and tap water. Prior approval from the Central Animal Ethical Committee of the Banaras Hindu University was obtained (Dean/2014/CAEC/607; dated May 30, 2014) for the experimental protocol. All the experimental groups were tested on the same days of a given experiment, and they were handled, weighed and observed by blind observers using the same lab equipments.
Drugs and chemicals
Aspirin, 2-hydroxybenzoic acid, 3-hydroxybenzoic acid, 4-hydroxybenzoic acid, and fluoxetine were purchased from-HiMedia Laboratories Pvt. Ltd. Mumbai, India. Carboxymethyl Cellulose (CMC) was obtained from Central Drug House Pvt. Ltd., New Delhi, India and diazepam was acquired from Lupin Ltd., Jammu, India.
Animal grouping and drug administration
In mice dose finding experiments, the animals were randomly allotted to different experimental groups of six animals each. Standard drugs fluoxetine (10 mg/kg), diazepam (5 mg/kg) and graded oral doses (3, 10, 30, 100 and 300 mg/kg) of test agents were suspended in 0.3% CMC and orally administered for 11 consecutive days (application volume 10 ml/kg). Other details of bioassay procedure used were similar to those reported earlier [11], and are summarized in Figure 1(a).
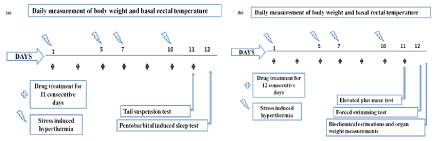
Figure 1: Summary of the experimental proceduers used in (a) the pilot dose finding experiments in mice, and (b) for evaluating the anxiolytic and anti-depressant
effects in stressed rats.
For the rat experiment, six randomly selected animals were allotted to each of the six experimental groups. One of them (control group) was treated once daily for 12 consecutive days with the vehicle (0.3% CMC), and four others were similarly treated with 20 mg/kg daily oral doses of aspirin or of one of the three mono-hydroxybenzoic acids. The sixth diazepam treated reference group was also similarly treated with its 5 mg/kg daily dose. One hour after treatments and recording their rectal temperatures, all animals were subjected to foot shock stress induced hyperthermia test on the 1st, 5th, 7th and 10th days of the experiment, and to elevated plus maze test for anxiolytics and forced swimming test for antidepressants on the 11th and 12th observational days. Immediately after the forced swimming test all rats were sacrificed by decapitation and their blood samples were collected for glucose, insulin and cortisol estimations. Immediately thereafter, adrenal glands and spleen of the animals were removed and weighed. Further details of this experiment are summarized in Figure 1(b).
Methodology
Stress induced hyperthermia
An individual animal of a group was placed in a black box (24 x 29 x 40 cm) with a grid floor for 1 min. After the animal had stayed there for 10 seconds, five consecutive foot shocks (2mA, 50 Hz of 2 ms duration) at 10 s intervals were delivered through the grid floor. At the end of the minute, the animals were placed back in their home cage, and 10 min thereafter its rectal temperature was recorded [12]. The difference in the basal rectal temperature of the animal on that day and 10 min after receiving foot shocks was considered as stress induced hyperthermic response of the animal on that day.
Tail suspension test
One hour after recording basal rectal temperature and treatment, each mouse was hung on a wire in an upside down posture. After initial vigorous movements, the mouse assumes an immobile posture and the period of immobility during a 5 min observation period was noted [13].
Pentobarbitone induced sleep test
Onset of sleep (loss of righting reflex) and duration of sleep induced by pentobarbitone induced hypnosis [14] was evaluated 24 hours after the last oral treatments. Pentobarbitone sodium (40 mg/kg) was administered intraperitoneally (application volume was 10 ml/kg). The rectal temperatures and body weights of mice were recorded before pentobarbitone administration.
Elevated plus maze test
The method described by Pellow and File (1986) was followed. The maze had opposite arms (50×10 cm) crossed with two enclosed arms of the same dimension but having 40 cm high walls. The arms were connected with a central square (10×10 cm) giving the apparatus the shape of plus sign. The maze was kept in dimly light room and elevated 50 cm above the floor. Rats were placed individually in the centre of maze, facing an enclosed arm. Thereafter, the number of entries and the time spent on the open and enclosed arms were recorded during the next 5 min [15]. An arm entry is defined when all four paws of rat were in the arm. Neutral blind observer made observations.
Forced swimming test
The method described by Willner (1984) was followed. In short, a rat was individually placed in a cylinder (45×20 cm) containing 38 cm water (25±2░C), so that it could not touch the bottom of the cylinder with its hind limb or tail, or climb over the edge of the chamber. Two swim sessions were allotted to each rat; an initial 15 min pretest session on day 11 of drugs treatment followed by a 5 min test session on the next day (i.e. on day 12 of the experiment). Period of immobility (i.e. the total time the animal remained floating in water without struggling and making only those movements necessary to keep its head above water) during the 5 min test period was measured [16].
Plasma glucose, insulin, cortisol level and organs weights
After decapitation, blood was collected directly by cardiac puncture in EDTA coated tubes and centrifuged at 1000×g for 20 min at 4oC to separate plasma (Compufuge CPR-30 Plus, with Rotor No. 8; REMI, India). Plasma glucose level was estimated by using biochemical enzyme test kit (ERBA diagnostics Mannheim GmbH, Germany), plasma insulin level by using Enzyme-Linked Immunosorbent Assay (ELISA) test kit (Chemux Bioscience, Inc, USA), and plasma cortisol by using ELISA kit (DSI S.r.l., Italy). All estimations were done by using absorbance microplate reader (iMarkTM- Bio-Rad Laboratories, California, USA) according to instructions manual of respective enzyme test kit. Immediately after blood collections, adrenal glands and spleen of the animals were dissected out, washed slowly under running tap water, excess water was removed using filter paper and weighed [17].
Statistical analysis
Means ± Standard Errors of Means (SEM) were calculated for the observed values in each experimental group. Statistical analysis was performed by Analysis of Variance (ANOVA) followed by Bonferroni post hoc test and t-test when stated. Graph Pad Prism-5 (Graph Pad Software Inc., La Jolla, California, USA) was used for statistical analysis. Origin-Pro 8 (Origin Lab Corporation, Massachusetts, USA) software was used for making graphs. P-value less than 0.05 was considered as statistically significant.
Results
Dose finding experiments in mice
Doses of aspirin and 3-hydroxybenzoic acid, and those of the reference drugs, were similar or the same as those used in earlier dose finding experiments conducted with salicylic and parahydroxybenzoic acid [11].
Body weights
Mean body weights of control and other treated groups of male mice during the course of the two experiments are summarized in Figures 2(a) and 2(b). These results are analogous to those made earlier with salicylic and 4-hydroxybenzoic acids and reconfirm that the mean body weights of CMC treated control groups consistently decrease during 12 observational days of the bioassay used. In both the experiments, mean body weights of the control groups recorded on the first observational day were not always significantly (p<0.05) different from those of the group recorded on subsequent observational days, but the mean body weight losses of both the control groups observed during the course of the experiments continue to increase significantly (p<0.05) during the cores of the experiments. Body weights of the fluoxetine or diazepam treated reference groups remained almost constant up to the 10th days of the experiments, but they also had some losses in mean body weights on the two subsequent days. Mean body weights of all aspirin or 3-hydroxybenzoic acid treated groups also remained constant up to the 10th day of the experiment, but after their 30 mg/kg or more daily doses mean body weights of the treated groups increased dose dependently on the last observational days. These observations reveal that, like 2- and 4-hydroxybenzoic acids, or like fluoxetine and diazepam, daily oral treatments with aspirin and 3-hydroxybenzoic acids protect the animals against occasional one minute duration of unavoidable foot shock stress triggered body weight losses, and that the minimally effective daily oral doses of aspirin and 4-hydroxybenzoic in protecting stress triggered body weight losses in male mice are 3 mg/kg/day or lower, and that unlike the psychoactive drugs fluoxetine or diazepam, daily oral treatments with aspirin or 3-hydroxybenzoic acid have longer lasting protective effects against occasional exposures to foot shock stress triggered body weight losses.
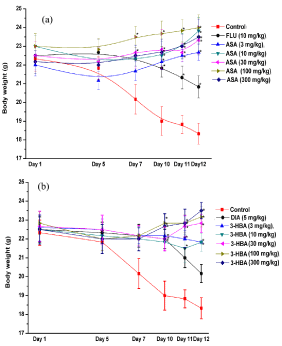
Figure 2: Effects of occasional foot shock stress on body weights of male
mice once daily treated either with aspirin (a) or with 3-hydroxybenzoic
acid (b) for 11 consecutive days. Abbreviations: ASA = Aspirin, 3-HBA =
3-hydroxybenzoic acid, FLU = Fluoxetine, DIA = Diazepam. Values are mean
± SEM (n=6). * denotes statistically significant difference (Two way ANOVA
followed by Bonferroni post hoc test) relative to the corresponding vales of the
control groups on a given observational day (*=p<0.05).
Basal core temperatures
These results are summarized in the Figures 3(a) and 3(b). As expected from earlier observations made under similar experimental conditions, the mean basal rectal temperatures of both the vehicles treated control groups increased on 5th observational days and continued to increase further up to the last days of experiments. These intermittent foot-shock induced elevations of core temperatures were antagonized significantly (p<0.05) both by aspirin and 3-hydroxybenzoic acid and also by the reference drugs. Unlike in the control groups, basal core temperature of fluoxetine or diazepam, or aspirin or 3-hydroxybenzoic acid treated groups remained almost constant on all observational days. A notable exception was the 3 mg/ kg daily 3-hydroxybenzoic acid treated group, the mean basal rectal temperature of which was somewhat higher on the 5th observational day. But this mean value of the group was statistically not significantly different from the mean value of the group observed on the first day of the experiment.
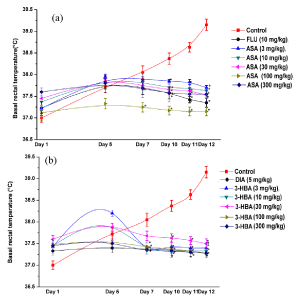
Figure 3: Effects of occasional foot shock stress on basal rectal temperature
of male mice once daily treated either with aspirin (a) or with 3-hydroxybenzoic
acid (b) for 11 consecutive days. Abbreviations: ASA = Aspirin, 3-HBA =
3-hydroxybenzoic acid, FLU = Fluoxetine, DIA = Diazepam. Values are mean
± SEM (n=6). * denotes statistically significant difference (Two way ANOVA
followed by Bonferroni post hoc test) relative to the corresponding vales of the
control groups on a given observational day (*=p<0.05).
Stress induced hyperthermia
Despite ca. 10% body weight losses and slight elevations of mean basal rectal temperature of the vehicle treated control groups during the course of the experiments, their transient hyperthermic responses triggered by one minute duration of unavoidable foot shocks remained almost constant on all observational days. These responses of the reference drugs fluoxetine (10 mg/kg/day) and diazepam (5 mg/kg/ day) treated groups were always lower than the corresponding control groups on all observational days, but their statistically significant inhibitory effects were observed only after their seven or more daily doses. Fluoxetine like significant inhibitory effects of aspirin against foot shock stress triggered transient hyperthermia were observed only after its seven or more daily 100 and 300 mg/kg oral doses and on the 10th and last observational day statistically significant effects of even 30 mg/kg/day dose of aspirin was also observed (Figure 4a). However, on all observational days the mean values of foot shock stress triggered hyperthermic responses all aspirin treated groups continued to be dose dependently decreased and this efficacy of aspirin continued to increase with increasing number of treatment days. Somewhat analogous were also the observations made with 3-hydroxbenzoic acid and diazepam (Figure 5a). From the log dose response curves of aspirin and 3-hydroxybenzoic acid shown in Figures 4(b) and 5(b), it is apparent that, statistically significant minimally effective doses of both of them in this test after their 10 daily oral doses is 30 mg/kg.
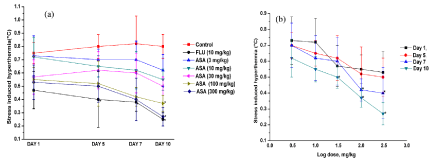
Figure 4: (a) Effects of daily oral doses of aspirin in mice footshock stress induced hyperthermia test on the 1st, 5th, 7th and 10th observational days of the dose
finding experiment and (b) its log dose response curves drawn from the observe values on different observational days. Abbreviations: ASA = Aspirin, FLU =
Fluoxetine. Values are mean ± SEM (n=6). * denotes statistically significant difference (Two way ANOVA followed by Bonferroni post hoc test) relative to the
corresponding vales of the control group on a given observational day (*=p<0.05).
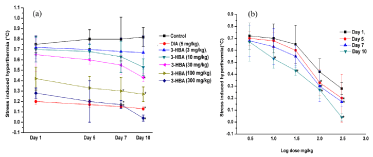
Figure 5: (a) Effects of daily oral doses of 3-hydroxybenzoic acid in mice footshock stress induced hyperthermia test on the 1st, 5th, 7th and 10th observational
days of the dose finding experiment and (b) its log dose response curves drawn from the observe values on different observational days. Abbreviations: 3-HBA =
3-hydroxybenzoic acid, DIA = Diazepam. Values are mean ± SEM (n=6). * denotes statistically significant difference (Two way ANOVA followed by Bonferroni post
hoc test) relative to the corresponding vales of the control groups on a given observational day (*=p<0.05).
Tail suspension test
In this test, often used for detecting antidepressant like effects of test agents, mean immobility period of the fluoxetine treated and the 30 mg/kg and higher daily aspirin treated groups were significantly (p<0.05) lower than the control group (Figure 6a). However, the efficacy of even the highest dose of aspirin tested was much lower than that of fluoxetine. As expected, diazepam had no significant effects in this test, but statistically significant effects of 3-hydroxybenzoic acid was observed even after its lowest tested daily dose tested (Figure 6b). Observed efficacies of the acid increased with its increasing dose, but effects of its highest dose tested were also quantitatively much lower than that of fluoxetine observed in the other experiment.
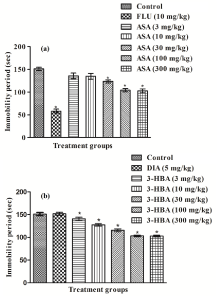
Figure 6: Effects of 11 daily oral doses of (a) aspirin or (b) 3-hydroxybenzoic
acid on immobility period in tail suspension test on male mice. Abbreviations:
ASA = Aspirin, 3-HBA = 3-hydroxybenzoic acid, FLU = Fluoxetine, DIA =
Diazepam. Values are mean ± SEM (n=6). * denotes statistically significant
difference (one way ANOVA followed by student t-test) relative to control
group (*=p<0.05).
Pentobarbitone induced sleep test
In the mouse bioassay used in this and our earlier studies for estimating pharmacologically interesting doses and dosing regimen, this test is conducted 24 hour after the last treatment for assessing possible long lasting effects of test agents as sedatives or modulators of drug metabolizing enzymes. No statistically significant effects of as aspirin and 3-hydroxybenzoic acid, or the reference drugs diazepam and fluoxetine, on the onset time of sleep induces by pentobarbitone were observed (results not shown). However, duration of pentobarbitone induced sleep was significantly shorter in the fluoxetine treated group and that of the diazepam treated one significantly longer than the corresponding control groups. Statistically significant and fluoxetine like shortening of duration of sleep were observed in the aspirin treated groups only after its two highest daily doses (100 and 300 mg/kg/day) tested (Figure 7a). Statistically significant and diazepam like prolongation of pentobarbitone induced sleep duration was observed for 3-hydroxybenzoic acid after its 30 mg/kg and higher daily doses only (Figure 7b).
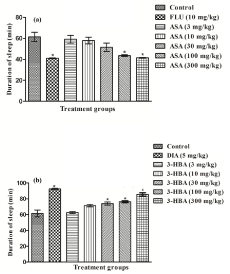
Figure 7: Effects of 11 daily oral doses of (a) aspirin or (b) 3-hydroxybenzoic
acid on pentobarbitone induced duration sleep in of male mice. Abbreviations:
ASA = Aspirin, 3-HBA = 3-hydroxybenzoic acid, FLU = Fluoxetine, DIA =
Diazepam. Values are mean ± SEM (n=6). * denotes statistically significant
difference (one way ANOVA followed by student t-test) relative to control
group (*=p<0.05).
Anxiolytic and anti-depressant activity in rats
Except for their protective effects against foot shock stress triggered body weight losses and slight elevation in basal core temperatures of stressed mice, diverse other statistically significant effects of aspirin and the hydroxybenzoic acid in all other tests conducted during the dose finding experiments were also observed after their 30 mg/ kg daily oral doses. Therefore, these experiments were conducted to experimentally verify the possibility whether even their lower daily oral doses (20 mg/kg/day) possess stress response suppressing and anxiolytic and antidepressants like activities in stressed rats or not.
Body weights and basal rectal temperatures
Mean body weights of the vehicle treated control group on the day 5th and the subsequent observational days were lower, and their mean basal rectal temperatures on those days were slightly higher than those recorded on the first treatment day of the experiment (Figure 8a and 8b). Body weight losses during the course of the experiment were also observed in the diazepam treated group, but mean basal rectal temperatures of this group remained almost constant on all observational days. Observed protective effects of aspirin and all the three hydroxybenzoic acids against occasional unpredictable short duration (1 min) of foot shock stress triggered body weight losses and slight elevation in basal core temperatures were almost identical. From the 7th and subsequent treatment days onwards, mean body weights of the aspirin or hydroxybenzoic acids treated groups steadily increased (Figure 8a). Although mean rectal temperatures of these groups also increased somewhat on the 5th observational day, this increase was quantitatively less, than those observed in the vehicle treated control groups, and on subsequent observational days their mean basal temperatures steadily decreased towards the values recorded for them on the 1st day of the experiment (Figure 8b). These results were quite analogous to those observed in mice dose finding experiments, and reveal that daily 20 mg/kg oral doses of aspirin and all the three hydroxybenzoic acids are high enough for counteracting mild unpredictable stress triggered body weight losses and slight elevation of basal rectal temperatures in rats as well.
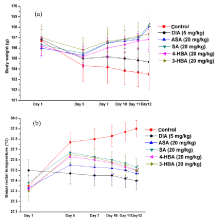
Figure 8: Effects of occasional foot shock stress on (a) body weights and
(b) basal rectal temperatures of male rats once daily treated either with
aspirin or the three hydroxybenzoic acids. Abbreviations: DIA = Diazepam,
ASA = Aspirin, SA = Salicylic acid, 4-HBA = 4-hydroxybenzoic acid, 3-HBA =
3-hydroxybenzoic acid. Values are mean ± SEM (n=6). * denotes statistically
significant difference (Two way ANOVA followed by Bonferroni post hoc
test) relative to the vales of the control groups on a given observational day
(*=p<0.05).
Stress induced hyperthermia
Except for the diazepam treated group, magnitudes of transient hyperthermic responses triggered by short duration of unavoidable foot shocks of the vehicle treated control and all other groups after their single oral doses were not statistically significantly different from each other (Figure 9). Unlike in mice dose finding experiments, statistically significant effects of 5 mg/kg/day diazepam treated group were observed on all treatment and observational days, and its efficacy in this test remained almost constant on all those days. Although statistically significant observed effects of aspirin and salicylic acid on the 5th observational day were quantitatively a bit higher than those of 3- or 4- hydroxy benzoic acids, these differences disappeared one hour after their 7 and 10 daily doses. Quantitatively, the observed effect of 10 daily 20mg/kg/day doses of aspirin or of the three hydroxybenzoic acids was almost identical to those observed for 5 mg/kg/day diazepam.
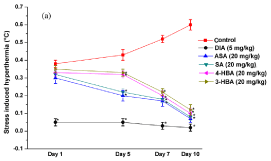
Figure 9: Effects of daily oral doses of aspirin or the three hydroxybenzoic
acids in rat footshock stress induced hyperthermia test on the 1st, 5th, 7th
and 10th observational days of further experiments. Abbreviations: DIA =
Diazepam, ASA = Aspirin, SA = Salicylic acid, 4-HBA = 4-hydroxybenzoic
acid, 3-HBA = 3-hydroxybenzoic acid. Values are mean ± SEM (n=6). *
denotes statistically significant difference (Two way ANOVA followed by
Bonferroni post hoc test) relative the vales of the control groups on a given
observational day (*=p<0.05).
Elevated plus maze test
The mean percentages of entries in the open arm and time spent there of the 11 daily 5 mg/kg/day oral diazepam treated or 20 mg/kg/ day aspirin or salicylic acid treated groups were significantly higher and than those of the vehicle treated control one. Quantitatively the anxiolytic efficacies of diazepam observed in this test was almost identical to those of aspirin or salicylic acid (Figure 10a). However, in this test no significant effects of 3- or 4- hydroxybenzoic acids were observed. Therefore, it seems reasonable to assume that unlike 3- or 4-hydroxybenzoic acids, aspirin and salicylic acid also possess diazepam like anxiolytic activities after their repeated daily doses, and that 3- and 4- hydroxy benzoic acids are more specific foot shock stress triggered physiological responses involving homeostatic processes regulating body weight and temperature than aspirin or salicylic acid.
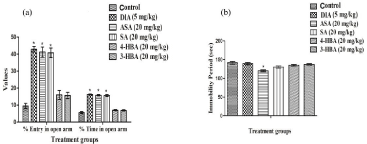
Figure 10: Effects of daily oral doses of aspirin or the three hydroxybenzoic acids on (a) elevated plus maze test and (b) forced swimming test on male rats.
Abbreviations: DIA = Diazepam, ASA = Aspirin, SA = Salicylic acid, 4-HBA = 4-hydroxybenzoic acid, 3-HBA = 3-hydroxybenzoic acid. Values are mean ± SEM
(n=6). * denotes statistically significant difference (One way ANOVA followed by student t- test) relative to control group (*=p<0.05).
Forced swimming test
The mean immobility period of the diazepam and 2- or 3- or 4-hydroxybenzoic acids treated groups in this test for antidepressants activity, conducted one hour after their 12 daily oral doses in stressed rats were not significantly different from the vehicle treated control group (Figure 10b). However, this value of the aspirin treated group in the test was the lowest and statistically significant different from that of the control group (p<0.05). These results indicate that under the experimental condition used, salicylic acid is not always the bioactive metabolite of aspirin, and aspirin also possess some antidepressant like activity even after its fairly low oral doses.
Biochemical estimation and organ weights
These results are summarized in the tables 1 and 2. As compared to the mean values of the vehicle treated control group, significantly lower mean plasma glucose and cortisol and higher insulin levels were observed in the diazepam treated or aspirin or 2- or 3- or 4-hydroxybenzoic acid treated groups (Table 1). Although the absolute adrenal gland and spleen weights of the vehicle treated control groups were also statistically significantly different from all other treated groups, such were not the cases for the relative organ weights (Table 2). These observations could indicate that daily oral treatments with diazepam or with low doses of aspirin and all the three mono-hydroxybenzoic acids modulate the contents of extracellular fluids in those organs and most probably have no effects on cellular growth in those organs. However, more detailed studies will be necessary to experimentally verify this possibility.
Treatment Groups
Glucose (mg/dl)
Insulin
(ÁIU/ ml)
Cortisol
(ng/ml)
Control
134.33±2.86
9.33±0.84
112.83±2.93
DIA (5mg/kg)
91.83±3.20*
18.00±0.63*
93.33±1.89*
ASA (20 mg/kg)
92.17±3.81*
15.00±1.32*
94.83±1.22*
SA (20 mg/kg)
90.83±2.77*
14.83±1.45*
95.33±1.09*
4-HBA (20 mg/kg)
114.17±3.91*
13.17±0.54*
99.83±2.79*
3-HBA (20 mg/kg)
116.50±3.80*
13.50±0.99*
99.67±2.88*
Abbreviations: DIA = Diazepam, ASA = Aspirin, SA = Salicylic Acid, 3-HBA = 3-Hydroxybenzoic Acid, 4-HBA = 4-Hydroxybenzoic Acid. Values are mean ± SEM (n=6). * denotes statistically significant difference (one way ANOVA followed by student t-test) relative to control group (*=p<0.05).
Table 1: Effects of 12 daily oral doses of aspirin and of the three hydroxybenzoic acids on plasma glucose, insulin and cortisol level in stressed male rats.
Treatment Groups
Absolute organ weight (mg)
Relative organ weight
(mg/gm of body weight)
Adrenal gland
Spleen
Adrenal gland
Spleen
Control
51.17±2.75
139.50±3.37
0.31±0.09
0.85±0.36
DIA (5mg/kg)
37.50±1.78*
195.33±4.89*
0.23±0.08
1.19±0.95
ASA (20 mg/kg)
38.67±1.52*
186.50±3.98*
0.23±0.05
1.12±0.12
SA (20 mg/kg)
40.67±1.38*
185.17±3.47*
0.24±0.09
1.10±0.93
4-HBA (20 mg/kg)
42.83±1.25*
169.67±2.35*
0.26±0.02
1.02±0.39
3-HBA (20 mg/kg)
43.50±1.61*
164.50±2.99*
0.26±0.03
0.98±0.48
Abbreviations: DIA = Diazepam, ASA = Aspirin, SA = Salicylic Acid, 3-HBA = 3-Hydroxybenzoic Acid, 4-HBA = 4-Hydroxybenzoic Acid. Values are mean ± SEM (n=6). * denotes statistically significant difference (one way ANOVA followed student by t-test) relative to control group (*=p<0.05).
Table 2: Effect of 12 daily oral doses of aspirin (ASA) and hydroxybenzoic acids on absolute and relative weights of adrenal glands and spleen of male rats subjected to stress induced hyperthermia on days 1, 5, 7 and 10 of treatments.
Discussion
Reported observations reveal and reconfirm that low dose (20 mg/ kg/day or lower) aspirin and 3-hydroxybenzoic acid are salicylic and 4-hydroxybezoic acids like desensitizers of diverse stress responses, and that in this respect they are almost equiactive after their repeated daily oral doses. Like numerous edible and other phytochemicals and drugs, their efficacies continue to increases with increasing numbers of treatment days, and their anxiolytic and antidepressant like efficacies in rodent models become apparent after their repeated daily doses only. However, their psychopharmacological activity profiles in rats and mice, or in different rodent models, do not seem to be identical. Thus, although low dose aspirin had anxiolytics and antidepressants like activities in both rats and mice, antidepressants like efficacy of all the three hydroxybenzoic acids were observed in mice tale suspension test only and not in rat forced swimming test. Similarly, although anxiolytics like efficacies of aspirin and all three hydroxybenzoic acids in foot shock stress induced hyperthermia tests in stressed rats and mice were observed, only salicylic acid and aspirin had significant diazepam like anxiolytics activities in stressed rat elevated plus maze test. Further dose response and other studies using the same behavioural models in rats and mice will be necessary for clarifying the observed differences in the psychopharmacological activity profiles of aspirin and the mono-oxygenated benzoic acids.
Observations reported in this communication are the very first ones revealing modulating effects of diazepam and low dose aspirin and mono-hydroxybenzoic acids on glucose and insulin homeostasis and on cortisol and adrenal gland and spleen weights in rats subject to occasional exposures to inescapable foot shock stress of short duration only (1 min). These metabolic or physiological biomarkers are reliable ones for estimating the functions of Hypothalamic Adrenal Axis (HPA) involved in regulating stress responses, eating behavior and thermoregulation as well [18,19]. Diazepam is well known for its anti-stress and anxiolytic activities and it has often been reported that it also modulates glucose homeostasis in patients as well as in rodents [20-23]. It is now well recognized also that low dose aspirin and salicylates also possess analogous clinical efficacies and that their regular intake are effective in preventing obesity and diabetes associated metabolic disorders and mental health problems, including depression and cognitive disorders [24-26]. Our observations strongly suggest that low dose 3- and 4-hydoxybenzoic acids are also salicylic acid like stress response desensitizing agents capable of modulating the biological processes and mechanisms involved in thermoregulation and body weight changes. Therefore, it can be expected that all mono-hydroxybenzoic acids are also potential therapy relevant bioactive constituents of numerous traditionally known herbal remedies often used in almost all traditionally known systems of medicine for prevention and cure of diverse lifestyle diseases, including obesity and diabetes.
Salicylic acid and 4-hydroxy-hydroxybenzoic acids are also the ultimate or penultimate metabolites of structurally diverse phytochemicals commonly consumed with every day meals or herbal remedies, and also of some essential aromatic amino acids. Therefore, it is apparent that their blood or urine levels observed after their oral intake are not very reliable predictors of their oral bioavailability, or of their pharmacological efficacies observed after their regular oral intakes. Due to their antimicrobial activity and acidity and other physicochemical properties, they can alter microbial ecology inside the gastrointestinal tract, and thus could also contribute to their therapeutically interesting bioactivities after their regular oral intake. Involvement of these biological processes in the modes of actions of food phytochemicals and other xeno- or syn-biotics are now well recognized [27], and it has recently been pointed out also that acclimatization-induced stress can also influences host metabolic and microbial composition [28].
However as yet no very convenient, reliable, pharmacologically well validated, and more holistic bioassay system is available for identifying the gut microbial ecology modulating phytochemicals potentially useful as drug leads urgently needed for prevention and cure of diabesity and other metabolic disorders spreading like epidemics in the 21st century [29,30]. The bioassay procedures used in this study evolved from numerous observations made during our ongoing efforts to define psychopharmacological activity profiles of plant extracts currently widely used in traditionally known systems of medicine and health care in India and other countries, and to identify their bioactive constituents [31]. Observations reported in this communication add further experimental evidences in support of the assumption that like numerous other phytochemicals all the three structurally simple mono-hydroxybenzoic acids often encountered in numerous edible and other plants can also contribute to the broad spectrums of pharmacological activity profiles of their medicinally used extracts. Moreover, they strongly suggest also that the clinically observed emotion modulating and other bioactivities of regular low dose aspirin intake [32,33] could as well be due to its stress response modulating effects and that its primary metabolite salicylic acid also contributes to its diverse, but not all, therapeutically interesting bioactivities. In addition, they reaffirm that the bioassay procedure used in this and in our earlier studies [13,34-41] is not only a convenient and reliable one for estimating the dose and dosing regimen of stress response suppressing agents in rats and mice, but also for clarifying their structure activity relationship necessary for more rational designing of drug leads urgently needed for prevention and cure of mental health problems accompanying almost all inflammatory chronic diseases. Therefore, it seems reasonable to suggest that diverse versions of this bioassay system could as well be used as a starting point not only for obtaining more realistic information on the bioactive principles and modes of actions of traditionally known herbal remedies, but also for discovering and developing novel drug leads and pharmacological targets urgently needed for preventions and cure of diverse spectrums of stress triggered pathologies and life threatening diseases.
In any case, it seems certain that salicylic acid is the major bioactive metabolite of low dose aspirin involved in its many (but not all) therapeutically observed beneficial effects against mental health problem, and that both 3- and 4-hydroxybenzoic acids can also be considered as potential therapeutic leads for prevention and cure of stress triggered psychopathologies. However therapeutically the more interesting or urgent questions whether salicylic acid is also involved in ulcerogenic effects of aspirin, or whether low dose 3- and 4-hydroxybenzoic acids also possess aspirin like anti-inflammatory, analgesic, and ulcerogenic potentials still remain open. Therefore, efforts are now being made in our laboratories to compare the efficacies of low dose mono-hydroxybenzoic acids and aspirin in animal models of inflammation, pain and gastric ulcers. Results of these efforts could eventually lead to pharmaceuticals or polypills containing combinations of low dose aspirin with mono-hydroxybenzoic acids, which could have broader therapeutic potentials and safety margins than low dose aspirin itself. Since unlike aspirin, salicylic acid (and most probably the other two mono-hydroxybenzoic acids?) does not acetylate prostaglandin synthesizing enzymes and other biological targets and yet possess aspirin like stress response desensitizing and other therapeutically interesting bioactivities, possibility of success in such ventures can be considered as fairly high. Moreover, these efforts will certainly be useful for better understanding of pharmacological principles behind traditionally known medicinal uses of numerous plants, and thus could enable more rational modernizations of traditionally known herbal remedies.
Such remedies still continue to be the major therapeutic possibilities for a vast majority of global population, and during more recent decades their popularities have also increased in almost all economically more developed countries as well. Numerous plant derived products now often used in such remedies posses stress response modulating or adaptogenic properties, and the numbers of reports identifying salicylic or 4-hydroxybenzoic acids as their bioactive constituents have also continued to increase during more recent decades. Pivotal roles of stress and adaptive physiological processes in health and diseases are now well recognized [42], and our observations reveal and reconfirm that all mono-hydroxybenzoic acids possess adaptogenic or physiological stress resistance inducing properties. Therefore, it seems reasonable to suggest that further efforts to define their sites and modes of actions could not only be useful for better understanding of pharmacological principles behind traditionally known medicinal uses of traditionally known herbal remedies, but also could eventually lead to the identification of novel pharmacological targets urgently needed for prevention and cure of diverse spectrums of stress triggered co-morbid mental health problems.
Conclusion
All three naturally occurring mono-hydroxybenzoic acids are low dose aspirin like inducers of stress resistance against chronic, unavoidable, and short durations of exposures to footshock stress triggered alterations in thermoregulatory mechanisms and body weight changes in rodents. Bioactivity profiles of low dose aspirin are analogous, but not identical, to those of low dose salicylic and the 3- and 4-hydroxybenzoic acids are more specific stress response regulators than salicylic acid or aspirin.
References
- Rena G, Sakamoto K. Salicylic acid: old and new implications for the treatment of type 2 diabetes? Diabetology International. 2014; 5: 212-218.
- Goldfine AB, Conlin PR, Halperin F, Koska J, Permana P, Schwenke D, et al. A randomised trial of salsalate for insulin resistance and cardiovascular risk factors in persons with abnormal glucose tolerance. Diabetologia. 2013; 56: 714-723.
- Paterson JR, Lawrence JR. Salicylic acid: a link between aspirin, diet and the prevention of colorectal cancer. QJM. 2001; 94: 445-448.
- Jankowski JA, Limburg PJ. Aspirin therapy for cancer: it is never too late. Br J Cancer. 2011; 105: 1105-1106.
- Macalister CJ, Bradshaw TR. The use of salicylic acid as a preservative in food. The Lancet. 1903; 161: 717-720.
- Liu C, Kuei C, Zhu J, Yu J, Zhang L, Shih A, et al. 3,5-Dihydroxybenzoic acid, a specific agonist for hydroxycarboxylic acid 1, inhibits lipolysis in adipocytes. J Pharmacol Exp Ther. 2012; 341: 794-801.
- Offermanns S. Free Fatty Acid (FFA) and Hydroxy Carboxylic Acid (HCA) receptors. Annu Rev Pharmacol Toxicol. 2014; 54: 407-434.
- Sekiguchi H, Kasubuchi M, Hasegawa S, Pelisch N, Kimura I, Ichimura A. A novel antidiabetic therapy: free fatty acid receptors as potential drug target. Curr Diabetes Rev. 2015; 11: 107-115.
- Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A, Kimura I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients. 2015; 7: 2839-2849.
- Kumar V, Chatterjee SS. Single and Repeated Dose Effects of Phytochemicals in Rodent Behavioural Models. EC Pharmaceutical Science. 2014; 1: 16-18.
- Langstieh AJ, Verma P, Thakur AK, Chatterjee SS, Kumar V. Desensitization of mild stress triggered responses in mice by a Brassica juncea leaf extract and some ubiquitous secondary plant metabolites. Pharmacologia. 2014; 5: 326-338.
- Zethof TJ, Van der Heyden JA, Tolboom JT, Olivier B. Stress-induced hyperthermia in mice: a methodological study. Physiol Behav. 1994; 55: 109-115.
- Thakur AK, Chatterjee SS, Kumar V. Antidepressant-like effects of Brassica juncea L. leaves in diabetic rodents. Indian J Exp Biol. 2014; 52: 613-622.
- Ojima K, Matsumoto K, Tohda M, Watanabe H. Hyperactivity of central noradrenergic and CRF systems is involved in social isolation-induced decrease in pentobarbital sleep. Brain Res. 1995; 684: 87-94.
- Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav. 1986; 24: 525-529.
- Willner P. The validity of animal models of depression. Psychopharmacology (Berl). 1984; 83: 1-16.
- Salman TM, Alagbonsi IA, Biliaminu SA. Blood glucose-lowering effect of Telfairia Occidentalis: A preliminary study on the underlying mechanism and responses. Biokemistri. 2013; 25: 133-139.
- Sinha R, Jastreboff AM. Stress as a common risk factor for obesity and addiction. Biol Psychiatry. 2013; 73: 827-835.
- Bazhan N, Zelena D. Food-intake regulation during stress by the hypothalamo-pituitary-adrenal axis. Brain Res Bull. 2013; 95: 46-53.
- Garabadu D, Krishnamurthy S. Diazepam potentiates the antidiabetic, antistress and anxiolytic activities of metformin in type-2 diabetes mellitus with cooccurring stress in experimental animals. Biomed Res Int. 2014; 2014: 693074.
- al-Ahmed FA, el-Denshary ES, Zaki M, el-Sawaf HA, Abu-Jayyab AR. Interaction between diazepam and oral antidiabetic agents on serum glucose, insulin and chromium levels in rats. Biosci Rep. 1989; 9: 347-350.
- Nade VS, Kawale LA, Naik RA, Yadav AV. Adaptogenic effect of Morus alba on chronic footshock-induced stress in rats. Indian J Pharmacol. 2009; 41: 246-251.
- Kulkarni MP, Juvekar AR. Attenuation of Acute and Chronic Restraint Stress-induced Perturbations in Experimental Animals by Nelumbo nucifera Gaertn. Indian J Pharm Sci. 2008; 70: 327-332.
- Simpson SH, Gamble JM, Mereu L, Chambers T. Effect of aspirin dose on mortality and cardiovascular events in people with diabetes: a meta-analysis. J Gen Intern Med. 2011; 26: 1336-1344.
- Berk M, Dean O, Drexhage H, McNeil JJ, Moylan S, O'Neil A, et al. Aspirin: a review of its neurobiological properties and therapeutic potential for mental illness. BMC Med. 2013; 11: 74.
- Rahola JG. Somatic drugs for psychiatric diseases: aspirin or simvastatin for depression? Curr Neuropharmacol. 2012; 10: 139-158.
- Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, et al. Host-gut microbiota metabolic interactions. Science. 2012; 336: 1262-1267.
- Yap IK, Kho MT, Lim SH, Ismail NH, Yam WK, Chong CW. Acclimatisation-induced stress influenced host metabolic and gut microbial composition change. Mol Biosyst. 2015; 11: 297-306.
- Farag YM, Gaballa MR. Diabesity: an overview of a rising epidemic. Nephrol Dial Transplant. 2011; 26: 28-35.
- Zimmet P. Diabesity–the biggest epidemic in human history. Med Gen Med. 2007; 9: 39.
- Chatterjee SS, Kumar V. Holistic psychopharmacology and promiscuous plants and principles of ayurveda. Am J Plant Sci. 2012; 3: 1015-1021.
- Ketterer MW, Brymer J, Rhoads K, Kraft P, Lovallo WR. Is aspirin, as used for antithrombosis, an emotion-modulating agent? J Psychosom Res. 1996; 40: 53-58.
- Bosma-den Boer MM, van Wetten ML, Pruimboom L. Chronic inflammatory diseases are stimulated by current lifestyle: how diet, stress levels and medication prevent our body from recovering. Nutr Metab (Lond). 2012; 9: 32.
- Thakur AK, Chatterjee SS, Kumar V. Adaptogenic potential of andrographolide: An active principle of the king of bitters (Andrographis paniculata). J Tradit Complement Med. 2014; 5: 42-50.
- Thakur AK, Chatterjee SS, Kumar V. Neuropsychopharmacology of a therapeutically used Andrographis paniculata extract: a preclinical study. Orient Pharm Exp Med. 2014; 14: 181-191.
- Thakur AK, Dey A, Chatterjee SS, Kumar V. Reverse Ayurvedic pharmacology of Ashwagandha as an adaptogenic anti-diabetic plant: A pilot study. Curr Trad Med. 2015; 1: 51-61.
- Khan SA, Chatterjee SS, Kumar V. Comparison of stress response desensitizing efficacies of aspirin and 3-hydroxybenzoic acid in mice. Indian J Pharmacol. 2014; 46: S93.
- Shakya A, Chatterjee SS, Kumar V. Role of fumarates in adaptogenics like efficacies of traditionally used Fumaria indica extracts. TANG [Humanitus Medicine]. 2015; 5: 28-37.
- Shivavedi N, Chatterjee SS, Kumar V. Evaluation of pharmacologically interesting dose range of ascorbic acid in mice. SAJ Neurol. 2014; 1: 1-8.
- Shivavedi N, Chatterjee SS, Kumar V. Stress response modulating effects of lactic acid in mice. Ther Targets Neurol Dis. 2014; 1: e418.
- Verma S, Chatterjee SS, Kumar V. Metformin like stress response modulating effects of Turmeric curcuminoids in mice. SAJ Neurol. 2015; 1: 102.
- McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998; 840: 33-44.