
Review Article
Austin J Pharmacol Ther. 2016; 4(1).1077.
Citicoline: A Potential Breakthrough in Cerebrovascular Disorder
Shaiba Sana Qureshi, Jeetendra Kumar Gupta* and Pradeep Mishra
Institute of Pharmaceutical Research, GLA University Mathura, India
*Corresponding author: :Gupta JK, Department of Pharmacology, GLA University, Institute of Pharmaceutical Research, Mathura, India
Received: December 02, 2015; Accepted: January 29, 2016; Published: February 15, 2016
Abstract
Citicoline, CDP Choline or Cytidine diphophocholine is a novel drug with a broad spectrum of benefits for condition associated with neurological dysfunction. Citicoline is an endogenous compound and an essential intermediate in the synthesis of phosphatidylcholine (cell membrane phopholipid). It is launched worldwide (outside of US and Canada only) as a drug for stroke and head injuries. It plays important roles in structural integrity, signaling of cell membrane and plays important role in human physiology. It supports the synthesis of Acetylcholine and Betaine, a methyl donor to generate phospholipids. Citicoline attenuates the production of free radicals in Ischemic condition and also stimulates the activity of glutathione reductase and has the ability to promote learning and improve cognitive impairment in Parkinson’s and Alzheimer’s disease. Citicoline administration reduces the severity of mental and motor deficits associated head injuries and supports eye health and mental health. Pharmacokinetics suggests that it is well absorbed and high bioavailable orally. A dose of 500mg to 2000 mg per day is an effective based on clinical trials and is safe for use in elderly population and pediatrics. It has the ability to improve phospholipid metabolism, with a consequent improvement in the deteriorated axonal flow of dopamine.
Keywords: Citicoline; Phosphatidylcholine; Betaine; Choline; Neurological dysfunction
Abbreviations
CDP: Cytidine Diphosphocholine; US: United States; GABA: Gamma Amino Butyric Acid; ATP: Adenosine Tri Phosphate; BBB: Blood Brain Barrier; NE: Nor Epinephrine; IM: Intra Muscular
Introduction
Citicoline is a mononucleotide which is composed of choline, cytosine, pyrophosphate and ribose. It is an essential intermediate in the synthesis of cell membrane phospholipids i.e. Phosphatidylcholine and Acetylcholine, a key neurotransmitter. Phospholipids are essential constituents of cell and have a high turnover rate, which requires a continuous synthesis of these compounds to ensure the adequate function of cell membrane [1,2]. Citicoline first identified in 1955 by Kennedy et al. and was synthesized in 1956. Initially it was developed in Japan to treat stroke patients. Later, Interneuron obtained its marketing and manufacturing license for Canada and US in 1993. By September 1997, a patent application had been filed by Interneuron worldwide, for the use of Citicoline [3]. It is widely available as an approved drug for the treatment of neurological disorder. When administered, Citicoline is hydrolyzed in the intestinal tract and in circulation to form choline and cytidine. Choline is a component of the diet and is produced in the brain in small amount. It plays several essential roles in human physiology, including signaling of cell membrane, support synthesis of betaine, a methyl donor and Acetylcholine. Studies in neuronally related cell lines have also shown that Cytidine administration increased the incorporation of choline into membrane phosphatidylcholine. Citicoline lowers the toxicity index an additional 20 fold and has low level of toxicological index [4,5]. It prevents the accumulation of free fatty acids and generation of free radicals at the site of ischemia, thereby prevents the initiation of proinflammatory cascade of events. Citicoline has been shown to act as a dopaminergic agonist. In addition, it also has some effects upon the other monoamines, serotonin and nor epinephrine, muscarinic receptors, and glutamate and GABA [6]. It inhibit catabolism of cerebral phospholipids and has a protective effect upon membrane ATPase and enzymes involved in brain energy metabolism, particularly succinyl dehydrogenase and citrate synthetase [7-9].
Bioavailability / Pharmacokinetics
Administration of 300mg dose to healthy adults shows nearly complete absorption, with less than 1% of excretion in feces [10]. The main route of excretion was found to be via a respiratory route, with significant excretion occurring through urine. A confirmatory study, using radioactive Citicoline in rats, found 62.8% of total radioactivity distributed in brain tissues as phospholipids, including phosphatidylcholine and sphingomyelin. Metabolites of orally administered Citicoline resynthesize endogenous Citicoline, which yields beneficial effects for the synthesis and incorporation of neuronal phospholipids [11-13]. Only a small percentage of total Citicoline crosses the BBB as choline and Cytidine, the utilization of these precursors is extremely efficient [14].
Mechanism of Action
Citicoline has beneficial effects on neurological functions; it acts by increasing the synthesis of phosphatidylcholine, the primary neuronal phospholipid and enhancing the production of acetylcholine [15]. Brain phospholipid synthesis is impaired following stroke and ischemic events. Oral citicoline administration increases the plasma levels of choline and cytidine, building blocks used to restore neuronal membrane integrity. Citicoline seems to have different effects on phosphatidylcholine synthesis in younger versus older adults. Phosphatidylcholine is an essential compound for cell membrane integrity and reduces in brain as a result of aging. Clinical data suggest that uridine and choline are the circulating substrates through which citicoline facilitates an increase in brain membrane phospholipid synthesis. Uridine crosses the blood-brain barrier and is converted to uridine triphosphate. Uridine directly gets converted to cytidine triphosphate intracellularly. The frontal lobe is the preferred site for the deposition of Citicoline [16-19].
This area of the brain contributes to memory function by supporting attention, memory capacity, and by reducing mental fatigue. Citicoline has benefit in patients experiencing ischemia by decreasing the accumulation of free fatty acids at the site of the lesion, which occurs as a result of neuronal cell damage and death. After the initiation of ischemia, there is a significant increase in arachidonic acid, glycerols, and free fatty acids caused by the breakdown of neuronal membranes (Figure 1) [20-22].
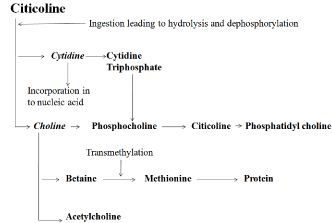
Figure 1: Major metabolic pathway of citicoline.
Toxic metabolites such as prostaglandins, thromboxanes, and free radicals get accumulated, leading to further damage. Intracerebral administration of citicoline prior to induction of ischemia reduces the rise in free fatty acids, arachidonic acid, and other toxic metabolites, attenuating free radical damage and restoring membrane function [23].
Citicoline normalizes neurotransmitter release pattern. In conditions of cerebral hypoxia and ischemia, NE (Nor Epinephrine) release may decrease, while the release of dopamine may increase. Citicoline administration to rats kept in a chronic hypoxic state reduces behavioral deteriorations and increased survival time. It is able to increase the dilation of blood vessels in animals with cerebral microcirculation injury, significantly increasing cerebral blood flow. Citicoline administration to rats increases striatal dopamine synthesis lesions and have shown to regenerate nerve cells. Citicoline enhances the preservation of an inner mitochondrial membrane component known as cardiolipin, which is an important regulatory factor for preservation of mitochondrial function [24-27]. It also facilitates the preservation of sphingomyelin, which promotes signal transduction in nerve cells and attenuates lipid peroxidation. It has been shown to have direct free-radical suppressive effects. Citicoline treatment significantly increases the length and branch points of dendrites, increasing the overall surface area occupied by neurons, which leads to an increased efficiency of sensory information processing [28-31].
Clinical Applications
Citicoline serves as a precursor for neuronal membrane phospholipids and an important nutritional substance for supporting learning ability and memory functions [32]. Neuroprotection of citicoline has been described since 1978 and several mechanism of action has been proposed [33]. Citicoilne pre-treatment prevents excitotoxic death caused by excessive glutamate exposure in an in vivo focal cerebral ischemia model [34]. Experiments in animals and humans provide evidence of its ability to promote these important cognitive processes. Citicoline at a dosage of 1,000 mg/day for 30 days is very effective in alzheimer’s disease, traumatic brain injury and vascular dementia [35]. It enhances cholinergic neural transmission, activate repair mechanisms to rejuvenate neuronal membranes and has regulatory effect on parameters associated with blood flow and circulation. It has been found that citicoline have moderate antidepressant effect in patients with Alzheimer’s disease and multiinfarct dementia. Citicoline has been reported to decrease infarct volume by 27.8% and to reduce brain edema with either a single therapy or combination with other agent like nimodipine [36].
Citicoline at a dosage of 1,000mg/day for 15 days, followed by 500 mg/day, has benefits in Parkinson’s disease. Levodopa is used in the long-term management of Parkinson’s disease; however, prolonged use of levodopa decreases efficacy and the development of dyskinesia. Citicoline has been found to have a levodopa-sparing effect and an ability to increase dopamine synthesis. 1000 mg/day IM dose of citicoline for 15 days show beneficial effects on eye health, specifically in cases of amblyopia and glaucoma [37-39]. It has been considered for the treatment of cocaine dependence because of its ability to repair neuronal membranes, which are damaged by cocaine use, and its ability to increase central nervous system dopamine levels [40]. Citicoline play as an adjunctive therapeutic agent for the treatment of disease arising from an infectious etiology including sequelae of sepsis, parasitic infection such as cerebral malaria by disregulating the host immune response. It has the ability to enhance cognitive function at 2,000 mg/day for 6 weeks [41].
Conclusion
Citicoline is a novel compound with a very broad spectrum of benefits in conditions associated with symptoms of neurological dysfunction. It maintains neural health and optimal cognitive function. Citicoline promotes cholinergic and dopaminergic functions and supports phospholipid synthesis and incorporation into cell membranes. Citicoline also enhances antioxidant mechanisms in the body, while suppressing the damaging effects of free radicals on neural tissue. It also promotes anti-inflammatory activities and optimizes patterns associated with the release of neurotransmitters. Given its widespread activity on neural tissue, citicoline is considered as a comprehensive therapeutic agent for supporting brain health [42- 44]. Citicoline has no adverse events. Minor transient adverse effects are rare and most commonly include stomach pain and diarrhea [45].
References
- Kennedy EP, Weiss SB. The function of cytidine coenzymes in the biosynthesis of phospholipides. J Biol Chem. 1956; 222: 193-214.
- Chida N, Shimizu Y. Biosynthesis of myelin lipids of cultured nervous tissues--incorporation of choline and CDP-choline into myelin phospholipids. Tohoku J Exp Med. 1973; 111: 41-49.
- Sreenivasa Reddy Y, Dinakar A and Srinivas L. Design development and evaluation of citicoline controlled release tablets. Der Pharmacia Lettre. 2013; 5: 296-311.
- Weiss GB1. Metabolism and actions of CDP-choline as an endogenous compound and administered exogenously as citicoline. Life Sci. 1995; 56: 637-660.
- Jackowski S, Wang J, Baburina I. Activity of Phosphatidylcholine biosynthetic pathway modulates the distribution of fatty acids in the glycerolipids in proliferating cells. Biochem Biophys Acta. 2000; 1483: 301-315.
- Dowd SR, Bier ME, Patton-Vogt JL. Turnover of phosphatidylcholine in Saccharomyces cerevisiae. The role of the CDP-choline pathway. J Biol Chem. 2001; 276: 3756-3763.
- Henneberry AL, Wright MM, McMaster CR. The major sites of cellular phospholipid synthesis and molecular determinants of Fatty Acid and lipid head group specificity. Mol Biol Cell. 2002; 13: 3148-3161.
- Secades JJ, Lorenzo JL. Citicoline: pharmacological and clinical review. Clin Pharmacol. 2006; 28: 1-56.
- Jambou R, El-Assaad F, Combes V, Grau GE. Citicoline (CDP-choline): What role in the treatment of complications of infectious diseases. Int J Biochem Cell Biol. 2009; 41: 1467-1470.
- Weiss GB. Metabolism and actions of CDP-choline as an endogenous compound and administered exogenously as citicoline. Life Sci. 1995; 56: 637-660.
- D'Orlando KJ, Sandage BW Jr. Citicoline (CDP-choline): mechanisms of action and effects in ischemic brain injury. Neurol Res. 1995; 17: 281-284.
- Babb SM, Appelmans KE, Renshaw PF. Differential effect of CDP-choline on brain cytosolic choline levels in younger and older subjects as measured by proton magnetic resonance spectroscopy. Psychopharmacology (Berl). 1996; 127: 88-94.
- Wurtman RJ, Regan M, Ulus I, Yu L. Effect of oral CDP-choline on plasma choline and uridine levels in humans. Biochem Pharmacol. 2000; 60: 989-992.
- Silveri MM, Dikan J, Ross AJ, Jensen JE, Kamiya T, Kawada Y, et al. Citicoline enhances frontal lobe bioenergetics as measured by phosphorus magnetic resonance spectroscopy. NMR Biomed. 2008; 21: 1066-1075.
- Secades JJ, Frontera G. CDP-choline: pharmacological and clinical review. Methods Find Exp Clin Pharmacol. 1995; 17: 1-54.
- Drago F, Mauceri F, Nardo L, Valerio C, Genazzani AA, Grassi M. Effects of cytidine-diphosphocholine on acetylcholine-mediated behaviors in the rat. Brain Res Bull. 1993; 31: 485-489.
- Adibhatla RM, Hatcher JF, Dempsey RJ. Citicoline: neuroprotective mechanisms in cerebral ischemia. J Neurochem. 2002; 80: 12-23.
- Adibhatla RM, Hatcher JF. Citicoline decreases phospholipase A2 stimulation and hydroxyl radical generation in transient cerebral ischemia. J Neurosci Res. 2003; 73: 308-315.
- Rema V, Bali KK, Ramachandra R, Chugh M, Darokhan Z, Chaudhary R. Cytidine-5-diphosphocholine supplement in early life induces stable increase in dendritic complexity of neurons in the somatosensory cortex of adult rats. Neuroscience. 2008; 155: 556-564.
- Gupta JK, Qureshi Shaiba Sana. Potential benefits of Methylcobalamin; A Review. Austin Journal of Pharmacology and Therapeutics. 2015; 3: 1-4.
- Petkov VD, Mosharrof AH, Kehayov R, Petkov VV, Konstantinova E, Getova D. Effect of CDP-choline on learning and memory processes in rodents. Methods Find Exp Clin Pharmacol. 1992; 14: 593-605.
- Petkov VD, Kehayov RA, Mosharrof AH, Petkov VV, Getova D, Lazarova MB, et al. Effects of cytidine diphosphate choline on rats with memory deficits. Arzneimittelforschung. 1993; 43: 822-828.
- Teather LA, Wurtman RJ. Dietary cytidine (5')-diphosphocholine supplementation protects against development of memory deficits in aging rats. Prog Neuropsychopharmacol Biol Psychiatry. 2003; 27: 711-717.
- Teather LA, Wurtman RJ. Dietary CDP-choline supplementation prevents memory impairment caused by impoverished environmental conditions in rats. Learn Mem. 2005; 12: 39-43.
- Spiers PA, Myers D, Hochanadel GS, Lieberman HR, Wurtman RJ. Citicoline improves verbal memory in aging. Arch Neurol. 1996; 53: 441-448.
- Alvarez XA, Laredo M, Corzo D, Fernández-Novoa L, Mouzo R, Perea JE, et al. Citicoline improves memory performance in elderly subjects. Methods Find Exp Clin Pharmacol. 1997; 19: 201-210.
- Franco-Maside A, Caamaño J, Gómez MJ, Cacabelos R. Brain mapping activity and mental performance after chronic treatment with CDP-choline in Alzheimer’s disease. Methods Find Exp Clin Pharmacol. 1994; 16: 597-607.
- Caamaño J, Gómez MJ, Franco A, Cacabelos R. Effects of CDP-choline on cognition and cerebral hemodynamics in patients with Alzheimer's disease. Methods Find Exp Clin Pharmacol. 1994; 16: 211-218.
- Fernández-Novoa L, Alvarez XA, Franco-Maside A, Caamaño J, Cacabelos R. CDP-choline-induced blood histamine changes in Alzheimer's disease. Methods Find Exp Clin Pharmacol. 1994; 16: 279-284.
- Cacabelos R, Alvarez XA, Franco-Maside A, Fernández-Novoa L, CaamaÑo J. Effect of CDP-choline on cognition and immune function in Alzheimer's disease and multi-infarct dementia. Ann N Y Acad Sci. 1993; 695: 321-323.
- Cacabelos R, CaamaÑo J, Gómez MJ, Fernández-Novoa L, Franco-Maside A, Alvarez XA. Therapeutic effects of CDP-choline in Alzheimer's disease. Cognition, brain mapping, cerebrovascular hemodynamics, and immune factors. Ann N Y Acad Sci. 1996; 777: 399-403.
- Ramaker C, Marinus J, Stiggelbout AM, Van Hilten BJ. Systematic evaluation of rating scales for impairment and disability in Parkinson's disease. Mov Disord. 2002; 17: 867-876.
- Martí Massó JF, Urtasun M. Citicoline in the treatment of Parkinson's disease. Clin Ther. 1991; 13: 239-242.
- Overgaard K, Meden P. Citicoline--the first effective neuroprotectant to be combined with thrombolysis in acute ischemic stroke? J Neurol Sci. 2006; 247: 119-120.
- Pietro Gareri, Alberto Castagna, Antonino Maria Cotroneo, Salvatore Putignano, Giovambattista De Sarro, Amalia Cecilia Bruni. The role of citicoline in cognitive impairment: pharmacological characteristics, possible advantages, and doubts for an old drug with new perspectives. Clin Interv Aging. 2015; 10: 1421-1429.
- Alvarez-Sabín J, Román GC. The role of citicoline in neuroprotection and neurorepair in ischemic stroke. Brain Sci. 2013; 3: 1395-1414.
- Eberhardt R, Birbamer G, Gerstenbrand F, Rainer E, Traegner H. Citicoline in the treatment of Parkinson's disease. Clin Ther. 1990; 12: 489-495.
- Cubells JM, Hernando C. Clinical trial on the use of cytidine diphosphate choline in Parkinson's disease. Clin Ther. 1988; 10: 664-671.
- Agnoli A, Ruggieri S, Denaro A, Bruno G. New strategies in the management of Parkinson's disease: a biological approach using a phospholipid precursor (CDP-choline). Neuropsychobiology. 1982; 8: 289-296.
- Tazaki Y, Sakai F, Otomo E, Kutsuzawa T, Kameyama M, Omae T, et al. Treatment of acute cerebral infarction with a choline precursor in a multicenter double-blind placebo-controlled study. Stroke. 1988; 19: 211-216.
- Qureshi I, Endres JR. A novel therapeutic agent with neuroprotective, neuromodulatory and neuroregenerative properties. Natural Medicine Journal. 2010; 2: 22.
- Wurtman RJ, Regan M, Ulus I, Yu L. Effect of oral CDP-choline on plasma choline and uridine levels in humans. Biochem Pharmacol. 2000; 60: 989-992.
- Secadesm JJ, Lorenzo JL. Citicoline:pharmacological and clinical review. Clin Pharmacol. 2006; 28: 1-56.
- Agut J, Font E, Sacristán A, Ortiz JA. Bioavailability of methyl-14C CDP-choline by oral route. Arzneimittelforschung. 1983; 33: 1045-1047.
- European Food Safety Authority. Scientific Opinion on the safety of “citicoline” as a Novel Food ingredient. EFSA Journal. 2013; 11: 17.