
Research Article
Austin J Pharmacol Ther. 2016; 4(1).1079.
Intradialytic Complications Found in Patients at a Tertiary Care Hospital
Mehmood Y¹*, Ghafoor S², Ashraf MI³, Riaz H4, Atif SR4 and Saeed M5
1Faculty of Pharmacy, University of Central Punjab, Pakistan
2Department of Chemistry, GC University, Pakistan
3Pharmacology Department, Rashid Latif Medical College, Pakistan
4Rashid Latif College of Pharmacy, Pakistan
5Department of Pharmacy, Riphah International University, Pakistan
*Corresponding author: :Yasir Mehmood, Faculty of Pharmacy, University of Central Punjab, Pakistan
Received: December 20, 2015; Accepted: March 01, 2016; Published: March 04, 2016
Abstract
Introduction: Hemodialysis is a continuous process completed in almost 4 hours. During this procedure toxic substances are removed from the body by simple diffusion technique through a dialysis machine. During the procedure a lot of changes in the fluid and mineral compartment occur due to which patients suffer from various complications. The aim of the study was to observe those complications and their management. Services hospital Lahore was selected for this study and a convenience sampling technique was used for collecting data.
Methods: A cross-sectional study was conducted at Nephrology unit of a tertiary care hospital from 15 June, 2014 to 15 December, 2014. A total of 40 patients on regular hemodialysis were included in the study. Patients with acute renal failure were excluded from the study.
Results: Most commonly observed complication were hypotension 37.5%, Cramps 12.5%, Itching 15%, Vomiting 22.5% and dialysis reaction 5%. Normal saline and 5% dextrose were used for managing the complications. For dialyzer reaction hydrocortisone Sodium Succinate was given stat intravenously and session was postponed. Hemodialysis is a not totally risk free although it is beneficial for saving patient life.
Conclusion: Although intradialytic complications are life threatening but they are manageable and their frequency is low.
Keywords: Hemodialysis; Vomiting; Dialysis; Complications
Introduction
Hemodialysis is a method of removing toxic substances (impurities or wastes) from the blood when the kidneys are unable to do so [1]. Dialysis is from Greek word “dialusis” mean dissolution, “dia” meaning through, and “lusis” mean loosening [2].
It removes waste products such as potassium and urea [3], and free water from the blood when the kidneys are unable to do so in renal failure. Other renal replacement therapies are renal transplant and peritoneal dialysis.
When kidneys stop working hemodialysis replaces most of their filtration function [4]. Hemodialysis removes those substances from blood which could cause death if kidneys stop working [4]. Since hemodialysis is not a constant process, it cannot monitor body functions as do normal kidneys [5], but it can eliminate waste products and restore electrolyte and pH levels when hemodialysis session is done.
The basic principle of hemodialysis is diffusion across a semi permeable membrane [6]. Hemodialysis utilizes a diffusion mechanism called counter current flow, where the dialysate flow in the opposite direction to blood flow the extracorporeal circuit. Counter-current flow maintains the concentration gradient across the membrane at a maximum and increases the efficiency of the hemodialysis [6].
Fluids are removed (ultra filtration) by applying hydrostatic pressure in the dialysate compartment, causing free water and some dissolved solutes to move out [7]. The dialysis solution used is a sterilized solution of mineral ions. Urea and other waste products, potassium, and phosphate diffuse into the dialysis solution. However, concentrations of sodium and chloride are kept similar to those of normal plasma to prevent loss [8]. sodium bicarbonate is added in a higher concentration than plasma to correct the blood acidity. In hemodialysis, three primary methods are used to gain access to the blood: and I/V catheter, an Arteriovenous (AV) fistula and a synthetic graft [9]. The type of access is influenced by factors such as the expected time course of a patient’s renal failure and the condition of his or her vasculature. Patients may have multiple accesses, usually because an AV fistula or graft is maturing and a catheter is still being in use [10].
Most preferred method is AV (Arteriovenous) fistula. For creating AV fistula a vascular surgeon joins an artery and a vein together through anastomosis. This junction bypasses the capillaries and blood flows rapidly through the fistula. Its advantages are lower risk of infection and thrombosis [11]. An AV fistula takes about 6 month to mature from surgery to first dialysis. If a fistula has a very high blood flow a Steal syndrome can occur. Where the blood not enters the limbs and circulate between fistula and general circulation. This results in cold extremities cramping pain and severe tissue damage (Figure 1).
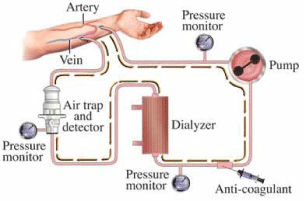
Figure 1: Pattern of dialysis [12].
Materials and Methods
Study place: Hemodialysis unit 1 of Services hospital Lahore.
Study duration: Duration of study was 15 days.
Sample size: A sample size of 40 patients who was on regular hemodialysis was selected.
Sampling Technique: To evaluate the sample size, a sampling technique was used namely “convenience sampling”.
convenience sampling is a method in which those subjects are selected for study that is easily available. This is sampling technique that selects the subjects from population based on accessibility. This technique was used because the data was taken of those patients who came in hemodialysis ward regularly.
Inclusion criteria: The patients on regular hemodialysis were included both of morning and evening shifts.
Exclusion criteria: Emergency and HCV positive patients were excluded.
Main aim of study was to observe intradialytic complications and see their management.
Working procedure:
- Selection of topic “Intradialytic complications”
- Development of questionnaire
- Hospital visits for data collection
- Graphical representations of data
- Discussions
Results
The study was conducted at Services Hospital Lahore to observe intradialytic complications. For this case history of 40 patients who were on regular hemodialysis twice or thrice a week was taken.
Hemodialysis is a four hour procedure. Hemodialysis session of each patient was observed during the study period. Most common complications observed were Hypotension, Cramps, Chills, Fever, Dialyzer reaction, Itching, Nausea and Vomiting. Almost all the complications were managed locally in the hospital.
Hypotension, one of the major complications was found to be 37.5%. The major causes of hypotension were loss of fluid and minerals specially Ca+2Na+1. Management was done by giving Normal Saline, 5% Dextrose Sol., by stopping the ultra-filtration or by reducing the flow rate per ml and placing the patient in a Trendelenburg position.
Cramps were reported to be 12.5% and stretching exercises was given for relief.
Nausea and vomiting was observed in 17.5% patients and the patients were given antiemetics mostly Domperidone inj IV. Itching in about patients 15%. Antihistamines were given.
Dialyzer reactions were experienced by 5% patients. On the basis of type of reactions the patients might be at risk of anaphylactic shock. The reaction was immediately stopped by clamping the tubing. Patients were given IV corticosteroids the process was resumed after symptoms were cured. The patients of acute renal failure 62% and of chronic renal failure were 38%.
Time period for hemodialysis of most of patients was two year or less. Maximum duration found was up to 5 years in this study duration.
Patients with multiple hemodialysis sessions were more stable with few intradialytic complications than the patients with few hemodialysis sessions that showed more complications.
The patients who took excessive fluid between the dialysis sessions had a weight gain of more than 3 kg and suffer from more intradialytic complications [12].
All the hemodialysis sessions end with intensive weakness.
Most patients undergoing hemodialysis from one year or less are 25%.Up to three years is 62.5%.Up to five years are12.5% (Figure 2).
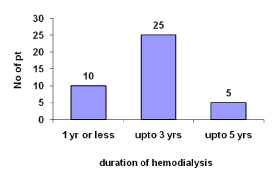
Figure 2: Time period of hemodialysis.
Major causes were diabetes 25% and hypertension 37.5%.About 7.5% have a history of excessive antibiotic use and other causes 17.5% were surgical operations and deep vein thrombosis etc (Figure 3).
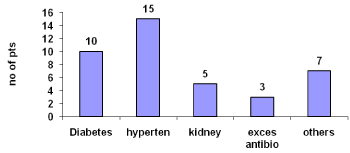
Figure 3: Causes of renal failure.
Acute cases were 62.5% and chronic were 37.5% (Figure 4).
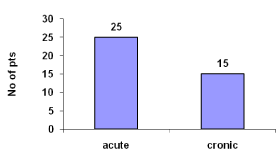
Figure 4: Types of renal failure.
About 97.5% hemodialysis was on bicarbonate (Figure 5).
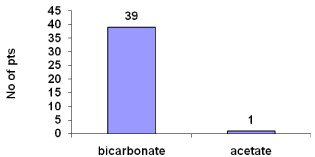
Figure 5: Mode of hemodialysis.
About 95% patients under go dialysis two times in a week and 5% three times in a week (Figure 6).
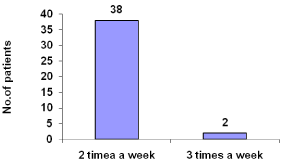
Figure 6: Frequency of hemodialysis per week.
Hypotension was reported to be 37.5% (Figure 7).
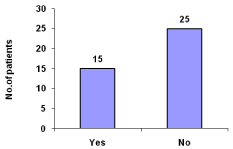
Figure 7: Hypotension during hemodialysis.
About 15% patients suffer from chills (Figure 8).
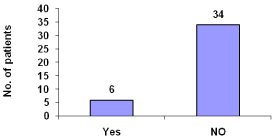
Figure 8: Itching during hemodialysis.
Patients suffer from chills were 7.5% (Figure 9).
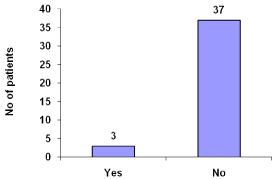
Figure 9: Chills during hemodialysis.
Nausea and vomiting reported were 17.5% (Figure 10).
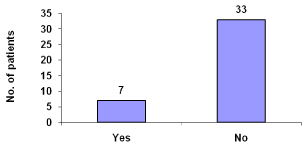
Figure 10: Nausea and vomiting during dialysis.
Cramps were reported by 12.5% (Figure 11).
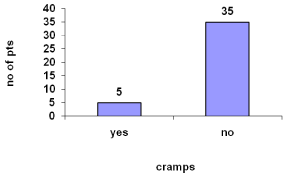
Figure 11: Cramps during hemodialysis.
Fever was reported by 2.5% (Figure 12).
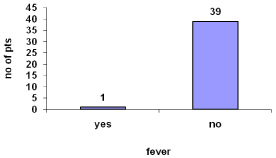
Figure 12: Fever during hemodialysis.
Dialyzer reactions reported were 5% (Figure 13).
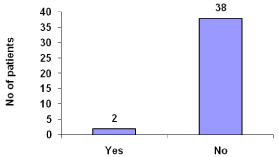
Figure 13: Dialyzer reactions.
About 75% patients use dietary supplement (Figure 14).
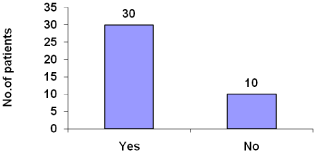
Figure 14: Dietary supplement intake.
Heparin was used in all hemodialytic processes (Figure 15).
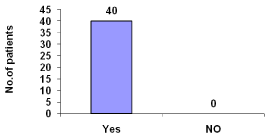
Figure 15: Heparin used during hemodialysis.
Discussion
Intradialytic complications continue to be a leading problem, and its incidence varies from 20 to 30% (Figure 16). Intradialytic complications causes discomfort. The population at risk includes individuals with diabetes (autonomic dysfunction); individuals with Left Ventricular Hypertrophy (LVH) and diastolic dysfunction, a history of prior myocardial infarction, or cardiovascular intervention; individuals with symptomatic coronary heart disease; individuals with high interdialytic weight gains (>3% of body weight); and dialysis patients who are anephric. Intradialytic hypotension continues to be a leading problem, and its incidence varies from 20 to 30%. Intradialytic hypotension not only causes discomfort, but also increases mortality. Risk factors for IDH are both age and co morbidity dependent the basic principle of hemodialysis is diffusion across a semi permeable membrane [6]. Hemodialysis utilizes a diffusion mechanism called counter current flow, where the dialysate flow in the opposite direction to blood flow the extracorporeal circuit. Counter-current flow maintains the concentration gradient across the membrane at a maximum and increases the efficiency of the hemodialysis [6].
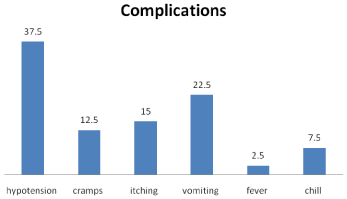
Figure 16: Complications during hemodialytic process.
New dialysis patients started on HD in the United States have an approximate average safe zone UF of 1.8 to 2.3 kg/treatment based on mean baseline weight across all age categories of age and yet the standard kilogram interdialytic weight gain pattern that typical patients present with requires UF rates higher than the accepted safe zone, thus increasing their risk for IDH. Incorrectly assessed ideal body weight followed by excess removal of UF is the commonest cause IDH (33%) in our study. IDH episodes may resolve with treatment in the unit or persist even after the patient leaves the unit and returns home. Theoretically, persistent hypotension after treatment completion may provide an explanation for patients who sustain an insult or die sometime later at home. Patients experiencing IDH who are usually hypertensive may resume antihypertensive medications at home when the BP is still low. This could lead to further nocturnal decreases in BP that increase the risk for an ischemic event. Improper dosage and frequency of antihypertensive medications administration accounted for 11% of IDH in our patients. Detailed hemodynamic analysis of IDH suggests 2 important things. (1) There is no sudden decrease in plasma volume just before a hypotensive episode. (2) IDH seems to be due to a decrease in cardiac output engendered by reduced cardiac filling. Most of the 3 L or so of plasma volume resides in the veins, and several organ perfusion systems, notably skin and splanchnic, contain veins that can markedly alter their capacity.
Headache was reported by just over 50% of patients, in keeping with other studies. Typically, headache occurs after 3–4 h of dialysis, although some believe that it is nitric oxide mediated, which is often initially generated at the start of the dialysis session. However, headache can also be associated with raised intracranial and intraocular pressures. One earlier study also reported an association between longer dialysis session times and headache, with increased symptomatic headache when patients had their treatment times increased from 4 to 5 h, despite decreased intradialytic hypotension .
Itching during dialysis was reported by 15% of patients. Pruritus is a very common symptom in chronic kidney disease patients and has many potential causes. Although itching could be due to contact sensitization linked to vascular access cleansing solutions and needles or reactions to components of the extracorporeal circuit , it could also be simply due to the patient being relatively immobile for several hours during dialysis and being unable to distract themselves, so become more aware of their generalized pruritus. Similarly, the increased reporting of backache could be related to relative immobility in a dialysis chair for several hours, in patients with underlying renal muscle and bone disorders.
Most of the patients undergoing Hemodialysis (HD) were in poor condition. Complications occurring during HD increased patient morbidity and mortality and led to termination of some dialysis sessions. The commonest complications were chill, hypotension, vomiting cramps, fever and convulsions. However unlike most studies, hypertension, not hypotension was the commonest complication reported. One reason for this could be the large proportion of children with AKI as such patients usually have fluid overload which contributes to the 30 development of intradialytic hypertension (Figure 17).
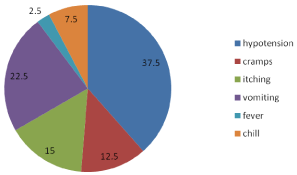
Figure 17: Complications during hemodialytic process.
Conclusion
Hemodialysis is a beneficial procedure for removal of wastes but not completely free of risks. A lot of complications are associated with the process which can be overcome by taking careful measures, skilled staff and patients compliance. Hemodialysis is a four hour procedure. I closely observed hemodialysis session of each patient during my study period. Most common complications I observed were Hypotension, Cramps, Chills, Fever, and Dialyzer reaction, Itching, Nausea and Vomiting. Almost all the complications were managed efficiently by the Medical staff and technicians in the unit.
References
- Schilthuizen SF, Batenburg LF, Simonis F, Vercauteren FF. Device for the removal of toxic substances from blood: Google Patents. 2015.
- Preus A. Historical dictionary of Ancient Greek philosophy: Rowman & Littlefield. 2015.
- Soykan O, Schu C, Chaffin KA. Method and device to treat kidney disease: Google Patents. 2014.
- Singbartl K, Kellum JA. Urinary Biomarkers for Predicting Long-Term Dialysis: Google Patents. 2014.
- Chamney P, Moissl U, Wabel P, Amato C, Stuard S, Menzer M, et al. Haemodialysis techniques and adequacy 2. Nephrology dialysis transplantation. 2014; 29.
- Wong J, Vilar E, Farrington K. Haemodialysis. Medicine. 2015; 4: 478-483.
- Reddenna L, Basha SA, Reddy KSK. Dialysis Treatment: A Comprehensive Description. International Journal of Pharmaceutical Research & Allied Sciences. 2014; 3.
- Wrazel JS, Curtis JR, Nonn L, Peterson RB, Hailei W, Ingram-Goble R, et al. Dialysis system: Google Patents. 2014.
- Tan CS, Wu S, Kalva SP. Declotting of Dialysis Vascular Access Dialysis Access Management. 2015; 145-158.
- Santoro D, Benedetto F, Mondello P, Pipitò N, Barillà D, Spinelli F, et al. Vascular access for hemodialysis: current perspectives. International journal of nephrology and renovascular disease. 2014; 7: 281.
- Walker, C. Dialysis Access Management. Vascular disease management. 2014; 11: 145-155.
- Hemonnot CY, Mauermann M, Herrmann H, Koester S. The assembly of simple epithelial keratin filaments: Deciphering the ion-dependence in filament organization. Biomacromolecules. 2015; 16: 3313-3321.