
Research Article
Austin J Pharmacol Ther. 2016; 4(1).1081.
Comparative Evaluation of Antidiabetic and Antioxidant Activities of Aqueous Fruit Peel and Leaf Extracts of Annona Squamosa on High Fat Diet and Multiple Low Dose Streptozotocin Mouse Model of Diabetes
Sahu M¹*, Sahoo NK¹, Alagarsamy V¹ and Rath BP²
¹Department of Pharmacology, MNR College of Pharmacy, Fasalwadi, Sangareddy, Medak, Telangana, India
²Department of Pharmacology, Sherwood College of Pharmacy, Uttar Pradesh, India
*Corresponding author: :Madhusmita Sahu, Department of pharmacology, MNR College of Pharmacy, Fasalwadi, Sangareddy, Medak, Telangana, 502294, India
Received: May 20, 2016; Accepted: July 15, 2016; Published: July 19, 2016
Abstract
Annona squamosa possess varied medicinal properties. It is traditionally used for antidiabetic and antihyperlipidemic activities. The study emphasizes on comparative evaluation of antidiabetic and antioxidant activities of the fruit peel and leaf extracts of the plant on diabetes induced male albino mice by high fat diet with multiple low dose streptozotocin from 14th day of 28 days study regimen. Fourty male albino mice (4weeks of age, 20- 25gm body weight) were selected with division into five groups such as non-diabetic control, diabetic control, diabetes treated with Glibenclamide, diabetes treated with Aqueous Fruit Peel Extract Of Annona Squamosa (AFPEAS) and diabetes treated with Aqueous Leaf Extract Of Annona Squamosa (ALEAS) respectively. At 28th day of the study, all the biochemical parameters like plasma glucose, triglyceride, total cholesterol, glucose utilisation, insulin tolerance and muscle antioxidant level were measured. All the parameters were compared with the diabetic control group. The aqueous leaf extract of Annona squamosa showed marked and significant improvement in plasma glucose, triglyceride, total cholesterol, glucose utilisation, insulin tolerance activity in comparison to that of aqueous fruit peel extract of Annona squamosa. However on the basis of result of antioxidant level, aqueous fruit peel extract of Annona squamosa showed marked free radical scavanging activity than other treated groups. Both AFPEAS and ALEAS treated groups showed significant improvement in skeletal muscle coefficient (*p < 0.05), whereas significant reduction (*p < 0.05) in liver coefficient compared with diabetic controlled mice was noticed in case of ALEAS.
Keywords: Anti diabetic; Antioxidant; Annona squamosa; Streptozotocin; Glibenclamide; High fat diet
Introduction
Diabetes mellitus (DM) is a heterogeneous disease that is characterised by high blood glucose resulting from insulin resistance with relative impairment in insulin secretion and is often accompanied by hypertension [1,2]. In Diabetes increased lipid peroxidation is also associated with hyperlipidemia. Diabetes is often initially managed by increasing exercise and dietary modification. As the condition progresses, medications may be needed. Diabetes mellitus is primarily due to lifestyle factors and genetics [2]. A number of lifestyle factors are known to be important for the development of diabetes mellitus. In one study, those who had high levels of physical activity, a healthy diet, without smoking and non-alcoholic had an 82% lower rate of diabetes. Body weight plays a major role in diabetes mellitus. Obesity has been found to contribute to approximately 55% of type II diabetes [2]. Decreasing the consumption of saturated fats and trans fatty acids while replacing them with unsaturated fats may decrease the risk [2].
Many traditional or herbal treatments for diabetes are used throughout the world. This research focuses on the role of traditional therapeutic and natural medicines from traditional medicinal plants for diabetes. Traditional medicines from readily available medicinal plants offer great potential for the discovery of new anti diabetic drugs [3-6]. Most of plants contain glycosides, alkaloids, terpenoids, flavonoids, carotenoids, etc., which are frequently implicated as having antidiabetic effect. Plant drugs and herbal Formulation are frequently considered to be less toxic, free from side effects and having good antioxidant properties than synthetic one; those are frequently implicated as having anti diabetic effects by showing specific modes of action in the treatment of diabetes [7].
Annona squamosa also called as custard apple in English and sharifa in Hindi belongs to family Annonaceae. This plant possesses different medicinal properties. The roots, leaves, fruits, seeds & bark of plant have multiple uses and are pharmacologically active. The fruit is juicy and creamy-white. Chemically it contains annonaine an alkaloid, flavonoids, glycosides, aporphine alkaloids, tetrahydro isoquinoline alkaloids, terpens etc. Annona squamosa is also used in hyperthyroidism, as an antimicrobial and insecticidal, antidiabetic, uterotonic, antifertility, antitumour, antioxidant, antispasmodic, analgesic, antiinflammatory, antiulcer, abortifacient etc [8]. The Fruits are having high calorific value, rich source of vitamins and minerals. The seeds are having insecticidal properties and are useful for removing head lice. The leaves are shown to have anti-diabetic and hepatoprotective properties. Fruits are having haematinic, sedative, stimulant & expectorant activity [9]. Annona squamosa is traditionally used for antidiabetic and antihyperlipidemic activities [10].
The present investigation was conducted for comparative evaluation of antidiabetic and antioxidant activities of aqueous fruit peel and leaf extracts of Annona squamosa on high fat diet and multiple low dose Streptozotocin (MLDS) induced diabetic mice. The effect produced by both the extracts of Annona squamosa on different parameters were compared with the group treated with Glibenclamide, as reference drug.
Materials and Methods
Plant material
The fruit and leaves of Annona squamosa were obtained from Sangareddy in the month of October and were authentificated from Department of Botany (JNTU). After identification the fruit peels and leaves were cleaned well with water and dried in a shadow place. After complete drying, the fruit peel and leaves were powdered separately. Then 75 g powder from each was extracted in 1 litre of boiling water for 2-3h and concentrated to half of the volume by boiling in a water bath. The resulting dark-brown extract was cooled and filtered using Whatman No. 1 filter paper. The filtrate was centrifuged at 10,000 rpm at room temperature (25°C) and the sediment was discarded. The supernatant extract was concentrated up to 100 ml on rota vapour under reduced pressure and then stored at room temperature, and protected from direct sunlight. The Phytochemical screening gave positive tests for flavonoids, terpenoids, alkaloids and glycosides [11].
Experimental animals
Fourty male albino mice (4 weeks of age, 20- 25gm) were selected from the stock of animal house (Regd. No. 285/CPSCSEA) of MNR College of pharmacy, Sangareddy, Hyderabad, India. They were divided into 5 experimental groups, each group containing eight animals in it. The animals were kept in polystyrene cages under standard laboratory conditions i.e. at temperature of 24±2°C, relative humidity of 60±2% and were exposed to a 12h photo period. The animals were fed normal chew pellet (procured from Rayan’s biotecghnologies Pvt. Ltd. Hydrabad, India) and water ad libitum. After one week of acclimatization the protocol of diabetes induction and drug treatment was followed as per the protocol.
Selection of doses and route of administration of the drugs
Different doses (50–2500mg/kg, p.o) prepared from the aqueous extracts of fruit peel and leaves of Annona squamosa were administered to the different groups of rats and were observed continuously for 1 hour and then at half - hourly intervals up to 4 hours. The gross behavioural changes were observed up to 72 hours, followed by observation of mortality rate up to 14 days as per the OECD Guideline 425. From the toxicity study; it was observed that plant extract is non-toxic and caused no death up to the dose of 2500 mg/kg (1/10th of the maximum dose i.e. 250 mg/kg was selected). It is safe and used for further experiment. The dose and route of the administration of drugs were selected from the literature and acute toxicity study and are as follows:
- Glibenclamide - 0.5 mg/kg [12]
- Aqueous Fruit Peel Extract of Annona squamosa (AFPEAS) -250mg/kg orally [12]
- Aqueous Leaf Extract of Annona squamosa (ALEAS) - 250mg/kg orally [12]
- Group 1: Non-diabetic Control fed with normal chew diet and treated with normal saline daily.
- Group 2: Diabetic control (High fat diet + multiple low dose Streptozotocin), 1% sodium carboxy methyl cellulose was administered daily.
- Group 3: Diabetic control (High fat diet + multiple low dose Streptozotocin), treated with 0.5 mg/kg Glibenclamide and was given orally
- Group 4: Diabetic (High fat diet + multiple low dose Streptozotocin), treated with of Aqueous Fruit Peel Extract of Annona squamosa, 250 mg/kg which is prepared in 1 % sodium carboxy methyl cellulose and was given orally.
- Group 5: Diabetic (High fat diet + multiple low dose Streptozotocin), treated with of Aqueous leaf Extract of Annona squamosa, 250 mg/kg which is prepared in 1 % sodium carboxy methyl cellulose and was given orally
Grouping of animals
The experimental animals were divided into the following groups such as:
Induction of type II diabetes in mice
After one week of acclimatization, the mice were divided into five groups. The non diabetic group animals were fed with a normal chow diet whereas the other group animals were fed with high fat diet for 28 days. The basic composition of high fat diet was (g/ 100 g high fat diet): Casein 28.9%, DL- Methionine 0.33%, corn starch 20.73%, sucrose 9.05%, vegetable hydrogenated oil 27.41%, corn oil 1.6%, cellulose 2.66%, vitamin and mineral mix 3%, calcium carbonate 0.67%. The drugs were solubilised in 1% CMC and administered by oral gavages between 11.00–12.00 h from day 14 to day 28. On day 15, the animals (Mice) were injected with low dose of Streptozotocin (40 mg/kg, i.p.) dissolved in citrate buffer (pH 4.5) for five consecutive days. The mice were kept on the above treatments and fed with the high-fat diet until day 28 [13].
At the end of the week 4, plasma glucose, triglyceride (TG), Total Cholesterol (TC), Oral Glucose Tolerance Test (OGTT), Insulin Tolerance Test (ITT) were measured. Antioxidant activity (Melonaldehyde) was estimated in gastrocnemious muscles of mice.
Collection of blood and determination of biological parameters
Animals (Male albino mice) were fasted overnight for a period of 18 h. Blood (0.5 ml) was withdrawn via the retro-orbital sinus under mild ether anaesthesia and was collected in micro tubes previously filled with 10% EDTA solution (20 μl of 10% EDTA/ ml of blood). The micro tubes were centrifuged at 4000 rpm at 4oC for 20 min to obtain clear plasma. The plasma was then analyzed for glucose, triglyceride, total cholesterol in the auto analyser using commercially available biochemical kits [14].
Oral Glucose Tolerance Test (OGTT): The OGTT was performed in overnight (18 h) fasted albino mice. The animals (Mice) were however allowed to drink water and the treatment groups were administered the respective drugs at the regular time. Glucose (2 g/ kg) was fed to each animal 10 min after collecting blood at 0 min. 0.1 ml blood was withdrawn from the retro-orbital sinus under mild ether anaesthesia at 0min, 30min, 60 min and 120 min and was collected in micro tubes previously filled with 10% EDTA solution (20 μl of 10% EDTA/ ml of blood). The micro tubes were centrifuged at 4000 rpm at 4°C for 20 min to obtain clear plasma. The plasma was then analyzed for glucose through the above mentioned method [15].
Insulin Tolerance Test (ITT): The ITT was performed in overnight (12 h) fasted mice. The animals (Mice) were however allowed to drink water and the treatment groups were administered the respective drugs one hour before blood collection. Insulin 0.75U/ kg was administered intraperitonialy to each animal 10 min after collecting blood at 0 min. Blood was withdrawn from the retroorbital sinus under mild ether anaesthesia at 0min, 30min, 60 min and 120 min and was collected in micro tubes previously filled with 10% EDTA solution (20 μl of 10% EDTA/ ml of blood). The micro tubes were centrifuged at 4000 rpm at 4°C for 20 min to obtain clear plasma. The plasma was then analyzed for glucose through the above mentioned method [16].
Estimation of Malonaldehyde (MDA) level in Skeletal muscle: The animals (Mice) were sacrificed at the end of the treatment week and then skeletal muscle (Gastrocnemius muscle left, gastrocnemius muscle right, soleus muscle left, soleus muscle right) were isolated. The weight of the muscle was taken immediately after drying in a blotting paper. Gastocnemius muscle and soleus muscle was transferred for homogenization for estimation of Malondialdehyde (followed by Esterbauer and Cheeseman method) [17].
Statistical analysis: Data were expressed as mean ± Standard Error of the Mean (SEM). Data were Analyzed by Using One-Way Analysis of Variance (ANOVA) followed by dunnett’s t-test as post hoc analysis. Statistical significance was determined at 5% level of confidence (p<0.05).
Result
Percentage yield of the extracts
After drying, the dried mass of the aqueous extract of Fruit Peel and leaf extract of Annona squamosa were weighed in a digital balance. The yields were found to be 20.08 gm (Fruit peel) and 18.1 gm (leaf) from 75gm of Annona squamosa. The percentage yields were found to be 26.77% and 24.13% respectively.
Plasma glucose, triglyceride and total cholesterol level
Plasma glucose level: The fasting plasma glucose level of non diabetic group was found to be normal at week 4. After high fat diet and Multiple low dose of STZ (MLDS), plasma glucose level of diabetic control groups were profoundly increased (227.62 ±5.91,## p<0.01) up to 200 - 230mg/dl at 28 day. on prior treatment with aqueous fruit peel extract of Annona squamosa, plasma glucose level was significantly (197.87± 12.16, *p<0.05) reduced at week 04.Whereas aqueous leaf extract of Annona squamosa treated group showed better reduction (161.25±11.96,**p<0.01) in fasting plasma glucose level as shown in Figure 1.
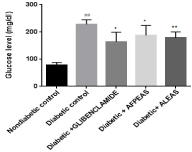
Figure 1: Effect of aqueous extract of Annona squamosa (Fruit peel and
Leaf) on plasma glucose level at week 04. N=08; Values are expressed as
mean ± SEM; AFPEAS: Aqueous Fruit Peel Extract of Annona squamosa;
ALEAS: Aqueous Leaf Extract of Annona squamosa; ##p<0.01 vs. non
diabetic group; *p<0.05 and **p<0.01 vs. diabetic control.
Plasma triglyceride level: The fasting plasma TG level of nondiabetic group was found to be normal at week 4. Diabetic control mice showed significantly (86.87±1.77, #p<0.05) increased in TG in comparison to nondiabetic mice. On treatment with aqueous fruit peel extract of Annona squamosa and aqueous leaf extract of Annona squamosa, fasting plasma TG level was significantly (80.0±1.76 and 73.0±3.24 respectively, *p<0.05) attenuated as compared to diabetic control at week 4 (Figure 2). The animals treated with Glibenclamide also showed significant (75.87± 2.10, *p<0.05) reduction in TG level.
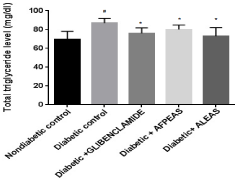
Figure 2: Effect of aqueous extract of Annona squamosa (Fruit peel and
Leaf) on plasma Triglyceride level at week 04. N=08; Values are expressed
as mean ± SEM; AFPEAS: Aqueous Fruit Peel Extract of Annona squamosa;
ALEAS: Aqueous Leaf Extract of Annona squamosa; #p<0.05 vs. non
diabetic group; *p<0.05 vs. diabetic control.
Plasma total cholesterol level: The fasting plasma total cholesterol level was normal in nondiabetic group at week 4. Diabetic control mice showed significantly (155.87±14.39, #p<0.05) increased total cholesterol in comparison to nondiabetic mice. On treatment with aqueous fruit peel extract of Annona squamosa and aqueous leaf extract of Annona squamosa, fasting plasma total cholesterol level was significantly (97.0±5.54 and 92.87±6.14, *p<0.05) attenuated as compared to diabetic control at week 4 (Figure 3). All the groups demonstrated significant (*p<0.05) reduction in plasma total cholesterol at week 04 in comparison to diabetic control. However, the Glibenclamide treated animals showed significantly reduction (90.25±3.07, * p<0.05) in total cholesterol level.
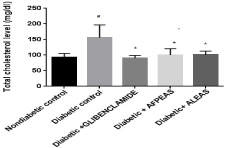
Figure 3: Effect of aqueous extract of Annona squamosa (Fruit peel and Leaf)
on plasma Total cholesterol level at week 04. N=08; Values are expressed
as mean ± SEM; AFPEAS:Aqueous Fruit Peel Extract of Annona squamosa;
ALEAS: Aqueous leaf Extract of Annona squamosa; #p<0.05 vs. non diabetic
group; *p<0.05 vs. diabetic control.
Oral Glucose Tolerance Test (OGTT): Oral glucose tolerance test test was carried out after week 04. In diabetic control group, plasma glucose level was significantly (## p<0.01) high in both the phases (30 and 60 min) as compared to normal chew diet fed mice. In all treatment groups, the slope of first phase was unchanged as compared to diabetic control mice. However, the plasma glucose level was signed (*p<0.05) low as compared to the diabetic control group at 0 and 30 min. AFPEAS and ALEAS treated groups showed significant (*p<0.05) lowering of glucose curve as compared to diabetic control. Whereas the ALEAS treated mice showed effective glucose utilisation at both 60 and 120 min as compared to diabetic control group. At 60 min of OGTT test all the treated group showed significant (*p<0.05) glucose utilisation as compared to diabetic control group. The results are shown in Figure 4.
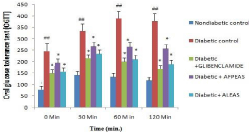
Figure 4: Effect of aqueous extract of Annona squamosa (Fruit peel and
Leaf) on OGTT at week 04. N=08; Values are expressed as mean ± SEM;
AFPEAS: Aqueous Fruit Peel Extract of Annona squamosa; ALEAS: Aqueous
Leaf Extract of Annona squamosa; ##p<0.01vs. non diabetic group; *p<0.05
vs. diabetic control.
Insulin tolerance test: In diabetic control mice, the plasma glucose was very slowly but not effectively reduced (##p<0.01) at both 30 and 60 min after insulin injection as compared to nondiabetic control mice.Whereas the plasma glucose level was again normalised at 120 min. However, both the extract (AFPEAS and ALEAS) treated groups showed significant (*p<0.05) glucose disposal in response to insulin but the ALEAS was more effective than other extract (Figure 5).
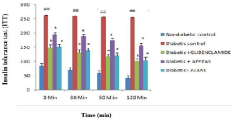
Figure 5: Effect of aqueous extract of Annona squamosa (Fruit peel and
Leaf) on ITT at week 04. N=08; Values are expressed as mean ± SEM;
AFPEAS:Aqueous Fruit Peel Extract of Annona squamosa; ALEAS: Aqueous
Leaf Extract of Annona squamosa; ##p<0.01vs. non diabetic group; *p<0.05
vs. diabetic control.
Estimation of skeletal muscle coefficient: A significant (0.007125±0.0007, ##P < 0.01) decrease in skeletal muscle coefficient was seen in animals treated with high-fat- MLDS as compared with normal chow diet fed mice. However both the AFPEAS treated animals showed significant (0.00975±0.0007258,*P< 0.05) increase in gastrocnemious and soleus muscle weight as compared to diabetic mice. Whereas the ALEAS treated animals showed more significant (0.012±0.000534522,**P<0.01) increase in gastrocnemious and soleus muscle coefficient than AFPEAS treated animals (Figure 6).
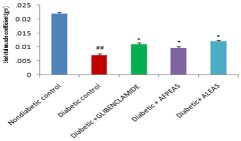
Figure 6: Effect of aqueous extract of Annona squamosa (Fruit peel and Leaf)
on Skeletal muscle weight at week 04. N=08; Values are expressed as mean
± SEM; AFPEAS:Aqueous Fruit Peel Extract of Annona squamosa; ALEAS:
Aqueous leaf Extract of Annona squamosa; ##p<0.01vs. non diabetic group;
*p<0.05 vs. diabetic control.
Estimation of liver coefficient: A significant (0.067±0.0011, ## P < 0.01) increase in liver coefficient were seen in diabetic control group as compared to nondiabetic mice. However both Glibenclamide and ALEAS treated animals produce significant decrease in (0.06±0.0012 and 0.05±0.0022 respectively, *P < 0.05) in liver coefficient as compared to diabetic mice (Figure 7). Whereas the AFPEAS treated animals showed (0.061±0.0012) decrease in liver weight but insignificantly.
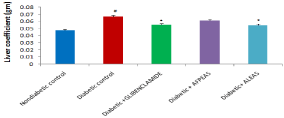
Figure 7: Effect of aqueous extract of Annona squamosa (Fruit peel and
Leaf) on Liver weight at week 04. N=08; Values are expressed as mean ±
SEM; AFPEAS:Aqueous Fruit Peel Extract of Annona squamosa; ALEAS:
Aqueous leaf Extract of Annona squamosa; ##p<0.01vs. non diabetic group;
*p<0.05 vs. diabetic control.
Antioxidant level: Reduction in melonaldehyde (MDA) level causes reduction in oxidative stress. Because due to oxidative stress in diabetes, a significant (11.38 +-0.40 nm/gm of tissue, ##p < 0.01) increase in antioxidant level (MDA) were seen in animals treated with high-fat MLDS as compared to non diabetic mice. Though Annona squmosa fruit peel and leaf extract are having flavonoid content, it shows good antioxidant properties and showed a significant (8.00 ± 0.59 and 8.73±0.88 nm/gm of tissue respectively, * p < 0.05) decrease in MDA level in the skeletal muscle of mice when it compared with vehicle (diabetic control) treated animals but AFPEAS showed more effective free radical scavenging activity than other groups (Figure 8). Whereas the standard drug treated (glibenclamide) mice also decrease (10.17+-0.49 nm/gm of tissue) the MDA level but not significantly.
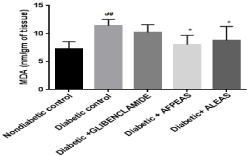
Figure 8: Effect of aqueous extract of Annona squamosa (Fruit peel and
Leaf) on MDA level at week 04. N=08; Values are expressed as mean ± SEM;
AFPEAS:Aqueous Fruit Peel Extract of Annona squamosa; ALEAS: Aqueous
leaf Extract of Annona squamosa; ##p<0.01vs. non diabetic group; *p<0.05
vs. diabetic control.
Discussion
Diabetes is a chronic lifelong disease, which is characterised by high level of sugar or glucose in the blood. Type-2 diabetes is the most common form. Insulin is a hormone produced in the pancreatic beta cell, which is required to move the glucose into the cell. Inside the cell glucose is stored and later is used for energy.
In Type-2 diabetes, the fat cells, liver and muscle cells do not respond correctly to insulin. As a result, blood sugar does not get into the cell to be stored for energy. When sugar cannot enter cells, a high level of sugar builds up in the blood. This is called hyperglycemia. Type 2 diabetes usually occurs slowly over time. Most people with the disease are overweight or obese when they are diagnosed. Increased fat makes it harder for your body to use insulin the correct way [18,19].
Increasing evidence suggests that oxidative stress plays a role in the pathogenesis of diabetes mellitus and its complications [20]. Hyperglycemia increases oxidative stress, which effects the impairment of insulin action and insulin secretion. In addition, antioxidant mechanisms are diminished in diabetic patients, which may further augment oxidative stress [21,22]. Whereas the antidiabetic drugs showed antidiabetic activities but does not produced significant free radical scavenging properties. Several studies have addressed the possible participation of dietary antioxidants, such as vitamins and herbal formulations in ameliorating the diabetic state and retarding the development of diabetes complications [23,24].
There are many effective anti diabetic drugs are available for therapeutic use. However, an agent which can improve glucose uptake and free radial scavenging properties can be an add-on therapy to the anti-diabetic agents. This can help the patient by reducing the antidiabetic dose, possibility of hypoglycemia and other side effect caused by available drugs. In market a varieties of synthetic antidiabetic drugs are available. But these synthetic chemical moieties are having more side effects than therapeutic properties.
On the basis of literature review, oral administration of Annona squamosa aqueous extract to diabetic rats for 30 days significantly reduced blood glucose, urea, uric acid and creatinine, but increased the activities of insulin, C-peptide, all marker enzymes to near control levels and produce antidiabetic and antihyperlipidamic activities [25].
Annona squamosa is reported to possess varied medicinal properties such as insecticidal, antiovulatory and antitumour activities [26]. The present investigation reports the hypoglycaemic and antidiabetic effect of water extract of leaves of this plant. The observation and preliminary idea of the mechanism of its action reported here offer scientific explanation for the potential use of this plant for the treatment of diabetes mellitus. The plant is reported to contain glycoside, alkaloids, saponins, flavonoids, tannins, carbohydrates, proteins, phenolic compounds, phytosterols, amino acids. The flavonoid content of the plant is having antidiabetic activity. The antidiabetic activity of Annona squamosa may be due to increased release of insulin from β-cells of pancreas or it may potentiate the effect of insulin [27].
The primary aim of the present investigation was to comparatively evaluate the antidiabetic and antioxidant activities from both the aqueous fruit peel and leaf extracts of Annona squamosa while other reported methods used either leaf or fruit peel extract [10,12]. With this background information the present study was designed and studied for improvement of glucose uptake and free radical scavenging properties of natural or herbal drugs. In this present study the AFPEAS showed significantly reduction in plasma glucose. Triglyceride and total cholesterol level after 2 weeks of treatment but the ALEAS showed better disposal of glucose, triglyceride and total cholesterol level than diabetic control group compared with results obtained for reported methods after 3 weeks12. However at different (0, 30, 60.120 min) time interval, both the extract treated animals were showed significant utilization in plasma sugar level in both OGTT and ITT test. The AFPEAS and ALEAS treated mice showed significant increase in skeletal muscle coefficient as compared to diabetic control group. In case of estimation of liver coefficient, only ALEAS extract treated mice showed significant reduction in liver weight coefficient only after three weeks of treatment. Previously reported methods used induction of Diabetes by streptozotocin or alloxan while the present method used high fat diet and multiple low dose streptozotocin for the first time. From the above result it was conformed that the Annona squamosa plant extracts are having antidiabetic and hepatoprotective properties. On the basis of above biochemical estimation, it was conformed that the aqueous fruit peel extract of Annona squamosa is having good free radical scavenging activity than others drug treated mice.
Conclusion
Conclusively the present study demonstrated that the Aqueous Leaf Extract of Annona squamosa (ALEAS) showed significant reduction in plasma glucose, triglyceride and total cholesterol level along with better glucose tolerance in Oral Glucose Tolerance Test (OGTT) and insulin responsiveness for Insulin Tolerance Test (ITT) than Aqueous Fruit Peel Extract of Annona squamosa (AFPEAS). But the aqueous fruit peel extract of Annona squamosa is having better free radical scavenging or antioxidant properties than aqueous leaf extract of this plant. However, Future study is necessary to evaluate and isolate the active principle of fruit peel and leaves of Annona squamosa, responsible for the above activity.
Acknowledgement
The authors would like to acknowledge the contributions of MNR College of Pharmacy, Fasalwadi, Sangareddy, Medak, Telangana, India for providing necessary facilities to carry out the research work.
References
- DeFronzo RA. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabet. 2009; 58: 773-795.
- Risérus U, Willett WC, Hu FB. Dietary fats and prevention of type 2. prog lipid res. 2009; 48: 44-51.
- Jung M, Park M, Lee HC, Kang YH, Kang ES, Kim SK. Antidiabetic agents from medicinal plants. Curr Med Chem. 2006; 13: 1203-1218.
- Malviya N, Jain S, Malviya S. Antidiabetic potential of medicinal plants. Acta Pol Pharm. 2010; 67: 113-118.
- Singh LW. Traditional medicinal plants of Manipur as anti-diabetics. J Med Plant Res.2011; 5: 677-687.
- Patel DK, Prasad SK, Kumar R, Hemalatha S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac J Trop Biomed. 2012 ; 2: 320-330.
- Shah R. Pharmacognosy and Pharmacology of Annona squamosa: A review. Int J Pharm Life Sci. 2011; 2: 1183-1189.
- Mittal M, Juyal V, Singh A. Antidiabetic and cytoprotective effect of annona squamosa leaves of diabetic wistar albino rats. Pharmacol online. 2010; 2: 908-913.
- Kappor LD. “Handbook of Ayurvedic Medicinal Plants”, CRC Press, London, 2005; 41-42.
- Rajesh KG. Achyut NK. Hypoglycaemic and antidiabetic effect of aquous extract of leaves of Annona squamosa (l.) in experimental animal. Curr Sci. 2005; 88:1244-1254.
- Gupta RK, Kesari AN, Watal G, Murthy PS, Chandra R, Maithal K, Tandon V. Hypoglycaemic and antidiabetic effect of aqueous extract of leaves of Annona squamosa (L.) in experimental animal. Curr Sci. 2005; 88: 1244-1254.
- Sharma A, Chand T, Khardiya M, Yadav KC, Mangal R, Sharma AK. Antidiabetic and Antihyperlipidemic Activity of Annona Squamosa Fruit Peel in Streptozotocin Induced Diabetic Rats. Inter J Toxicol Pharmacol Res. 2013; 5: 15-21.
- Arulmozhi DK, Kurian R, Bodhankar SL, Veeranjaneyulu A. Metabolic effects of various antidiabetic and hypolipidaemic agents on a high-fat diet and multiple low-dose streptozocin (MLDS) mouse model of diabetes. J Pharm Pharmacol. 2008; 60: 1167-1173.
- Richman JG, Kanemitsu-Parks M, Gaidarov I, Cameron JS, Griffin P, Zheng H. Nicotinic acid receptor agonists differentially activate downstream effectors. J Biol Chem. 2007; 282: 18028-18036.
- Bonner-Weir S. Morphological evidence for pancreatic polarity of beta-cell within islets of Langerhans. Diabetes. 1988; 37: 616-621.
- Thounaojam MK, Jadeja RN, Devkar ARV, Ramachandran AV. Prevention of high fat diet induced insulin resistance in C57BL/6J mice by Sida rhomboidea Roxb. Extract J Health Sci. 2010; 56: 92-98.
- Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990; 186: 407-421.
- American Diabetes Association. Standards of medical care in diabetes -- 2014. Diabetes Care. 2014; 37: 14-80.
- Buse JB, Polonsky KS, Burant C. Type 2 diabetes mellitus. Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, editors. In: Williams Textbook of Endocrinology. 12th Edn. Philadelphia, Pa: Saunders Elsevier; 2011.
- Shinde SA, Deshmukh AD, Suryakar AN, More UK, Tilak MA. The levels of oxidative stress and antioxidants in diabetes mellitus before and after diabetic treatment with or without antioxidants. Ind J Basic Appl Med Res. 2014; 3: 455-460.
- Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med. 2011; 50: 567-575.
- Maritim AC, Sanders RA, Watkins JB. Diabetes, oxidative stress, and antioxidants: A review. Journal of Biochemical and Molecular Toxicology. 2003; 17: 24.
- Sheikh-Ali M, Chehade JM, Mooradian AD. The antioxidant paradox in diabetes mellitus. Am J Ther. 2011; 18: 266-278.
- Cuerda C, Luengo LM, Valero VA, Burgos R, Calvo FL. Antioxidants and diabetes mellitus: review of the evidence. Nutr Hosp. 2011; 26: 68-78.
- Kaleem M, Medha P, Ahmed QU, Asif M, Bano B. Beneficial effects of Annona squamosa extract in streptozotocin-induced diabetic rats. Singapore. Med J. 2008; 49: 800.
- Damaseeno DC. Effect of Annona squamosa extract on early pregnancy in rats. Phytomedicine. 2002; 9: 667-672.
- Patel DJ, Kumar V. Annona squamosa L.: Phytochemical analysis and Antimicrobial Screening. J pharma Res. 2008; 1: 34-38.
Citation: Sahu M, Sahoo NK, Alagarsamy V and Rath BP. Comparative Evaluation of Antidiabetic and Antioxidant Activities of Aqueous Fruit Peel and Leaf Extracts of Annona Squamosa on High Fat Diet and Multiple Low Dose Streptozotocin Mouse Model of Diabetes. Austin J Pharmacol Ther. 2016; 4(1).1081. ISSN: 2373-6208.