
Research Article
Austin J Pharmacol Ther. 2016; 4(2).1082.
Anticancer Activity of Fruit and Leaf Extracts of Averrhoa Bilimbi on MCF-7 Human Breast Cancer Cell Lines: A Preliminary Study
Maya S. Nair*, Kamala Soren, Virendra Singh and Bibari Boro
Department of Biotechnology, Indian Institute of Technology, India
*Corresponding author: Maya S Nair, Department of Biotechnology, Indian Institute of Technology, India
Received: May 04, 2016; Accepted: July 22, 2016; Published: July 27, 2016
Abstract
The fruit and leaf extracts of Averrhoa bilimbi were analyzed for the presence of phytochemicals, flavonoid content and in vitro cytotoxic potential. The methanolic extract of the fruit showed the presence of various phytoconstituents viz flavonoids, saponins, tannins, terpenoids etc. on the basis of the qualitative chemical tests while the ethanolic leaf extract showed the presence of phenols, alkaloids and flavonoids. The total flavonoid content of the fruit extract was found as 358 ± 0.7 μg rutin /g plant extract while for leaf extract, it was 47 ± 1.2μg/g only. The methanolic fruit extract exhibited significant cytotoxic potential against MCF-7 human breast cancer cell lines with an IC50 value of 154.9 μg/ ml whereas an IC50 value of 668 μg/ml observed for ethanolic leaf extract. The present findings indicate that the methanolic fruit extract could be considered as a source of novel anticancer compounds.
Keywords: Averrhoa bilimbi; Flavonoid; Cytotoxicity; Rutin; MTT assay; MCF-7 cell lines
Introduction
Though cancer has been considered as a group of diseases affecting the more developed countries, its incidence in many different forms is now rapidly increasing worldwide. It represents the largest cause of mortality in the world. Although many drugs are there in active development and many are in clinical trials, there is an urgent need to develop much more effective and less toxic drugs. It has been reported that about 50% of all drugs in clinical use are derived from natural products, many of which have the potential to induce apoptosis in various human cell lines [1].
Plants and their secondary metabolites have always been an exemplary source of medicine from time immemorial. A rapid progress has been made in the phytochemical analysis of the plant products. Natural products are formulated to develop different drugs having anticancer properties. Several plant extracts and their bioactive components are well recognized for their ability to exert anticancer effects [2]. Some of the most clinically useful chemotherapeutic agents developed from natural products include vincristine, podophyllotoxin, paclitaxel and camptothecin [3,4]. Flavonoids are polyphenolic compounds having free radical scavenging activity, inhibition of hydrolytic and oxidative enzymes, anti-inflammatory activity like properties and have been isolated from plants.
Averrhoa bilimbi plant belonging to Oxalidaceae family has been reported to have many pharmacological effects. Averrhoa bilimbi fruit and leaf have antibacterial, antiscourbutic, astringent, postpartum protective efficiency and has been used for the treatment of fever, mumps, inflammation of rectum, diabetes, rheumatism, whooping cough, hypertension etc in traditional medicine. The fruit juice is also helpful in removing stains and rust from clothes [5,6]. The hypoglycemic and hypolipidemic activity of the semi purified leaf extract of Averrhoa bilimbi on high fat diet streptozotocin induced diabetic rat was studied by Tan and group [7] and hypoglycemic activity by performing oral glucose tolerance test by Pushparaj et al. [8]. Their study also showed the hypotriglyceridemic, anti-lipid peroxidative, anti-atherogenic properties of the fruit extract in STZ diabetic rats. The fruit and its water extract are shown to have antihypercholesterolemic activity in rats through another study [9].
The ability of Averrhoa bilimbi fruit to heal gingival wound by formation of fibroblast was reported by Hartini [10]. The cytotoxic potential of the hydromethanolic fruit extract of Averrhoa bilimbi was demonstrated by Chowdhury et al. [11] using brine shrimp lethal bioassay with an IC50 value of 5.011μg/ml. However, to our knowledge investigating effect of the anticancer activity of Averrhoa bilimbi extract is lacking so far. The present study was carried out to estimate the total flavonoid content of the fruit and leaf extract of Averrhoa bilimbi (A. Bilimbi) and to demonstrate their anticancer potential against human breast cancer cell line.
Materials and Methods
Plant material and extraction
The fruits and leaves of A.Bilimbi were obtained from Kerala, India. The botanical identification was done by the local ayurvedic physician Dr. Ashwin Raj (Sreekumar Pharmacy, Thrickodithanam, Kerala).
Leaf extract
Shade dried leaves were crushed into powder and were extracted successively with petroleum ether, chloroform, ethyl acetate, 80% ethanol and water in a soxhlet apparatus.
Fruit extract: The fruits were cut into pieces and dried in a hot air oven at 40°C. The dried fruits were ground into fine powder. A methanolic extract was prepared by mixing 10g of the fruit powder with 80% methanol at a solid: liquid ratio of 1:10. The mixture taken in a conical flask was agitated at 50°C for 2 hours, filtered to obtain a clear solution and was used for further investigation.
For biological assay, the crude extracts obtained were lyophilized and redissolved in dimethyl sulphoxide to a concentration of 100 mg/ ml stock solution and was mixed with culture media to obtain the desired concentration.
Chemicals
Rutin hydrate was obtained from Sigma (St.Louis, USA). Minimal Essential Medium eagle (MEM), Foetal Bovine Serum (FBS), Trypsin, Phosphate Buffer Saline (PBS), Ethylthiazolyldiphenyl-Tetrazolium Bromide (MTT), Acridine Orange, Ethidium Bromide, Dimethyl Sulphoxide, Trypan blue dye were from Himedia (Mumbai, India). Aluminium chloride was from SD Fine (RFCL, Mumbai), Methanol and ethanol from Qualigens (GSK, NewDelhi). All chemicals used were of analytical grade.
Phytochemical screening
Phytochemical components of fruit and leaf extracts of A. blilimbi were identified by qualitative analysis. Freshly prepared methanolic fruit extract was qualitatively tested for the presence of tannin, phlobatannins, saponin, flavonoids, terpenoids cardiac glycosides and anthraquinones using the method described elsewhere [12]. The ethanolic leaf extract was tested for the presence of phenols, alkaloids and flavonoids using the protocol described by Kumar et al [13].
Total flavonoid content
The total flavonoid content of the ethanolic leaf extract and methanolic fruit extract of A. bilimbi was determined according to the protocol by Mervart et al. [14] 100 μl of fruit extract in 900 μl of methanol was taken and mixed with 1 ml of aluminium chloride (2% in methanol). The mixture was vigorously shaken and absorbance at 367 nm was read using a UV spectrophotometer (Cary Bio100, Varian, USA) after 10 minutes of incubation. Aqueous ethanol (80%) was used as reference for the leaf extract and aqueous methanol (80%) was used for fruit extract. The absorption of standard rutin solution (1 mg.ml-1) was measured under the same conditions. The flavonoid concentrations in both leaf and fruit extracts were calculated from the rut in calibration curve and expressed as rutin equivalent (RE) in mg of rutin /g of plant extract [15].
Cell lines and cell cultures
MCF-7 human breast cell line was obtained from National Center for Cell Science (NCCS), Pune, India. Cells were cultured in Minimum Essential Medium Eagle (MEM) medium supplemented with 10% (v/v) fetal bovine serum (FBS) and 1% antibiotic-antimycotic mix (100U/ml of pencillin and 100U/ml streptomycin). The cells were grown in tissue culture flask at 37°C in the presence of humidified 5% CO2.
Trypan blue dye exclusion assay
The dye exclusion test is used to determine the number of viable cells present in a cell suspension. The cells were treated with appropriate concentrations of crude ethanolic leaves extracts and incubated for 24 hrs at 37° C. After treatment the cells were collected and suspended in 1 ml Phosphate Buffer Saline (PBS). 0.4% trypan blue dye solution was added to this suspension and incubated for 1 minute at room temperature [16]. After incubation the cells were counted using haemocytometer (Superior Marienfield, Germany) and the percentage viability was determined using the formula as follows:
Cytotoxicity assay (MTT assay)
Cytotoxic effect of the leaf and fruit extracts was determined by MTT assay, as previously described [17]. The cells were seeded on 96-well plate at a density of 5× 104 cells per ml per well and treated with different concentrations of crude leaf extract ranging from 600μg/ml upto 1000μg/ml. The cells were then incubated for about 36 hours. After incubation the medium was changed and MTT solution (5mg/ml) was added and incubated for 4-8 hours in dark. MTT was removed without disturbing the formazan crystals and 100μl of DMSO was added into the wells. The contents of the plate were mixed for 5 minutes to dissolve the crystals properly and then absorbance was measured on ELISA reader (Molecular Devices, Spectromax M2e, USA) at 570 nm [18]. Viability and inhibition of cell growth by the plant extract was calculated relative to the absorbance (OD) of control treated with DMSO for 100% cell viability [19].
Experiments were performed in triplicate. The IC50 values for fruit and leaf extracts were obtained using nonlinear regression in Graphpad prism as shown in Figure 2. Doxorubicin and DMSO were used as positive and negative controls respectively.
Acridine Orange/Ethidium Bromide staining
Acridine Orange (AO) and ethidium bromide (EtBr) are fluorescent dyes capable of binding to DNA. Based on the fluorescence emitted by these compounds upon excitation, differentiation of healthy, early and late apoptotic and necrotic cells can be done. The cells were seeded in a 24 well plate and treated with different concentrations of methanolic fruit extract and ethanolic leaves extract for 24 h. After incubation the media was removed and the cells were washed with PBS. 4 μl of Acridine orange/Ethidium bromide dye mixture (100 μg.ml-1 AO and 100 μg.ml-1 EB) was added to cells for staining and was observed under fluorescent microscope (Axiovert, 25 Carl Zeiss) for differential staining of live and dead cells [20].
Results
Phytochemical screening
Freshly prepared crude extracts from the fruit and leaves of A.bilimbi were tested for the presence of various chemical constituents qualitatively [12]. The results are as given in (Table 1). The fruit extract showed the presence of tannins, phlobatannins, saponins, flavonoids, terpenoids, cardiac glycosides and anthocyannins. The leaf extract contains flavonoid, alkaloids and phenols.
Total flavonoid content (μg rutin per g plant extract)
IC50 value
μg.ml-1
Phytochemicals present
Fruit
358 ± 0.7
154.9
Tannins, Phlobatannins, Saponins, Flavonoids
Terpenoids, Cardiac glycosides, Anthocyannins
Leaf
47 ± 1.2
668.3
Flavonoid, Alkaloids, Phenols
Table 1: Total flavonoid content, IC50 value and phytochemicals present in the fruit and leaf extracts of A.bilimbi.
Total flavonoid content
Flavonoids, terpenoids, alkaloids, phenols have been identified in plants having cytotoxic properties. Flavonoid content of the hydromethanolic extract of the fruit and 80% ethanolic leaf extracts were estimated. The fruit extract showed a higher content of flavonoid. The total flavonoid content in crude hydromethanolic fruit extract of A. bilimbi was found to be 358 ± 0.7 μg.g-1 plant extract (in Rutin equivalent). In the case of leaf extract, among all the extracts obtained, the total flavonoid content in 80% ethanol was found to be higher and is 47± 1.2 μg.g-1 of plant extract in Rutin equivalent. The standard curve of rutin is shown (Figure 1). Hence the ethanol extract of the leaf was studied for its cytotoxic effect along with the methanolic extract of fruit.
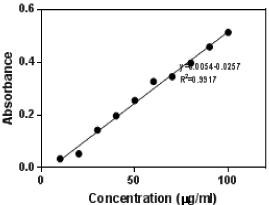
Figure 1: Rutin standard curve.
Cytotoxicity assays
MTT assay: Cytotoxic assays provide important preliminary information in identifying the extract with potential anti-neoplastic properties which can be used in future studies. The cytotoxic effect of fruit and leaf extracts were evaluated using MCF-7 tumor cell lines. Both fruit and leaf extracts were active on MCF-7 cell lines. Different concentrations of the extracts were used and dose dependent growth inhibition of cancerous cells was observed. IC50 value for fruit extract was 154.9 μg.ml-1 and a maximum inhibition of cell growth was obtained at 300 μg.ml-1. The ethanolic leaf extract of A.Bilimbi showed an IC50 value 668.3 μg.ml-1. The % inhibition for both the extracts is given in figure (Figure 2a and 2b). The fruit extract showed a greater potential of cytotoxic activity as compared to leaf extract. This could be attributed to the higher amount of flavonoids present in the fruit extract. The IC50 for positive control, doxorubicin could not be determined exactly as at concentration of 1 μg.ml-1, the cell viability was about 21%.
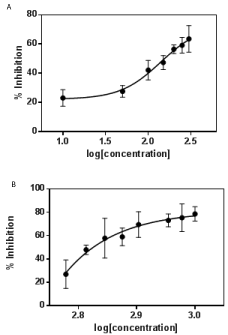
Figure 2: Growth inhibition curve for A Bilimbi a) fruit extract and b) leaf
extract against MCF-7 cell lines. Error bar represents mean ± standard
deviation of experiments performed in triplicate.
Acridine orange/ ethidium bromide dye staining: Acridine orange and ethidium bromide dye mixture differentially stains the live and dead cells. Acridine orange (AO) can permeate all cells and the nuclei will appear green. Ethidium bromide is taken up by cells whose cytoplasmic membrane integrity is lost, and stains the nucleus orange and also dominates over AO. Hence live cells have a normal green nucleus, early apoptotic cells have bright green nucleus with condensed or fragmented chromatin and late apoptotic cells show condensed and fragmented orange chromatin. In this study it is found that the control cells appeared green whereas the treated cells showed a mixture of green and orange colour stained cells for both fruit and leaf extracts (Figure 3). Though the proportion of the orange cells was more in the case of treated cells for both fruit and leaf extract, this was found to be larger in the case of methanolic fruit extract.
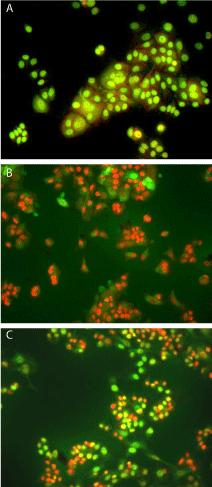
Figure 3: Acridine Orange/Ethidium Bromide stained nuclei of MCF-7 cells
(a) treated with DMSO only (b) treated with 400 μg/ml fruit extract (c) treated
with 750 μg/ml leaf extract.
Trypan blue dye exclusion assay: Apoptosis is a natural way to maintain cell morphology, tissue homeostasis and helps in prevention of cancer. The trypan blue dye exclusion test is based on the principle that live cells possess intact cell membranes that excludes this dye whereas dead cells do not. As a result viable cells have a clear cytoplasm whereas a nonviable cell will have a blue cytoplasm. When MCF-7 cells were treated with fruit extract, the cells appeared blue due to cytotoxic effect whereas the control cells showed a clear cytoplasm. The viability of cells was found to be around 58% for cells treated with 100 μg.ml-1 concentration of fruit extract and 37% for 300 μg.ml-1 concentrations. Cells were 50% and 20% viable when treated with 750 and 1000 μg.ml-1 of leaf extracts respectively.
Discussion
Total flavonoid content in the methanolic extract of A.bilimbi fruit extract was found to be higher compared to ethanolin leaf extraction. Flavonoids are the most common and widely distributed polyphenolic compounds present in plants. Plant flavonoids mostly exist in their glycosylated form and glycosylation can have different effects on their biological activities. Different studies report the effect of glycosylation and de-glycosylation of flavonoids and its effect on the biological activities. In some cases glycosylation increased the biological activity of the compounds [21-23] and in some other cases it showed a decrease in its efficiency. Rutin showed an IC50 value < 250 μg.ml-1 while its aglycone (hydrolysed rutin) showed a value ~2 μg.ml-1 against MCF-7 cell lines as reported in literature [24]. This implies that rutin in its deglycosylated form is more effective in inducing cell death than in its glycosylated form. The review article by Xiao et al. [23] demonstrates the glycosylation /deglycosylation effect of various flavonoids in various in vitro/in vivo studies. Both forms can have different pharmacokinetic behaviour. Lin and Harnly [25] in their work developed a screening method for identification of glycosylated flavonoids and polyphenolic compounds present in plant materials using different analytical and quantification techniques. Hence detailed investigation is needed for the form of flavonoids present in the extract and about the biological activity of them when present in glcosylated/deglycosylated forms.
Acridine orange and ethidium bromide dye staining results showed the cytotoxic effects of the water and methanol extracts and the effect was more for the methanolic extract of the fruit. The reason for this can be due to the presence of higher flavonoid content in the fruit extract, though the extracts contains a mixture of all types of flavonoids along with many other components. There can be a synergestic effect because of the presence of different molecules which will be exploited in future studies. The result agrees well with the various reports according to which flavonoids present in dietary plants are reported to exhibit cytotoxicity through modulation of principal elements involved in apoptotic process [26].
Conclusion
This study reports the preliminary results of the anticancer activity of crude extracts from A.bilimbi. The study showed that A.bilimbi fruit and leaves extract inhibit the proliferation of MCF-7 breast cancer cells with the involvement of apoptosis or programmed cell death. The fruit extract of A.bilimbi showed a better activity towards MCF-7cell lines. The results of this study reveal that A.bilimbi fruit extract could be considered as a promising chemotherapeutic agent in cancer treatment. Separation of different fractions of flavonoids by high performance liquid chromatography and cytotoxic analysis of each fraction will help in identification of the active component in A.bilimbi. Also, studies on the apoptotic transduction pathway in cancer cells upon treatment with this plant extract would throw light on reason behind cell death induction. Work is currently under progress in this direction.
Acknowledgement
Kamala Soren, Virendra Singh and Bibari Boro are thankful to the Department of Biotechnology (DBT, India) for providing student project grant. Authors are greatful to Prof. Partha Roy, Department of Biotechnology, IIT Roorkee for his valuable suggestions.
References
- Kim J, Park EJ. Cytotoxic anticancer candidates from natural resources. Curr. Med. Chem. Anticancer agents. 2002; 2: 485-537.
- Pezzuto JM. Plant-Derived anticancer agents. Biochem. Pharm 1997; 53: 121-133.
- Lee KH. Anticancer drug design based on plant derived natural products. J. Biomed. Sci. 1999; 6: 236-250.
- Cragg GM, Newman DJ. 2004/Rev.2006, Plants as a source of anti-cancer agents in Ethnopharmacology in Encyclopedia of life Support Systems (EOLSS), Edited by Elaine Elisabetsky and Nina L. Etkin (Eolss Publishers, Oxford, UK,).
- Abas F, Nordin H, Lajis, Israf DA, Khozirah S, KalsomYU. Antioxidant and nitric oxide inhibition activities of selected Malay traditional vegetables. Food Chem. 2006; 95: 566-573.
- Roy A, Geetha RV, Lakshmi T. Averrhoa bilimbi Linn–Nature’s Drug Store- A Pharmacological Review. Int. J. of Drug Develop. & Res. 2006; 3: 101-106.
- Tan BKH, Tan CH, Pushparaj PN. Anti-diabetic activity of semi-purified fractions of Averrhoa bilimbi in streptozotocin-diabetic rats. Life Sci. 2005; 7: 2827-2839.
- Pushparaj PN, Tan BKH, Tan CH. The mechanism of hypoglycemic action of the semi-purified fractions of Averrhoa bilimbi in streptozotocin-diabetic rats. Life Sci. 2001; 70: 535-547.
- Ambili S, Subramoniam A, Nagarajan NS. Studies on the Antihyperlipidemic Properties of Averrhoa bilimbi Fruit in Rats. Planta Med. 2009; 75: 55-58.
- Hartini IGAA. Tropical application of ethanol extract of star fruit leaves (Averrhoa bilimli Linn) increases fibroblasts in gingival wounds healing of white male rats, Indonesian J. Biomed. Sci. 2012; 6: 35-39.
- Chowdhury SS, Uddin GM, Mumtahana N, Hossain MM, Hasan SMR. In-vitro Antioxidant and Cytotoxic potential of Hydromethanolic extract of Averrhoa bilimbi L. Fruits. Int. J. of Pharm Sci. Res. 2012; 3: 2263-2268.
- Satyaprabha G, Kumaravel S, Paneerselvam A. Analysis of antioxidant activity, total phenol, total flavonoidand screening of phytocomponents in Plerots platypus and Pleurotus eous. J.of Chem. Pharmaceutical Res. 2011; 3: 1-6.
- Kumar A, Jha KK, Kumar D, Agrawal A, Gupta A. Preliminary Phytochemical Analysis of Leaf and Bark (Mixture) Extract of Ficus Infectoria Plant. The Pharma Innovation. 2012; 1: 83-89.
- Al Far MMM, Taie HAA. Antioxidant activities, Total anthocyannins, phenolics and flavonoids content of some sweet potato genotypes under stress of different concentrations of sugar and sorbitol, Aust. J. of Basic and Appl. Sci. 2009; 3: 3609-3616.
- Steenkamp V, Nkwane O, van Tonder J, Dinsmore A, Gulumian M. Evaluation of the phenolic and flavonoid contents and radical scavenging activity of three southern African medicinal plants, Afr. J. of Pharmacol. 2013; 7: 703-709.
- Giuseppe SA, Sorbello L, Guray S, Banerjee D, Bertino JR. Cytotoxicity and cell assays. Elsevier Sci, Cell Biology, Chapter 8. 2006; 315-324.
- Arulvasu C, Prabhu D, Manikandan R, Srinivasan P, Dinesh D, Babu G, et al. Induction of apoptosis by the aqueous and ethanolic leaf extract of Vitex negundo L. in MCF-7 human breast cancer cells. Int. J. of Drug Discovery. 2010; 2: 1-7.
- Ampasavate C, Okonogi S, Anuchapreeda S. Cytotoxicity of extracts from fruit plants against leukemic cell lines. Afr. J. of Pharm. Pharmacol. 2010; 4: 13-21.
- Wang X, Yuan S, Wang J, Lin P, Liu G, Lu Y, et al. Anticancer activity of litchi fruit pericarp extract against human breast cancer in vitro and in vivo. Toxicol. Appl. Pharmacol. 2006; 215: 168-178.
- Takahashi A, Matsumoto H, Yuki K, Yasumoto JI, Kajiwara A, Aoki M, et al. High-Let Radiation enhanced Apoptosis but not Necrosis regardless of p53 status. Int. J. Radiation Oncol Biol. Phys. 2004; 60: 591-597.
- Xiao J, Tamar S, Muzashvili TS, Georgiev MI. Advances in the biotechnological glycosylation of valuable flavonoids. Biotech. Adv. 2014; 32: 1145–1156.
- Dixon RA, Pasinetti GM. Flavonoids and Isoflavonoids: From Plant Biology to Agriculture and Neuroscience. Plant Physiol. 2010; 154: 453–457.
- Xiao J. Dietary Flavonoid Aglycones and Their Glycosides: Which Show Better Biological Significance?. Critical Rev Food Sci.Nutr. 2015.
- De Araújo MEMB, Moreira Franco YE, Alberto TG, Sobreiro MA, Conrado MA, Priolli DG, et al. Enzymatic de-glycosylation of rutin improves its antioxidant and antiproliferative activities. Food Chem. 2013; 141: 266-273.
- Lin L-Z, Harnly JM. A Screening Method for the Identification of Glycosylated Flavonoids and Other Phenolic Compounds Using a Standard Analytical Approach for All Plant Materials. J Agri Food Chem. 2007; 55: 1084-1096.
- Ramos S. Effects of dietery flavonoids on the apoptotic pathways related to cancer prevention. J. Nutr. Biochem. 2007; 18: 427-442.