
Research Article
Austin J Pharmacol Ther. 2016; 4(2).1086.
Anticataract Activity of Isoliquiritigenin Rich Fraction of Glycerrhiza glabra on Galactose Induced Cataractogenesis in Rats
Patel BV¹*, Johari S², Gandhi T² and Shah PK²
¹Department of Pharmacology, Sardar Patel College of Pharmacy, India
²Department of Pharmacology, Anand Pharmacy College, Near Town Hall, India
*Corresponding author: Patel BV, Department of Pharmacology, Sardar Patel College of Pharmacy, Bakrol, Gujarat, India
Received: June 18, 2016; Accepted: September 23, 2016; Published: September 29, 2016
Abstract
Objective: The specific objective of the present study was to evaluate effect of Isoliquiritigenin rich Fraction of Glycerrhiza glabra (IFGG) in galactose induced cataract in rats.
Methods: Sprague Dawley suckling rats of either sex (18 days; 40-50 gm) were selected and randomly allocated to six groups (n=6): normal control; Model control; Std treated with vitamin E (36 mg/kg) rest three groups treated with IFGG (5 mg/kg; 10 mg/kg; 20 mg/kg P.O; once daily respectively). All animals except normal control group were fed with galactose rich diet for 18 days starting from day 21 after parturition. Three days prior to the galactose feeding, animals were pre-treated with Vitamin E and IFGG and was continued till the end of study.
All the animals were checked daily for the appearance of cataract during experiments. The eyes were dilated with Tropicamide (0.8%) and was examined and photographed. At the end of the study lenses from all animals were isolated and homogenate was prepared and was used to estimate levels of Aldose Reductase (AR), Total protein, Sulfhydryl group (-SH), Malondialdehyde (MDA), Calcium, Soluble Protein content and Reduced Glutathione content (GSH).
Results: IFGG significantly prevented the galactose induced changes in level of AR, total protein, GSH, MDA, SH and calcium level.
Conclusion: IFGG inhibited the aldose reductase activity, by preventing the conversion of excess of glucose into the sorbitol and delayed the appearance and maturation of cataract. Thus it can be used as an alternative for prevention of cataract.
Keywords: Isoliquiritigenin; Cataract; Galactose; Aldose reductase
Abbreviations
IFGG: Isoliquiritigenin rich fraction of Glycerrhiza glabra; AR: Aldose Reductase; TP: Total protein; -SH: Sulfhydryl group; MDA: Malondialdehyde; GSH: Reduced Glutathione content; TCA: Trichloroacetic acid; DTNB: 5, 5’-Dithiobis-2-nitro benzoic acid; ARI: Aldose Reductase Inhibitors; TBA: Thiobarbituric acid
Introduction
Cataract is a major contributing factor of blindness. Cataract is defined as a clouding of the natural lens, or opacification of lens, a part of eye responsible for focusing and producing a clear and sharp image [1]. Cataract is a visual impairment caused due to opacification or optical dysfunction lens affecting more than 17 million people around the world. Cataract is mainly responsible for almost 80% of blindness cases in India [2]. The most recent data published by World Health Organization (WHO) showed that the total number of persons with visual impairment worldwide in 2010 was estimated to be 285 million, including 39 million blind people [3].
In the National Survey done in India in the year 2007, the prevalence of blindness was found to be 8%. The survey by Rapid Assessment of Avoidable Blindness shows the rise in prevalence of blindness and was found to be and 14.5% [4]. Considering current population (121crore) of India as per census 2011, approximately 62%, that is, 7.2 million are blind due to cataract. To the global target for elimination of preventable blindness by vision 2020: the right to sight initiative, India also handshakes in 1994 [5].
Various risk factors such as diabetes, oxidation of lens, dehydration, daylight, diet and lipid peroxidation attributes to the generation of lens opacification in elderly patients. Other factors such as smoking, environmental factor, lack of consumption of antioxidants, also increase risk of development of cataract [6].
In order to study the anticataract effects of varieties of agents, galactose induced rat model has been widely used and considered a good representation of human diabetic cataract [7,8]. Galactose model is commonly used as it produces large amount of reduced form, galactitol and finally into glucose. Furthermore, galactitol is not subsequently metabolised as compare to sorbitol. Three major mechanisms behind the formation of cataract are Oxidative stress, Polyol pathway and Non-enzymatic glycation [9,10].
Currently the only treatment for cataracts is surgery. It has been estimated that a 10-year delay in the onset and progression of cataract could reduce the need for cataract surgery by 50% [11].
WHO has recently defined traditional medicine (including herbal drugs) as comprising therapeutic practices that have been in existence, often for hundreds of years, before the development and spread of modern medicine and are still in use today. Herbal medicine is still the mainstay of about 75-80% of the world population, mainly in the developing countries, for primary health care because of better cultural acceptability, better compatibility with the human body and lesser side effects. However, the last few years have seen a major increase in their use in the developed world. In India, the herbal drug market is about $ one billion and the export of plant-based crude drugs is around $ 80 million [12].
Several herbal plants such as Adhatoda vasica, Allium cepa, Ginko biloba, Trigonella foenumgraceum, Vitex negundo and many more are screened for its anticataract activity [10].
Liquorice is the root of Glycerrhiza glabra L., Leguminosae. It is a widely used herbal medicine native to southern Europe and parts of Asia and has beneficial applications in both the medicinal and the confectionery sectors [13].
Recently, the flavonoids in liquorice have attained a considerable interest for their structural diversity and important pharmacological activities of the isolated flavonoids, including chalcones, isoflavones, isoflavans, flavonones, flavanonols, isoflavenes and arylcoumarins. Isoliquiritigenin in liquorice with a chalcone structure was reported to exhibit a variety of biological properties, such as anti-inflammatory, antioxidative and anti-tumor activities [14]. More recently, it reported that isoliquiritigenin, a substance purified from liquorice, is a new potent aldose reductase inhibitor [15].
A number of compounds, both natural and synthetic, have been found to inhibit aldose reductase. These so-called Aldose Reductase Inhibitors (ARIs) bind to aldose reductase, inhibiting polyol production. As a group, flavonoids are among the most potent naturally occurring ARIs. Several evaluations of in vitro animal lenses incubated in high sugar mediums have found flavonoids to inhibit aldose reductase. A group of researchers examined the effect of an orally administered ARI in inhibiting polyol accumulation [16].
Hence, the aim of current study is to evaluate the possible anticataractogenic effect of Isoliquiritigenin: an aldose reductase inhibitor is given in experimentally induced cataract in rats.
Material and Methods
Collection and authentification of Glycerrhiza glabra
Dried roots of Glycerrhizaglabra were obtained from commercial supplier of Anand. Botanical identification was done by Dr. D.B. Patel, Head and Professor of plant breeding department, Anand Agricultural University, Anand. A voucher specimen of collected roots of Glycerrhiza glabra was deposited at the herbarium of Anand Pharmacy College (Voucher APCH-46).
Isolation of Isoliquiritigenin from Glycerrhiza glabra
Dried root powder of Glycerrhiza glabra (1kg) was extracted with acetone at room temperature to obtain brown solid extract. Further this extract was fractionated using liquid-liquid extraction using HCl/CHCl3; its organic layer was collected and evaporated. Further it was extracted with hexane to obtain Isoliquiritigenin rich fraction of Glycerrhiza glabra (IFGG) [17]. IFGG was used to study its effect on cataractogenic rats. The percentage yield obtained is 0.5% and store in cool and dry place.
Chemicals
Acetone (Astron chemicals, India), Hydrochloric acid (Astron chemicals, India), Chloroform (Astron chemicals, India), Hexane (Sigma aldrich, India), Ether (Astron chemicals, India),Mercaptoethanol (Gujarat Chemicals, India), Ammonium Sulphate (Sulab reagents, India), HEPES buffer (Gujarat Chemicals, India), DL-glyceraldehyde (Sigma aldrich, India), Trichloroacetic acid (TCA) (S.D. Fine Chem, India), Disodium hydrogen phosphate, 5,5’-Dithiobis-2-nitro benzoic acid (DTNB) (S.D. Fine Chem, India), Sodium citrate (Sulab reagents, India), GSH standard (Loba chemie pvt. ltd., India), Sodium dodecyl sulphate (S.D. Fine Chem, India), Acetic acid (Sigma aldrich, India), Thiobarbituric acid (S.D. Fine Chem, India), sulfosalicylic acid (S.D. Fine Chem, India), Guanidine (Sulab reagents, India).
Animals
Sprague Dawley suckling rats of either sex (18 days; 40-50 gms) were selected for the experimental study. All animals were housed at ambient temperature (25±10°C) and relative humidity (55±5%) and 12h light/dark cycle. Animals were free access to standard pellet diet and water given ad libitum.
The experimental protocol was approved by Institutional Animal Ethical Committee as per the guidance of committee for the purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Social Justice and Empowerment, Government of India (Protocol No. APC/IAEC/1325).
Animal groups
Animals were randomly allocated to 6 groups, with n=6 animals in each group, as follows:
Group I Normal Control received normal rat chow diet
Group II Model Control received galactose rich diet
Group III Standard received galactose rich diet and Vitamin E 36mg/kg p.o. once daily
Group IV received galactose rich diet and IFGG 5 mg/kg p.o. once daily
Group V received galactose rich diet and IFGG 10 mg/kg p.o. once daily
Group VI received galactose rich diet and IFGG 20 mg/kg once daily
Induction of cataract by galactose
In the present study, Galactose rich diet was fed to all animals of all Groups except normal group animals for 18 days starting from day 21 after parturition.
The composition of diet was: Galactose (50%), Corn starch (20%), Casein (15%), Hydrogenated oil (9%), Salt mixture (NaCl) (1.4%), Cod liver oil (4.6%) [18].
Three days prior to the galactose fed diet, rats of Group III, IV, V and VI were treated with Vitamin E , IFGG (5 mg/kg; 10 mg/ kg; 20 mg/kg P.O; once daily) respectively and continued till the end of study. All animals were checked daily for the appearance of cataract during experiment. The eyes were dilated with Tropicamide (0.8%) and eyes of one animal from each group was examined and photographed on 0, 3rd, 6th, 9th, 12th, 15th, 18th day of galactose feeding.
Homogenate preparation and estimation of various biochemical parameters
At the end of study (18 days) lenses from all animals was isolated and homogenized in 1 ml 10mM chilled phosphate buffer (pH-7). Phosphate buffer (pH 7) was prepared according to Indian Pharmacopoeia 1996 [19]. The homogenate was used to estimate Aldose Reductase level by method proposed by Hayman et al [20].
Total protein content was estimated as described by Lowry et al [21] method; Sulfhydryl content was measured by Ellman’s method [22]; Malondialdehyde level was estimated by TBA reacting substances as proposed by Ohkawa et al [23]; and Calcium level was estimated by OCPC method as described by Bagainski ES [24]. The remaining homogenate was centrifuged at 7000 rpm for 15 min at 4°C to obtain the supernatant layer. The supernatant was used to estimate Soluble Protein content and Reduced Glutathione content was estimated by the method described by Lee et al [25].
Statistical analysis
All data were analysed using Graph Pad statistical software Version 5.5 and expressed as mean ± Standard Error of the Mean (SEM). Statistical analysis of various biochemical parameters was carried out using one way analysis of variance (ANOVA) followed by Dunnett’s Post hoc test. Data was considered statistically significant at P =0.05 and highly significant at P=0.001.
Results
Preliminary phytochemical screening
Preliminary phytochemical screening detected the presence of Flavanoids, streroids, terpinoids, alkaloids, triterpinoids in plant extract.
Effect of IFGG on lens morphology
Macroscopic evaluation of the lens was performed to observe the progression of cataract in Galactose fed model and the nuclear opacity was graded. All the lenses in normal group were transparent whereas model control group animals showed nuclear cataract grade 3. Incorporation of IFGG offered significant protection against cataract formation (Figure 1).
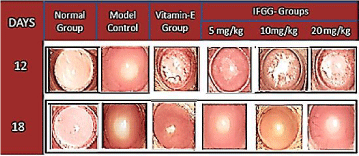
Figure 1: Macroscopic Evaluation of Progression of Cataract on 12th and
18th day.
Model control group showed Nuclear cataract grade 1 on 12th day and Grade
3 cataract on 18th day, whereas the progression of Cataract formation was
delayed in groups pre-treated with IFGG.
Effect of IFGG on total protein content and soluble protein content
The total Protein content and soluble protein content of lens was significantly decreased in model control group as compared to normal group. Groups pre-treated with IFGG prevented decrease in total protein content and soluble protein content of lens as compare to model control group (Table 1).
Parameters
Normal control
Model control
Vitamin E
(36 mg/kg)
IFGG
(5 mg/kg)
IFGG
(10 mg/kg)
IFGG
(20 mg/kg)
Total Protein content
0.3670.0106
0.231±0.0082##
0.343±0.0077*
0.305±0.00709*
0.325±0.0062*
0.307±0.0098*
Soluble protein content
0.3±0.0058
0.114±0.0006##
0.193±0.003*
0.163±0.003*
0.19±0.017*
0.176±0.012*
Table 1: Effect of IFGG on Total Protein content and Soluble Protein content in lens homogenate.
Effect of IFGG on Aldose Reductase (AR) and calcium level
Aldose reductase level in model control group (11.22± 0.276) was significantly increased as compare to model control group (8.25±0.095). In Vitamin E showed reduction in AR level (8.9±0.057). Treatment with IFGG (5, 10, 20mg/kg) showed significant reduction in AR level (9.202±0.037; 8.65±0.105; 8.75±0.076 respectively) (Figure 2).
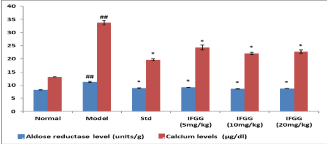
Figure 2: Effect of IFGG on aldose reductase level and Calcium levels in
lens homogenate.
The values were expressed as mean ± SEM. The statistical analysis was
carried out by one-way Analysis of Variance (ANOVA) followed by Dunnet’s
post hoc test.
P values <0.05 were considered significant.
*: Significantly different from Model control group at P<0.05.
##: Significantly different from Normal control group at P<0.001.
Calcium level was significantly elevated in model control group (0.033±0.0008 mg/dl) as compared to normal control group (0.0131±0.0007). Pre-treatment with Vitamin E (36 mg/ kg) reduced the calcium level to (0.019±0.0009) as compared to model control group. Pre-treatment with IFGG (5, 10, 20 mg/kg) significantly debased calcium level as compared to model control group (0.024±0.0009; 0.022±0.0004; 0.0227±0.0007 respectively) (Figure 2).
Effect of IFGG on Oxidative stress
MDA level in model control group showed significant elevation in lipid peroxidation as compare to normal control group, whereas pre-treatment with Vitamin E and IFGG, precluded the elevation in MDA levels (Figure 3).
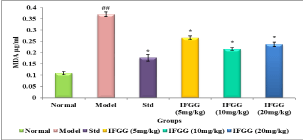
Figure 3: Effect of IFGG on Malondialdehyde (MDA) level in lens homogenate.
The values were expressed as mean ± SEM. The statistical analysis was
carried out by one-way analysis of variance (ANOVA) followed by Dunnet’s
post hoc test.
P values <0.05 were considered significant.
*: Significantly different from Model control group at P<0.05.
##: Significantly different from Normal control group at P<0.001.
GSH level and protein sulfhydryl content in model control group was declined as compare to normal control group, whereas the decline was prevented by pre-treatment with vitamin E and IFGG (Figure 4).
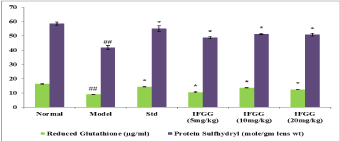
Figure 4: Effect of IFGG on reduced Glutathione level and Protein sulfhydryl
content in lens homogenate.
The values were expressed as mean ± SEM. The statistical analysis was
carried out by one-way Analysis of Variance (ANOVA) followed by Dunnet’s
post hoc test.
P values <0.05 were considered significant.
*: Significantly different from Model control group at P<0.05.
##: Significantly different from Normal control group at P<0.001.
Discussion
Cataract, defined as any opacity in the lens, is the leading cause of blindness around the world, affects up to 80% of the human population over the age of 70, and seriously impairs vision and quality of life. It has increased in prevalence in many countries as a result of a growing elderly population and the incidence of cataract is expected to rise in the future. Currently, the only effective treatment for cataract is surgical removal and replacement of the cataract with an artificial intraocular lens. However, cataract surgery may result in complications such as posterior capsular opacity, glaucoma, endophthalmitis, uveitis, retinal detachment and many more. In addition, the cost of surgery poses an economic burden on patients. Therefore, it is important to explore alternative pharmacological measures for the treatment of cataract [26].
Amongst various models for cataract, galactose induced model is widely used as it has resemblance with human diabetic cataract [7].
The key event in the galactose induced cataract is the activation of the polyol pathway, with the conversion of galactose into sorbitol by aldose reductase. Sorbitol accumulates in lens as the cellular membranes of the lens are impermeable to sorbitol, this leads to hyperosmotic cell swelling, which produces scattering of light and diminished lens transparency [8].
Macroscopic evaluation revealed that not only the appearance of cataract but also maturation pattern was delayed by pre-treatment with IFGG. Nuclear cataract appeared on 12th day in model control group while pre-treatment with IFGG delayed the appearance of nuclear cataract.
Galactosemic and diabetic cataractogenesis in experimental animals might be due to increased formation of polyols from reducing sugars by aldose reductase and Aldose Reductase (AR) is an important rate limiting enzyme that contributes to cataract induction in diabetic patient. It converts galactose to galactitol and glucose to sorbitol [11]. Aldose reductase inhibitors could be an effective strategy in prevention or delay of cataract [27]. Previous studies on experimental models have demonstrated a correlation between Aldose reductase inhibitors and prevention of diabetic cataract [28]. Kim J. et al carried out a preclinical study on galactose induced rats stated that AR activity was significantly elevated in model control group as compared to normal rats [11]. Moreover, in present study,Model control group showed significant elevation in aldose reductase level was elevated in as compared to normal control group. Pre-treatment with Vitamin E lowered the aldose reductase level up to 20.67% while pre-treatment with IFGG (5mg/kg, 10 mg/kg, 20 mg/ kg p.o; once daily) significantly lowered aldose reductase level up to 17.98%, 22.90%, 22.01% respectively as compared to model control group.
Oxidative stress is a common underlying mechanism of cataractogenesis and antioxidant defenses has been shown to prevent or delay cataract [27]. Reduced Glutathione is another mechanism behind formation of cataract [10]. The role of GSH in maintenance of lens clarity, as it serves as a major antioxidant in lens and keeps the protein in reduced form [6]. Glutathione levels were significantly reduced in model group when compare to normal rats [2]. Thiagarajanet al showed that model group animals showed reduced Glutathione level when compared with normal control rats [29]. In present study, there was significant decline in GSH level in model control group as compared to normal control whereas significant elevation was observed in Vitamin E and IFGG groups as compared to model control group.
It has been almost a century since it was shown that calcium is elevated in cataractous lenses and recent studies have confirmed that level is 23 fold is increased as compare to normal lenses. Elevated calcium level leads to protein synthesis and its aggregation, which shows its relation with rise in protein content [30]. Recently in 2013, a study on rats proposed that galactose feeding to rats leads to increase Calcium level in lens. Results in present study supported the data, model control rats have elevated calcium level and the rise was prevented by animals pre-treated with Vitamin E and IFGG [7].
A study on galactose induced cataract in rats showed that Total Protein content and soluble protein content was significantly decreased in model control group as compared to normal control group [31]. Present study also showed resembling results, model control group showed significant reduction in Total protein content and soluble protein content as compare to normal control group and the rise was averted by vitamin E and IFGG.
Yang et al stated that total sulfhydryl content in model group showed significant reduction when compared with normal control animals [26]. Also in the present study, total sulfhydryl content in model control group was decreased significantly as compared to normal control animals in contrast to animals pre-treated with Vitamin E and IFGG prevented reduction in SH content.
Thus, IFGG effectively improved the cataractogenic condition which was implicated by inhibiting the aldose reductase enzyme and it has antioxidant activity which was confirmed by inhibiting oxidative stress parameters. Hence it can be used as an alternative for prevention of cataract.
Acknowledgement
We would like to thank Anand Pharmacy College and most importantly my small and tiny rats.
References
- Shah NK, Pk P, Ba V, Sv J. Evaluation of Anti-Cataract Activity of Asparagus Racemosus Root Extract. Research Scholars (IJPRS). 2013; 19-26.
- Patel D, Prasad S, Kumar R, Hemalatha S. Cataract: A major secondary complication of diabetes, its epidemiology and an overview on major medicinal plants screened for anticataract activity. Asian Pacific J Trop Dis. 2011; 1: 323-329.
- Nowak MS, Smigielski J, Sahin A. The prevalence and causes of visual impairment and blindness among older adults in the city of lodz, poland. Medicine (Baltimore). 2015; 94: 505.
- Patil S, Gogate P, Vora S, Ainapure S, Hingane RN, Kulkarni AN, et al. Prevalence, causes of blindness, visual impairment and cataract surgical services in Sindhudurg district on the western coastal strip of India. Indian J Ophthalmol. 2014; 62: 240-245.
- Avachat SS, Phalke V, Kambale S. Epidemiological correlates of cataract cases in tertiary health care center in rural area of maharashtra. J Fam Med Prim care. 2014; 3: 45-47.
- Gupta KS, Kalaiselvan V, Srivastava S, Saxena R, Agrawal SS. Inhibitory Effect of Trigonella Foenum-Graecum on Galactose Induced Cataracts in a Rat Model; in vitro and in vivo Studies. J. Opthalmic Vis. Res. 2009; 4: 213- 219.
- Agarwal R, Iezhitsa I, Awaludin NA, Ahmad Fisol NF, Bakar NS, Agarwal P, et al. Effects of magnesium taurate on the onset and progression of galactoseinduced experimental cataract: in vivo and in vitro evaluation. Exp Eye Res. Elsevier Ltd; 2013; 110: 35-43.
- Kinoshita JH. Cataracts in galactosemia Investigate Ophthalmology St. Loui: USA. 1965: 786-799.
- Kinoshita JH. A thirty year journey in the polyol pathway. Experimental Eye Research. 1990; 50: 567-573.
- Patel P, Jivani N, Malaviya S, Gohil T, Bhalodiya Y. Cataract: A major secondary diabetic complication. Int Current Pharmaceutical J. 2012; 1: 180- 185.
- Kim J, Kim C-S, Lee YM, Sohn E, Jo K, Shin SD, et al. Scopoletin inhibits rat aldose reductase activity and cataractogenesis in galactose-fed rats. Evid Based Complement Alternat Med. 2013.
- Kamboj VP. Herbal medicine. 2000; 78: 35-51.
- Wang X, Zhang H, Chen L, Shan L, Fan G, Gao X. Liquorice, a unique “guide drug” of traditional Chinese medicine: A review of its role in drug interactions. J Ethnopharmacol. 2013; 1-10.
- Fu Y, Chen J, Li Y-J, Zheng Y-F, Li P. Antioxidant and anti-inflammatory activities of six flavonoids separated from licorice. Food Chem. 2013; 141: 1063-1071.
- Tawata M, Aida K, Noguchi T, Ozaki Y, Kume S, Sasaki H, et al. Anti-platelet action of isoliquiritigenin, an aldose reductase inhibitor in licorice. Eur J Pharmacol. 1992; 12: 87-92.
- Head K. Natural therapies for ocular disorders part two: Cataracts and glaucoma. Altern Med Rev. 2001; 6: 141-166.
- Vaya J, Belinky PA. Structure elucidation and antioxidative capacity. Elsevier Sci. Inc. 1997; 23: 302-313.
- Lee SM, Schade SZ, Doughty CC. Aldose reductase, NADPH and NADP+ in normal, galactose-fed and diabetic rat lens. Biochim. Biophys. Acta. 1985; 841: 247-253.
- Indian Pharmacopoeia. Ministry of Welfare, New Delhi. 1996; Vol. 1: 4th edition: 66.
- Hayman S, Kinoshita JH. Isolation and properties of lens aldose reductase. J Biol Chem. 1965; 240: 877-882.
- Lowry OH, Rosebbrough NJ, Farr AL, Randall RJ. Protein measurement with folin reagent. J Biol Chem.1951; 193: 265-275.
- Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Analytical biochemistry. 1968; 25: 192-205.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95: 351.
- Bagainski ES, Clin Chem Acta. 1973; 46.
- Lee AY, Chung SK, Chung SS. Demonstration that polyol accumulation is responsible for diabetic cataract by the use of transgenic mice expressing the aldose reductase gene in the lens. Proc Natl Acad Sci USA. 1995; 92: 2780-2784.
- Yang C, Yan H, Ding T. Hydrogen saline prevents selenite-induced cataract in rats. Mol Vis. 2013; 19: 1684-1693.
- Kalekar SA, Munshi RP, Kulkarni DK. Evaluation of anti-cataract potential of tinospora cordifolia and phyllanthus emblica in an invitro sugar induced cataracteric lens organ culture model. Int J Pharm Bio Sci. 2014; 5: 120-130.
- Kawakubo K, Mori A, Sakamoto K, Nakahara T, Ishii K. GP-1447, an inhibitor of aldose reductase, prevents the progression of diabetic cataract in rats. Biological and Pharmaceutical Bulletin. 2012; 35: 866-872.
- Thiagarajan G, Chandani S, Rao HS, Samuni AM, Chandrasekaran K, Balasubramanian D. Molecular and Cellular Assessment of Ginkgo Biloba Extract as a Possible Ophthalmic Drug. Exp Eye Res. 2002; 75: 421-430.
- Tang D. Influence of Age, Diabetes, and Cataract on Calcium, Lipid-Calcium, and Protein-Calcium Relationships in Human Lenses. Invest Ophthalmol Vis Sci. 2003; 44: 2059-2066.
- Suryanarayana P, Krishnaswamy K, Reddy GB. Effect of curcumin on galactose-induced cataractogenesis in rats. Mol. Vis. 2003; 9: 223-230.