
Case Report
Austin J Pharmacol Ther. 2017; 5(2).1092.
Successful Reversal of Anticoagulant Effect of Brodifacoum Poisoning with 4-Factor Prothrombin Complex Concentrate (PCC4)
Kusmierski KA*, Lackie CL and Scull JR
Department of Pharmacy, Millard Fillmore Suburban Hospital, USA
*Corresponding author: Kusmierski KA, Department of Pharmacy, Millard Fillmore Suburban Hospital, USA
Received: March 17, 2017; Accepted: April 05, 2017; Published: April 11, 2017
Abstract
Title: Successful reversal of anticoagulant effect of brodifacoum poisoning with 4-factor prothrombin complex concentrate (PCC4).
Introduction: Brodifacoum is a long-acting anticoagulant rodenticide that is similar to warfarin, but significantly more potent with prolonged half-life and potential for severe coagulopathy.
Case: A 43-year-old woman presented to the emergency department with non-traumatic bruising, abdominal pain, and hematuria. Initial evaluation revealed a severe coagulopathy and international normalized ratio (INR) greater than 13. PCC4 (Kcentra®) 25units/kg, phytonadione, and fresh frozen plasma (FFP) were administered with improvement in INR without additional intervention to control bleeding. A rebound increase in INR occurred within 24 hours. Brodifacoum toxicity was confirmed (brodifacoum level=300ng/mL) after patient admitted to possible cutaneous and aerosolized exposure to “rat poison.” The patient required FFP and high doses of phytonadione to control coagulopathy throughout her admission. She was discharged on phytonadione.
Discussion: PCC4 administration improved coagulation parameters rapidly with control of bleeding and no adverse effects. The rebound in coagulation parameters demonstrated the continued need for treatment of coagulopathy while awaiting confirmation of brodifacoum poisoning.
Conclusion: This case illustrates successful acute reversal of brodifacoum poisoning with Kcentra®, phytonadione, and FFP. In this patient, the addition of PCC4 rapidly reversed her severe coagulopathy without adverse events.
Keywords: Brodifacoum; Superwarfarin; Poisoning; Prothrombin complex concentrate; Bleeding; Rat poison
Introduction
Superwarfarins, a class of rodenticide, were developed to overcome resistance to warfarin in rodents. These long-acting anticoagulant rodenticides (LAARs) are highly lipid soluble with a longer duration of action and much greater potency than warfarin [1,2]. Brodifacoum, the most commonly used LAAR, is 100-fold more potent than warfarin, has a half-life of 16-36 days, and its anticoagulant effect may continue even after it is no longer measurable in the serum [3].
Due to the accessibility of superwarfarin compounds, both unintentional and intentional exposures have been reported. Standard treatment of superwarfarin poisoning involves the use of fresh frozen plasma (FFP) and phytonadione in the acute period, followed by long-term phytonadione administration until the LAAR effects resolve [1]. We describe a case of severe coagulopathy with bleeding due to brodifacoum poisoning successfully treated with 4-factor prothrombin complex concentrate (PCC4), Kcentra® (CSL Behring GmbH, Germany).
Case Presentation
A 43-year-old woman presented to the Emergency Department (ED) with a one week history of non-traumatic bruising on her lower extremities and distal upper extremities, right lower abdominal pain, and hematuria. Her medical history was significant for cauda equine syndrome following a traumatic spine injury and mixed mood disorder. Her home medications included baclofen, diazepam, diclofenac topical patch, gabapentin, hydrocodone/acetaminophen, omeprazole, multivitamin, and vortioxetine. She was a nonsmoker, denied recreational drug use, and admitted to only occasional social use of alcohol.
On admission, laboratory values revealed a severe coagulopathy which included an international normalized ratio (INR) greater than 13, prothrombin time (PT) greater than 100 seconds, and an activated partial thromboplastin time (aPTT) greater than 200 seconds. Her fibrinogen level was slightly elevated at 508mg/dL. Initial complete blood count (CBC) did not demonstrate anemia or thrombocytopenia with hemoglobin of 13.9g/dL, hematocrit of 40.4%, and platelet count of 218 x 109/L. Liver function tests and renal function was grossly normal. Urine toxicology screen was positive for benzodiazepines which was consistent with diazepam use prior to admission. Clotting factor studies were not obtained. Pertinent physical exam findings included a large hematoma in the right upper retromolar region, mild to moderate tenderness to palpation in the suprapubic and right lower quadrant region, multiple ecchymoses on her thighs and scattered on her distal lower extremities, and large ecchymoses involving the radial surfaces of the wrists bilaterally. A computerized tomography scan of the abdomen and pelvis demonstrated a retroperitoneal, intrarenal, perirenal, and pararenal hemorrhage. She denied a history of bleeding or clotting disorders as well as anticoagulant or overthe- counter (OTC) supplement use, any new medications, or OTC pain reliever used in excess. She denied the possibility of overdose of another family member’s warfarin, which was in the house.
Phytonadione, fresh frozen plasma (FFP), and PCC4 (Kcentra®) 25IU/kg (actual 23.4IU/kg, rounded to nearest vial size) were administered in the ED with initial improvement in coagulation laboratory values (INR=1.31, PT=15.8 seconds, and aPTT=35 seconds three hours post PCC4 administration), but a rebound increase occurred within 24 hours (Figure 1). The patient remained hemodynamically stable. Wanting to rule out warfarin toxicity, a warfarin level was drawn on hospital day two for completeness and resulted back without detection four days later. Brodifacoum toxicity was suspected after patient, on hospital day number three, admitted to cutaneous contact with “rat poison” about three weeks prior to admission after spilling it and sweeping it up by hand on two occasions. She also described possible aerosolized contact as she placed open bait beneath her bed. A brodifacoum level was drawn on hospital day four, sent to an outside laboratory, and resulted 20 days later which confirmed brodifacoum toxicity (brodifacoum level=300ng/mL). Patient required seven units of FFP and doses of up to 40mg/day of phytonadione to control coagulopathy throughout her twelve day admission. She was discharged on oral phytonadione 20mg daily with close follow-up with a hematologist. No thrombotic events occurred during her 12 day hospital stay.
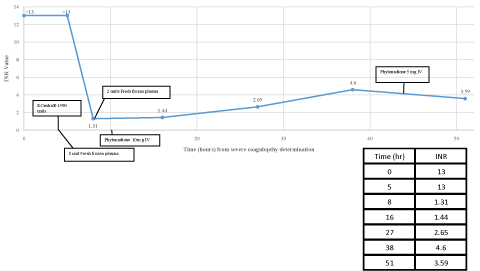
Figure 1: INR Response During Acute Coagulopathy Management.
Discussion
We present a case of severe coagulopathy, with ecchymoses and radiologically confirmed internal hemorrhage, treated with FFP, phytonadione, and 25IU/kg PCC4 (Kcentra®). Signs of bleeding stabilized and coagulation parameters improved rapidly as evidenced by the markedly reduced INR, first measured three hours post infusion. PCC4 has been shown to reverse coagulation factor deficiency induced by warfarin within 30 minutes and has been shown to be superior to FFP. The recommended dose for INR greater than six with acute major bleeding from warfarin is 50IU/kg, not to exceed 5000IU [4]. In our case, we demonstrate good INR reversibility with 25IU/kg, along with an initial 1 unit of FFP and 10mg intravenous (IV) phytonadione. Coagulation parameters rebounded within 24 hours post FFP, PCC4 and initial phytonadione administration, demonstrating the need for continued treatment with phytonadione for long-term management of coagulopathy.
Given the initial presentation of this patient, and delay in brodifacoum poisoning diagnosis which often accompanies such exposures, rapid reversal of severe coagulopathy and bleeding is warranted to prevent life-threating events or death [1]. Laboratory measurement of clotting factor activity prior to PCC4, while not available in our case, may have been beneficial in supporting early suspicion for brodifacoum poisoning, as levels were not available for 20 days. Severe bleeding from suspected or known brodifacoum exposure has been traditionally managed with blood products, such as FFP and red blood cell (RBC) transfusion, as well as initial IV phytonadione, followed by long term outpatient care and maintenance phytonadione for 3-6 months [1,2].
More recently, acute bleeding management with off-label use of various three or four factor prothrombin complex concentrate (PCC3 or PCC4, respectively) or recombinant activated factor VII have been described. Both offer potential advantages such as rapid onset, ease of administration (primarily due to avoidance of type and crossmatching and thawing time delays), and reduction of infused volume associated with multiple units of blood products [1, 5-7].
Haesloop et.al. present a recent case of intentional brodifacoum poisoning presenting with hemodynamic instability, coagulopathy and active bleeding. In this case, a PCC4 (Kcentra®, per author correspondence) was administered as 45IU/kg, along with 40mg IV phytonadione, 5 units FFP, 4 units RBC and 1 unit platelets. The patient received ongoing oral phytonadione for 1 month and there were no reported thrombotic events [6]. High doses of phytonadione were appropriately given in addition to PCC4 in this patient due to the known intentional brodifacoum exposure on admission to the ED. This contrasts with our patient’s initially unknown brodifacoum exposure, where a low dose of phytonadione was given in the ED. Haesloop et al’s case did not report a rebound in coagulation factors. Theoretically, administration of a larger dose of phytonadione may have blunted the rebound in coagulation factors that were seen in our case.
Despite the advantages offered by newer replacement coagulation factors, paradoxical thrombotic events have been associated with LAAR exposure, potentially due to early depletion of protein C and S as a result of acute exposure, and raise the need to use caution with coagulation factor replacement strategies [1]. Laposata, et al., present a case of a 40-year-old woman with severe coagulopathy and bleeding initially administered phytonadione, FFP and 5000IU PCC3. Subsequent management of this patient, three days later, included an additional 5000IU PCC3 without immediate complication. However, in a rapid series of events, the patient re-presented to the emergency department for loss of consciousness requiring intubation, bleeding and ecchymoses for which she received FFP, phytonadione, cryoprecipitate, and recombinant activated factor VII. Subsequent studies provided evidence for acute stroke, venous thrombosis, and disseminated intravascular coagulation. This case highlighted the potential for thrombosis and disseminated intravascular coagulation which has been associated with PCC [5].
We believe the PCC4, Kcentra®, may represent the best temporizing prothrombin complex concentrate since it has been shown to successfully reverse warfarin, which is a 4-hydroxucoumadin similar to brodifacoum. Kcentra® uniquely replaces factor II, VII, IX, X, as well as protein C and S, and has been shown to reverse warfarin, as evidenced by INR reversal, within 30 minutes [4]. It may have been valuable to determine a repeat INR earlier than three hours as in our case, especially if ongoing severe bleeding or surgical intervention dictated. This strategy could allow for additional PCC4 administrations to be considered. While further support is needed to determine the most effective dosing strategy during the acute management of coagulopathy from brodifacoum, we were successful with relatively conservative PCC4 dosing.
The patient initially denied ingestion of rodenticide, but eventually admitted to possible cutaneous and aerosolized exposure in her home. Brodifacoum is readily absorbed through the skin, but most poisonings occur via oral ingestion or inadvertent inhalation through smoking of tainted products [1]. It is unclear if cutaneous and/or aerosolized LAAR exposure described by our patient could cause the degree of elevation of brodifacoum level seen.
Conclusion
This case illustrates successful acute reversal of brodifacoum anticoagulant effects with PCC4 (Kcentra®). In this patient, it worked effectively and rapidly without adverse events. This case highlights the potential rebound in coagulation parameters with temporizing measures such as prothrombin complex concentrate and the need to maintain phytonadione at high doses for an extended duration.
References
- King N, Tran M. Long-Acting Rodenticide (Superwarfarin) Poisoning: A Review of Its Historical Development, Epidemiology, and Clinical Management. Transfus Med Rev. 2015; 29: 250-258.
- Chua JD, Friedenberg WR. Superwarfarin Poisoning. Arch Intern Med. 1998; 158: 1929-1932.
- Weitzel JN, Sadowski JA, Furie BC, Moroose R, Kim H, Mount ME, et al. Surreptitious ingestion of a long-acting vitamin K antagonist/rodenticide, brodifacoum: clinical and metabolic studies of three cases. Blood. 1990; 76: 2555-2559.
- CSL Behring GmbH. Kcentra® (prothrombin complex concentrate (Human)) package insert. Marburg, Germany. 2014.
- Laposata M, VanCott EM, Lev MH. Case 1-2007: A 40 year old woman with Epistaxis, hematemesis, and altered mental status. N Engl J Med 2007; 356: 174-182.
- Haesloop O, Tillick A, Nichol G, Strote J. Superwarfarin ingestion treated successfully with prothrombin complex concentrate. Am J Emerg Med. 2016; 34: 116.
- Kapadia P, Bona R. Acquired deficiency of vitamin K-dependent clotting factors due to brodifacoum ingestion. Conn Med. 2008; 72: 207-209.