
Research Article
Austin J Pharmacol Ther. 2021; 9(1).1126.
The Association between Co-Administration of Omeprazole and Clopidogrel and Cardiovascular Outcomes
Azab AN1,2,3*, Shmulevich E3,4, Gilutz H3, Shvartsur R1 and Friger M4
1Department of Nursing, Ben-Gurion University of the Negev, Israel
2Department of Clinical Biochemistry and Pharmacology, Ben-Gurion University of the Negev, Israel
3Department of Cardiology, Soroka University Medical Center, Israel
4Department of Public Health, Ben-Gurion University of the Negev, Israel
*Corresponding author: Azab AN, Department of Nursing, Ben-Gurion University of the Negev, Israel
Received: December 04, 2020; Accepted: January 22, 2021; Published: January 29, 2021
Abstract
Objective: To examine whether co-administration of clopidogrel and omeprazole affects the clinical outcomes of clopidogrel treatment.
Design and Methods: A retrospective cross-sectional study of 4078 patients after a percutaneous coronary intervention and stent implantation. Seven hundred twenty-three clopidogrel-treated patients who fulfilled the inclusion criteria of the study were included: 318 treated only with clopidogrel; 405 treated with clopidogrel and omeprazole (study group). The interaction between the drugs was examined in relation to adverse clinical outcomes such as all-cause mortality, Major Adverse Cardiovascular Events (MACE) and cardiac hospitalizations during one year.
Results: No significant difference was detected between the groups regarding the primary outcomes of the study. Regression models adjusted to basic characteristics and clinical variables showed a significant association between the study group and the primary outcomes through interactions with specific covariates: “all-cause mortality” through interaction with the covariate ethnicity (not-Jewish) (OR = 43.12, 95% CI 1.19-1567.8, P = 0.04), “MACE” through interaction with the covariates gender (female) and complicated angioplasty (OR= 9.36, 95% CI 2.04-42.94, P= 0.04); and “cardiac hospitalizations” through interaction with the covariates extent of artery stenosis and hypertension (OR= 2.65, 95% CI 1.043-6.76, P = 0.04).
Conclusion: Addition of omeprazole to clopidogrel may be associated with increased incidence of negative clinical outcomes, including death and MACE. These findings underscore the need for conduction of prospective randomized controlled trials that will examine the association between addition of omeprazole to clopidogrel and the incidence of clinical outcomes.
Keywords: Acute Coronary Syndrome; Clopidogrel; Major Adverse Cardiac Events; Omeprazole; Percutaneous Coronary Intervention
Abbreviations
ACS: Acute Coronary Syndrome; CABG: Coronary Artery Bypass Grafting; CYP-450: Cytochrome-P-450; DAPT - Dual Antiplatelet Therapy; GI: Gastrointestinal; LVEF: Left Ventricular Ejection Fraction; MACE: Major Adverse Cardiovascular Event; MI: Myocardial Infarction; NYHA: New York Heart Association; PCI: Percutaneous Coronary Intervention; PPI: Proton Pump Inhibitor; STEMI: ST-Segment Elevation Myocardial Infarction
Introduction
Dual Antiplatelet Therapy (DAPT) with aspirin and P2Y12 receptor antagonists - including clopidogrel, prasugrel and ticagrelor - is a well established treatment strategy for the reduction of Major Adverse Cardiovascular Events (MACE) among patients after Acute Coronary Syndromes (ACSs), especially those who underwent a Percutaneous Coronary Intervention (PCI) and stent implantation [1-11]. Low adherence to treatment (with) or early withdrawal of P2Y12 antagonists increase the risk of MACE [12-16]. Although the newer drugs prasugrel and ticagrelor are increasingly used in patients after ACS and PCI [17-19], clopidogrel is still regarded as a useful [20] and widely used drug [17,18,21,22]. These data underscore the crucial space that clopidogrel still occupies in the treatment of post-PCI patients.
Clopidogrel is a pro-drug that must undergo metabolism by cytochrome-P-450 (CYP450) enzymes (particularly CYP 2C19) in order to become active [23-30]. Thus, decreased function of CYP 2C19 - due to genetic or environmental reasons -is expected to reduce the antiplatelet activity of clopidogrel. These include polymorphisms of the CYP 2C19 encoding gene [5,24,26,28-37], interactions with drugs that inhibit CYP 2C19 [23,30,38], and with grapefruit juice [30,39].
Proton Pump Inhibitors (PPIs) are a family of drugs used for the treatment of gastric acid-related disorders [40,41], and are among the most widely used medications in the world [42-44]. PPIs inhibit H+/K+- ATPase leading to potent inhibition of gastric acid secretion. Usually, they are given to patients receiving DAPT in order to decrease the risk of dyspepsia and Gastrointestinal (GI) bleeding [45,46]. Similar to clopidogrel, PPIs are pro-drugs that require metabolism in order to become active (particularly through CYP2C19 and CYP3A4) and thus may inhibit the conversion of clopidogrel to its active metabolite and potentially alter its efficacy [30,47,48]. Among the clinically used PPIs it seems that only omeprazole significantly inhibits CYP2C19 and thus reduces the antiplatelet activity of clopidogrel [30,49-53].
In 2006, Gilard and co-authors were the first to report that administration of omeprazole together with clopidogrel was associated with a significant increase in platelet reactivity and decreased antiplatelet activity of clopidogrel [54]. It is thought that omeprazole attenuates the antiplatelet activity of clopidogrel by competitive inhibition of CYP2C19 (mainly) and reduction of the active metabolite of clopidogrel. Subsequently, numerous studies have reported that omeprazole attenuates the antiplatelet activity of clopidogrel [30,36,37,49-52,54-59].
Based on the findings of these observational studies, several medical agencies around the world have issued in the past safety announcements warning against concomitant use of clopidogrel and PPIs (particularly omeprazole) due to a potential drug-drug interaction that may attenuate the antiplatelet activity of clopidogrel [60,61]. However, a number of other observational studies, reported contradicting findings [25,59,62-67]. Furthermore, a large scale randomized clinical trial comparing omeprazole to placebo in DAPT users (the COGENT trial) showed that co-administration of omeprazole together with clopidogrel plus aspirin significantly decreased the incidence of adverse GI events without increasing the rate of MACE [68]. Post-hoc analyses of the COGENT trial [69] in patients undergoing PCI within 14 days of randomization and patients presenting with ACS (managed with or without PCI) reported similar results.
Over the years several reviews and meta-analyses addressed the question of concomitant clopidogrel and PPI treatment arriving at inconsistent conclusions [47,48,70-73]. Therefore, the aim of the present study was to examine the interaction between clopidogrel and omeprazole in order to elucidate whether omeprazole reduces the therapeutic efficacy of clopidogrel.
Methods
Design
A retrospective analysis of patients' cohort. The design of the study is presented in Figure 1.

Figure 1: Study design.
CABG: Coronary Arteries Bypass Grafting; MACE: Major Adverse Cardiac
Event; MI: Myocardial Infarction.
Study population
The study included patients who underwent a successful angioplasty with at least one stent implantation at Soroka University Medical Center hereafter, Soroka between 2005 to 2009, and were treated with clopidogrel with or without omeprazole. Exclusion Criteria: 1) Age under 18 years old; 2) low rate of adherence to pharmacotherapy – patients who purchased less than 50% of the predicted number of pills during the time of follow-up, or discontinued treatment with clopidogrel before/at three months according to pharmacy distribution recordings; 3) significant renal failure (serum creatinine > 2.0 mg/dl); 4) chronic liver disease; 5) left ventricular ejection fraction less than 25% or/and New York Heart Association functional class IV heart failure; 6) pregnancy or breast feeding during the study period; 7) oral anticoagulation treatment; 8) coexisting conditions that limited life expectancy to less than 12 months; 9) participation in other investigational trials at the time of the survey; and, 10) patients who were receiving any other drugs that inhibit or/and metabolized by CYP 2C19 (fluoxetine, sertraline, fluvoxamine, paroxetine, citalopram, escitalopram, venlafaxine, imipramine, amitriptyline, clomipramine, moclobemide, nelfinavir, nilutamide, phenytoin, phenobarbital, topiramate, diazepam, cyclophosphamide, fluconazole, chloramphenicol, ketoconazole, indomethacin and estrogen-containing oral contraceptives).
Sample size was calculated by incidence of clinical endpoint of 33% in the group of patients who were treated with clopidogrel plus omeprazole and 20% in the group who was treated only with clopidogrel, for a = 0.05 and power (1-β). Based on these assumptions, we calculated a sample size of 180 patients in each group. The sample size was calculated using the Power Sample Size Calculations (P.S. version 3.02) software.
Data collection
Data was collected from the hospital's computerized medical records. The data included demographic and clinical characteristics, cardiovascular risk factors and comorbidities, chronic medications, and PCI procedure data. Data collection was performed on the day of admission to the Cardiology department at which the stent implantation was performed and 12 months after admission.
Clinical endpoints
Three primary outcomes were defined: all-cause mortality; MACE; cardiac hospitalization for unplanned angiography or angina pectoris. MACE was defined as: cardiac death, non-fatal MI, cerebrovascular accident, CABG, unstable angina, and, catheterbased revascularization procedures.
Statistical analysis
Statistical analyses were completed using SPSS 18. For comparison between dependent variables (categorical variable) and independent categorical variables, we used chi-square test. For the same comparison with independent quantitative normally distributed variables, we used one-way ANOVA or group t-test, and for independent quantitative abnormally distributed variables we used Kruskal-Wallis ANOVA test or Mann-Whitney U test. For survival analysis, we used a Cox regression to evaluate the time period of adverse cardiac events in independent association between uses of omeprazole versus no omeprazole, as time varying covariates. We used binomial and multinomial logistic regression, adjusted for all variables (patient data, cardiac data, procedure data, angiographic data, and medication data) in order to assess the association between use of omeprazole, the independent variable of interest, and adverse outcomes among patients taking clopidogrel at the times of followup. Adjusted odds ratios and their 95% confidence intervals are presented. All tests of significance were two-tailed with the level of significance < 0.05.
Ethical aspects
The study was approved by the Institutional Ethics Committee in Soroka (authorization number 10550).
Results
Study population
A total of 4078 patients who underwent a successful angioplasty with stent implantation in Soroka during the years 2005 to 2009 were screened for inclusion in the study. Of those, 723 patients were finally included in the study out of which 318 received clopidogrel only and 405 received clopidogrel and omeprazole (Study Group) (Figure 2).

Figure 2: Screening and enrollment to the study. *Other drugs that inhibits or/
and are metabolized by CYP450 (see Methods).
CYP450: Cytochrome-P450; LVEF: Left Ventricular Ejection Fraction; NYHA:
New York Heart Association; PPI: Proton Pump Inhibitor.
Baseline and clinical characteristics of the study population are presented in Table 1. Patients in the Study Group were significantly older than those of the clopidogrel only group (64.2 + 11.9 vs. 61.3 + 11.7 year, respectively, P= 0.001) and had a higher incidence of respiratory disease (13.3% vs. 6.9%, P=0.005), hypertension (64.2% vs. 54.4%, P= 0.007), diabetes mellitus (36% vs. 28.9%, P= 0.04) and dyslipidemia (92.1% vs. 87.1%, P= 0.02). On the other hand, the clopidogrel only group included more male patients than the Study Group (83.6% vs. 72.6%, P= 0.001). Regarding PCI procedure, more patients in the clopidogrel only group underwent a bare metal stent insertion (28% vs. 18%, P = 0.001) while more patients in the Study Group underwent a drug eluting stent implantation (75.6% vs. 66.7, P= 0.008). Table 2 shows the pharmacotherapy of the groups. As seen, more patients in the clopidogrel only group were treated with angiotensin converting enzyme inhibitors (84% vs. 77.8%, P = 0.037, respectively). On the other hand, more patients in the Study Group were treated with bezafibrate (10.6% vs. 5.3%, P = 0.011) and polypharmacy - received at least five drugs simultaneously (97% vs. 92.5%, P= 0.005).
Clopidogrel Only (n=318), Number (%)
Clopidogrel + Omeprazole (n = 405), Number (%)
P
Age (Mean ± SD)
61.3 ± 11.7
64.2 ± 11.9
0.001
Body Mass Index (Mean ± SD)
28.07 ± 4.6
28.8 ± 4.86
0.72
Gender
Male
266 (83.6)
294 (72.6)
0.001
Ethnicity
Jew
279 (87.7)
327 (80.7)
0.036
Smoking
Yes
171 (53.8)
196 (48.4)
0.151
Alcohol Use
Yes
131 (41.2)
187 (46.2)
0.181
Respiratory Disease
Yes
22 (6.9)
54 (13.3)
0.005
Renal Disease
Yes
16 (5)
18 (4.4)
0.711
History of Stroke or Transient Ischemic Attack
Yes
13 (4.1)
24 (5.9)
0.262
Carotid or Vertebral Artery Disease
Yes
1 (0.3)
1 (0.2)
0.865
Diabetes Mellitus
Yes
92 (28.9)
146 (36)
0.04
Dyslipidemia
Yes
277 (87.1)
373 (92.1)
0.02
Hypertension
Yes
173 (54.4)
260 (64.2)
0.007
Cardiac Dysrhythmias
Yes
31 (9.7)
41 (10)
0.859
History of Heart Failure
Yes
124 (39)
151 (37)
0.657
Family History of CAD
Yes
107 (34)
115 (29)
0.134
Thrombolysis
Yes
20 (6.3)
22 (5.4)
0.631
CABG
Yes
43 (13.5)
59 (14.6)
0.679
ACS presentation
STEMI
73 (23)
71 (17.5)
0.116
NSTEMI
60 (18.9)
74 (18.3)
0.384
UA
62 (19.5)
95 (23.5)
0.289
Elective
123 (38.7)
165 (40.7)
-
Stent Type
BMS
89 (28)
73 (18)
0.001
DES
212 (66.7)
306 (75.6)
0.008
Pulmonary Edema
Yes
6 (1.9)
4 (1)
0.306
Cardiogenic Shock
Yes
0 (0)
3 (0.7)
0.124
Use of Clopidogrel Three Months Before the Index Admission
Yes
35 (11)
52 (12.8)
0.452
Abbreviations: ACS: Acute Coronary Syndrome; CABG: Coronary Artery Bypass Grafting; CAD: Coronary Artery Disease; ICCU: Intensive Cardiac Care Unit; NSTEMI: Non-ST Elevation Myocardial Infarction; STEMI: ST Elevation Myocardial Infarction; UA: Unstable Angina.
Table 1: Baseline and clinical characteristics of the study population.
Clopidogrel Only (n=318), Number (%)
Clopidogrel + Omeprazole (n=405), Number (%)
P
ARBs
Yes
28 (8.8)
50 (12.3)
0.128
ACEIs
Yes
267 (84)
315 (77.8)
0.037
Statins
Yes
310 (97.5)
387 (95.6)
0.167
Bezafibrate
Yes
17 (5.3)
43 (10.6)
0.011
Aspirin
Yes
300 (94.3)
385 (95.1)
0.666
Beta Adrenergic Blockers
Yes
251 (78.4)
312 (77)
0.543
Calcium Channel Blockers
Yes
47 (14.8)
82 (20.2)
0.057
Diuretic Drugs
Yes
40 (12.6)
63 (15.6)
0.256
Polypharmacy*
Yes
294 (92.5)
393 (97)
0.005
* Polypharmacy - use of five drugs groups simultaneously. Abbreviations: ACEIs: Angiotensin Converting Enzyme Inhibitors; ARBs: Angiotensin II Receptor Blockers.
Table 2: Treatment drugs of the study population at discharge from intensive cardiac care unit.
Study outcomes: Table 3 shows the results of the primary outcomes of the study. All-cause mortality did not significantly differ between the groups (P = 0.257). The occurrence of MACE (P = 0.292) and cardiac hospitalization (P = 0.249) also did not significantly differ between the groups. These results indicate that there was no significant difference between the groups regarding the primary outcomes of the study. Furthermore, there were no significant associations between subgroups (specific outcomes) or according to the stratification variables. However, it is important to mention that the sub-group analysis was limited by the small absolute numbers of specific events.
Clinical Endpoints
Clopidogrel Only (n=318), Number (%)
Clopidogrel + Omeprazole (n=405), Number (%)
P
All-Cause Mortality
5 (1.6)
11 (2.7)
0.257
MACE
Cardiac Death
1 (0.3)
2 (0.5)
0.672
Non-fatal MI
0 (0)
2 (0.5)
0.197
CVA
2 (0.6)
0 (0)
0.123
CABG
0 (0)
1 (0.2)
0.545
Catheter-based Revascularization
24 (7.5)
38 (9.4)
0.187
Unstable Angina
4 (1.3)
5 (1.2)
0.61
All
31 (9.7)
48 (12)
0.292
Cardiac Hospitalizations
Unplanned Angiography
21 (6.6)
32 (7.9)
0.248
Angina Pectoris
0 (0)
3 (0.7)
0.163
All
21 (6.6)
35 (8.6)
0.249
GI bleeding
6 (2)
36 (9)
0.001
CABG: Coronary Artery Bypass Grafting; CVA: Cerebrovascular Accident; MI: Myocardial Infarction; GI: Gastrointestinal.
Table 3: Specific clinical outcomes of the study population.
One of the major objectives of prescribing omeprazole together with antiplatelet drugs (particularly aspirin) is to reduce the risk of GI bleeding. We found that the rate of GI bleeding in the Study Group was significantly higher than that of the clopidogrel only group (9% vs. 2%, respectively; P = 0.001).
Furthermore, we performed a multivariate analysis to determine the association between the primary outcomes of the study and different covariates. A logistic regression adjusted to all covariates revealed that some covariates influenced the probability of each event. The covariates for each model were chosen based on their clinical importance and statistical significance (P< 0.05) in a univariate analysis and combined them with relevant interactions. We found that the association between the Study Group and “all-cause mortality” was strengthened and became significant (OR = 43.12, 95% CI 1.19-1567.8, P = 0.04) through an interaction with the covariate ethnicity “not-Jewish”. Similarly, it was found that the association between the Study Group and “MACE” was strengthened and became significant (OR= 9.36, 95% CI 2.04-42.94, P = 0.004) through an interaction with the covariates gender (female) and complicated angioplasty (defined as an angioplasty that resulted in acute vessel closure, stent thrombosis, perforation, or dissection). Moreover, the association between the Study Group and “cardiac hospitalizations” was strengthened and became significant (OR= 2.65, 95% CI 1.04- 6.76, P = 0.04) through an interaction with the covariates extent of artery stenosis and hypertension.
A Cox regression analysis adjusted to ethnicity, respiratory disease and diabetes revealed that treatment with clopidogrel plus omeprazole was associated with a similar probability for a one year survival (freedom from death) as compared to treatment with clopidogrel only (adjusted HR = 0.65, P = 0.82, Figure 3). However, a Cox regression analysis adjusted to ethnicity, respiratory disease and diabetes showed that the use of clopidogrel plus omeprazole was significantly associated with an increased risk for one year MACE as compared to use of clopidogrel only (adjusted HR= 6.13, P= 0.05, Figure 4). We examined the incidence of events during the first year after PCI in the two groups of the study. It was found that in both groups MACE was more frequent at one month after PCI as compared to all subsequent time-points during the first year after PCI. Moreover, a Cox regression analysis adjusted to ethnicity, respiratory disease and diabetes revealed that treatment with clopidogrel plus omeprazole was associated with a non-significant increase in the risk for one year cardiac hospitalizations as compared to treatment with clopidogrel only (adjusted HR = 2.08, P = 0.38, Figure 5).

Figure 3: Cox proportional hazard regresion for all-cause mortality. The HR
was adjusted to ethnicity, respiratory diseases, and diabetes mellitus.
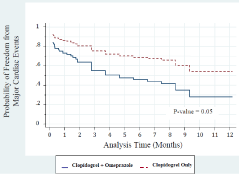
Figure 4: Cox proportional hazard regresion for major cardiac events. The
HR was adjusted to ethnicity, respiratory diseases, and diabetes mellitus.
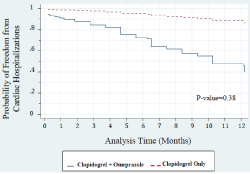
Figure 5: Cox proportional hazard regresion for cardiac hospitalizations. The
HR was adjusted to ethnicity, respiratory diseases, and diabetes mellitus.
Discussion
The major finding of the present study is that there was a significant association between the addition of omeprazole to clopidogrel and the primary outcomes all-cause mortality, MACE, and cardiac hospitalization through interactions with specific covariates. These results are consistent with previous studies [4,14,56,58,59,74- 76,23,31,36,37,49-51,55]. The increased incidence of the primary outcomes in the Study Group seems to derive from the fact that the patients of this group were sicker and probably had a poorer prognosis than those in the clopidogrel only group. As compared to the clopidogrel only group, the Study Group was significantly older, had a higher rate of patients who received a drug eluting stent, had a higher rate of co-morbidities including respiratory diseases, diabetes mellitus, hypertension and dyslipidemia (as presented by a higher percentage of patients who received bezafibrate for hypertriglyceridemia), and had a higher percentage of patients who were treated with at least five drugs (polypharmacy).
Although the rate of the primary outcomes did not differ significantly between the study groups (Table 3), a Cox regression analysis showed that the use of clopidogrel plus omeprazole was associated with an increased risk for one year MACE as compared to the use of clopidogrel only (adjusted HR= 6.13, P= 0.05, Figure 4). This result suggests that on the long-term, the addition of omeprazole to clopidogrel is associated with increased risk for MACE.
The major objective of prescribing omeprazole together with antiplatelet drugs (particularly aspirin) is to reduce the risk of GI bleeding. In the present study we found that the rate of GI bleeding in the Study Group was significantly higher than that of the clopidogrel only group (9% vs. 2%, respectively; P = 0.001, Table 3). The reason for this finding is not fully understood. A plausible explanation is that patients in the Study Group were given omeprazole as a preventive treatment due to a history of GI bleeding or/and high risk for GI bleeding. This is to say that omeprazole was not the leading cause for GI bleeding and that such patient may have suffered a bleeding event regardless of whether they were or were not administered with omeprazole. This is a temporal bias typical to retrospective studies in which it is difficult to determine the causative association between examined parameters. It is important to mention that treatment guidelines support the use of PPIs in patients after STEMI (and PCI) in two cases: patients with a history of GI bleeding and in patients with multiple risk factors for GI bleeding (such as advanced age, concurrent use of anticoagulants, steroids or non-steroidal antiinflammatory drugs including high dose aspirin, and helicobacter pylori infection) [77,78]. Similar to our findings, a retrospective study from Taiwan reported that patients who were treated with clopidogrel plus a PPI had a higher incidence of recurrent hospitalization for major GI complications than those who were treated with clopidogrel only [79].
Another hypothesis of our study was that the incidence of MACE will be more frequent at one month as compared to one year after PCI. This assumption relied primarily on previous evidence [80- 82] that the incidence of sub-acute thrombosis is higher in the first month post-PCI as compared to subsequent time points. Indeed, in this study the incidence of MACE was highest at one month after PCI, underscoring the need for a more extensive follow-up during this period of time, and possibly a more aggressive treatment regimen.
Regression models adjusted to all variables showed that there is a significant association between the Study Group and the primary outcome "all-cause mortality" through an interaction with ethnicity (not-Jewish). This result suggests that among Bedouin arab patients, the use of clopidogrel together with omeprazole may be associated with increased risk of death. The possible reasons for this result may include a particular genetic polymorphism and a low adherence to treatment among Bedouin patients, but also due to a high incidence of comorbidities [83-85] and low healthcare accessibility and utilization in this population [84-86]. Similarly, there was a significant association between the Study Group and the primary outcome “MACE” through an interaction with gender and complicated angioplasty. This result suggests that among female patients who had a complicated PCI, the use of clopidogrel with omeprazole may be associated with an increased risk for MACE. This finding may derive from differences in the anatomical and physiological characteristics of the coronary arteries and the cardiovascular system in general between women and men, making women more susceptible to coronary thrombotic events [87,88]. Moreover, there was a significant association between the Study Group and the primary outcome “cardiac hospitalizations” through an interaction with the covariates extent of artery stenosis and hypertension. This result suggests that among patients with hypertension and severe coronary artery stenosis the use of clopidogrel together with omeprazole may be associated with increased risk of cardiac hospitalizations. Hypertension and severe coronary stenosis are known risk factors for thrombotic cardiac events. Taken together, these three profiles may help predicting which patient may have a higher risk of adverse outcomes when given clopidogrel together with omeprazole [89-91].
Limitations
One of the limitations of the present study is that it is a retrospective cross-sectional study and, thus, has a low number of events, which decreases the statistical power of the study. Another limitation is the large number of patients who were excluded due to unknown/ low adherence to pharmacotherapy, missing data, as well as other reasons. The exclusion of these patients may have biased the results. However, since adherence to pharmacotherapy is a detrimental factor in treatment success, an otherwise analysis method of the data could also influence the outcomes of the study.
Conclusion
The results of this retrospective study suggest that there were significant associations between addition of omeprazole to clopidogrel and the primary outcomes all-cause mortality, MACE and cardiac hospitalizations among patients who underwent a PCI and stent implantation. Logistic regression models revealed that these associations occurred through interactions with specific covariates. It is important to mention that recent clinical practice recommendations, the results of studies on the concomitant use of clopidogrel and PPIs, and the introduction of prasugrel and ticagrelor has affected physicians’ prescribing habits. Studies report a steady decline in the prevalence of clopidogrel administration, with or without omeprazole. However, clopidogrel remains the most commonly used P2Y12 receptor antagonist among ACS inpatients, underscoring its importance in the treatment of post- PCI patients. Current treatment guidelines (which were published after the conduction of the present study) suggest that omeprazole does not reduce the therapeutic efficacy of clopidogrel and, thus, do not recommend against concomitant use of omeprazole together with clopidogrel. However, taking into account that numerous studies found that omeprazole may reduce the therapeutic efficacy of clopidogrel, further prospective, randomized, placebo-controlled, double-blind studies are necessary to elucidate the association between omeprazole and the clinical outcomes of clopidogrel treatment.
References
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001; 345: 494-502.
- Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise th. J Am Coll Cardiol. 2007; 50: e1-157.
- Schupke S, Neumann F-J, Menichelli M, Mayer K, Bernlochner I, Wohrle J, et al. Ticagrelor or Prasugrel in patients with acute coronary syndromes. N Engl J Med. 2019.
- Bhatt DL. Intensifying platelet inhibition - Navigating between Scylla and Charybdis. N Engl J Med. 2007; 357: 2078-2081.
- Abraham NS, Hlatky MA, Antman EM, Bhatt DL, Bjorkman DJ, Clark CB, et al. ACCF/ACG/AHA 2010 expert consensus document on the concomitant use of proton pump inhibitors and thienopyridines: A focused update of the ACCF/ ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID. Circulation. 2010; 122: 2619-2633.
- Wiviott SD, Antman EM, Gibson CM, Montalescot G, Riesmeyer J, Weerakkody G, et al. Evaluation of prasugrel compared with clopidogrel in patients with acute coronary syndromes: design and rationale for the Trial to assess Improvement in Therapeutic Outcomes by optimizing platelet Inhibition with prasugrel Thrombolysis In Myocardial Infar. Am Heart J. 2006; 152: 627-635.
- Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009; 361: 1045-1057.
- Cuisset T, Deharo P, Quilici J, Johnson TW, Deffarges S, Mence Bassez C, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J. 2017; 38: 3070-3080.
- Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: A report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2016; 134: e123-155.
- Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. Eur J Cardio-thoracic Surg. 2018; 53: 34-78.
- Steg PG, Bhatt DL, Simon T, Fox K, Mehta SR, Harrington RA, et al. Ticagrelor in patients with stable coronary disease and diabetes. N Engl J Med. 2019; 381: 1309-1320.
- Ackman ML, Graham MM, Hui C, Tsuyuki RT. Effect of a prior authorization process on antiplatelet therapy and outcomes in patients prescribed clopidogrel following coronary stenting. Can J Cardiol. 2006; 22: 1205-1208.
- Ho PM, Spertus JA, Masoudi FA, Reid KJ, Peterson ED, Magid DJ, et al. Impact of medication therapy discontinuation on mortality after myocardial infarction. Arch Intern Med. 2006; 166: 1842-1847.
- Ho PM, Peterson ED, Wang L, Magid DJ, Fihn SD, Larsen GC, et al. Incidence of death and acute myocardial infarction associated with stopping clopidogrel after acute coronary syndrome. JAMA - J Am Med Assoc. 2008; 299: 532-539.
- Jackevicius CA, Tu J V, Demers V, Melo M, Cox J, Rinfret S, et al. Cardiovascular outcomes after a change in prescription policy for clopidogrel. N Engl J Med. 2008; 359: 1802-1810.
- Sheehy O, LeLorier J, Rinfret S. Restrictive access to clopidogrel and mortality following coronary stent implantation. CMAJ. 2008; 178: 413-420.
- Kim K, Lee TA, Touchette DR, DiDomenico RJ, Ardati AK, Walton SM. Contemporary trends in oral antiplatelet agent use in patients treated with percutaneous coronary intervention for acute coronary syndrome. J Manag Care Spec Pharm. 2017; 23: 57-63.
- Farhat N, Haddad N, Crispo J, Birkett N, McNair D, Momoli F, et al. Trends in concomitant clopidogrel and proton pump inhibitor treatment among ACS inpatients, 2000-2016. Eur J Clin Pharmacol. 2019; 75: 227-235.
- Basra SS, Wang TY, Simon DJN, Chiswell K, Virani SS, Alam M, et al. Ticagrelor use in acute myocardial infarction: Insights from the National Cardiovascular Data Registry. J Am Heart Assoc. 2018; 7: e008125.
- Gimbel M, Qaderdan K, Willemsen L, Hermanides R, Bergmeijer T, de Vrey E, et al. Clopidogrel versus ticagrelor or prasugrel in patients aged 70 years or older with non-ST-elevation acute coronary syndrome (POPular AGE): the randomised, open-label, non-inferiority trial. Lancet. 2020; 395: 1374-1381.
- Esteve-Pastor MA, Ruiz-Nodar JM, Orenes-Pinero E, Rivera-Caravaca JM, Quintana-Giner M, Veliz-Martínez A, et al. Temporal trends in the use of antiplatelet therapy in patients with acute coronary syndromes. J Cardiovasc Pharmacol Ther. 2018; 23: 57-65.
- Khalid U, Bandeali S, Jones PG, Virani SS, Hira R, Hamzeh I, et al. Prescription patterns of Clopidogrel, Prasugrel, and Ticagrelor after percutaneous coronary intervention with stent implantation (from the NCDR PINNACLE Registry). Am J Cardiol. 2019; 124: 1807-1812.
- Amoah V, Worrall AP, Smallwood A, Armessilla AL, Nevill AM, Cotton JM. Clopidogrel and proton pump inhibitors: can near patient testing help in the tailoring of dual antiplatelet prescription? J Thromb Haemost. 2010; 8: 1422- 1424.
- Depta JP, Bhatt DL. Omeprazole and clopidogrel: Should clinicians be worried? Cleve Clin J Med. 2010; 77: 113-116.
- Ferreiro JL, Ueno M, Capodanno D, Desai B, Dharmashankar K, Darlington A, et al. Pharmacodynamic effects of concomitant versus staggered clopidogrel and omeprazole intake : Results of a prospective randomized crossover study. Circulation. 2010; 3: 436-441.
- Kenngott S, Olze R, Kollmer M, Bottheim H, Laner A, Holinski-Feder E, et al. Clopidogrel and Proton Pump Inhibitor (PPI) interaction: Separate intake and a non-omeprazole PPI the solution? Eur J Med Res. 2010; 15: 220-224.
- Kim K, Park P, Hong S, Park J-Y. The effect of CYP2C19 polymorphism on the pharmacokinetics and pharmacodynamics of clopidogrel: a possible mechanism for clopidogrel resistance. Clin Pharmacol Ther. 2008; 84: 236- 242.
- Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, et al. Cytochrome P-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009; 360: 354-362.
- Ogilvie BW, Yerino P, Kazmi F, Buckley DB, Rostami-Hodjegan A, Paris BL, et al. The proton pump inhibitor, omeprazole, but not lansoprazole or pantoprazole, is a metabolism-dependent inhibitor of CYP2C19: Implications for coadministration with clopidogrel. Drug Metab Dispos. 2011; 39: 2020- 2033.
- Wang ZY, Chen M, Zhu LL, Yu LS, Zeng S, Xiang MX, et al. Pharmacokinetic drug interactions with clopidogrel: Updated review and risk management in combination therapy. Ther Clin Risk Manag. 2015; 11: 449-467.
- Ohbuchi M, Noguchi K, Kawamura A, Usui T. Different effects of proton pump inhibitors and famotidine on the clopidogrel metabolic activation by recombinant CYP2B6, CYP2C19 and CYP3A4. Xenobiotica. 2012; 42: 633- 640.
- Jirungda S, Pussadhamma B, Komanasin N, Senthong V, Leuangwatthananon W. Interaction of CYP2C19 G681A polymorphism and omeprazole on clopidogrel responsiveness and impact in patients with acute coronary syndrome. Coron Artery Dis. 2019; 31: 266-272.
- de Mendonca Furtado RH, Giugliano RP, Strunz CMC, Cavalheiro Filho C, Ramires JAF, Kalil Filho R, et al. Drug interaction between clopidogrel and ranitidine or omeprazole in stable coronary artery disease: A double- blind, double dummy, randomized study. Am J Cardiovasc Drugs. 2016; 16: 275- 284.
- Ford NF. Clopidogrel resistance: pharmacokinetic or pharmacogenetic? J Clin Pharmacol. 2009; 49: 506-512.
- Simon T, Verstuyft C, Mary-Krause M, Quteineh L, Drouet E, Méneveau N, et al. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009; 360: 363-375.
- Furuta T, Iwaki T, Umemura K. Influences of different proton pump inhibitors on the anti-platelet function of clopidogrel in relation to CYP2C19 genotypes. Br J Clin Pharmacol. 2010; 70: 383-392.
- Arbel Y, Birati E, Abramowitz Y, Berliner S, Halkin A, Deutsch V, et al. Platelet inhibitory effect of clopidogrel in patients treated with omeprazole, pantoprazole, or famotidine A prospective randomized crossover study. J Am Coll Cardiol. 2012; 59: E1410.
- Lau WC, Waskell LA, Watkins PB, Neer CJ, Horowitz K, Hopp AS, et al. Atorvastatin reduces the ability of clopidogrel to inhibit platelet aggregation: A new drug-drug interaction. Circulation. 2003; 107: 32-37.
- Holmberg MT, Tornio A, Neuvonen M, Neuvonen PJ, Backman JT, Niemi M. Grapefruit juice inhibits the metabolic activation of clopidogrel. Clin Pharmacol Ther. 2013; 95: 307-313.
- Scarpignato C, Gatta L, Zullo A, Blandizzi C. Effective and safe proton pump inhibitor therapy in acid-related diseases - A position paper addressing benefits and potential harms of acid suppression. BMC Med. 2016; 14: 179.
- Krag M, Marker S, Perner A, Wetterslev J, Wise MP, Schefold JC, et al. Pantoprazole in patients at risk for gastrointestinal bleeding in the ICU. N Engl J Med. 2018; 379: 2199-2208.
- Pottegard A, Broe A, Hallas J, de Muckadell OBS, Lassen AT, Lødrup AB. Use of proton-pump inhibitors among adults: a Danish nationwide drug utilization study. Therap Adv Gastroenterol. 2016; 9: 671-678.
- Halfdanarson O, Pottegard A, Bjornsson ES, Lund SH, Ogmundsdottir MH, Steingrimsson E, et al. Proton-pump inhibitors among adults: a nationwide drug-utilization study. Therap Adv Gastroenterol. 2018; 11: 1-11.
- Boucherie Q, Rouby F, Frankel D, Roll P, Micallef J. Proton pump inhibitors prescriptions in France: Main trends from 2006 to 2016 on French health insurance database. Therapie. 2018; 73: 385-388.
- Khan SU, Lone AN, Asad ZUA, Rahman H, Khan MS, Saleem MA, et al. Metaanalysis of efficacy and safety of proton pump inhibitors with dual antiplatelet therapy for coronary artery disease. Cardiovasc Revascularization Med. 2019; 20: 1125-1133.
- Sehested TSG, Carlson N, Hansen PW, Gerds TA, Charlot MG, Torp- Pedersen C, et al. Reduced risk of gastrointestinal bleeding associated with proton pump inhibitor therapy in patients treated with dual antiplatelet therapy after myocardial infarction. Eur Heart J. 2019; 40: 1963-1970.
- Huang B, Huang Y, Li Y, Yao H, Jing X, Huang H, et al. Adverse Cardiovascular effects of concomitant use of proton pump inhibitors and clopidogrel in patients with coronary artery disease: A systematic review and meta-analysis. Arch Med Res. 2012; 43: 212-224.
- Bundhun PK, Teeluck AR, Bhurtu A, Huang WQ. Is the concomitant use of clopidogrel and proton pump inhibitors still associated with increased adverse cardiovascular outcomes following coronary angioplasty?: A systematic review and meta-analysis of recently published studies (2012-2016). BMC Cardiovasc Disord. 2017; 17: 1-11.
- Sibbing D, Morath T, Stegherr J, Braun S, Vogt W, Hadamitzky M, et al. Impact of proton pump inhibitors on the antiplatelet effects of clopidogrel. Thromb Haemost. 2009; 101: 714-719.
- Angiolillo DJ, Gibson CM, Cheng S, Ollier C, Nicolas O, Bergougnan L, et al. Differential effects of omeprazole and pantoprazole on the pharmacodynamics and pharmacokinetics of clopidogrel in healthy subjects: Randomized, placebo-controlled, crossover comparison studies. Clin Pharmacol Ther. 2011; 89: 65-74.
- Frelinger AL, Lee RD, Mulford DJ, Wu J, Nudurupati S, Nigam A, et al. A randomized, 2-period, crossover design study to assess the effects of dexlansoprazole, lansoprazole, esomeprazole, and omeprazole on the steady-state pharmacokinetics and pharmacodynamics of clopidogrel in healthy volunteers. J Am Coll Cardiol. 2012; 59: 1304-1311.
- Arbel Y, Birati EY, Finkelstein A, Halkin A, Kletzel H, Abramowitz Y, et al. Platelet inhibitory effect of clopidogrel in patients treated with omeprazole, pantoprazole, and famotidine: A prospective, randomized, crossover study. Clin Cardiol. 2013; 36: 342-346.
- Lin CF, Shen LJ, Wu FLL, Bai CH, Gau CS. Cardiovascular outcomes associated with concomitant use of clopidogrel and proton pump inhibitors in patients with acute coronary syndrome in Taiwan. Br J Clin Pharmacol. 2012; 74: 824-834.
- Gilard M, Arnaud B, Le Gal G, Abgrall JF, Boschat J. Influence of omeprazol on the antiplatelet action of clopidogrel associated to aspirin. J Thromb Haemost. 2006; 4: 2508-2509.
- Yun KH, Rhee SJ, Park HY, Yoo NJ, Kim NH, Oh SK, et al. Effects of omeprazole on the antiplatelet activity of clopidogrel. Int Heart J. 2010; 51: 13-16.
- Fontes-Carvalho R, Albuquerque A, Araujo C, Pimentel-Nunes P, Ribeiro VG. Omeprazole, but not pantoprazole, reduces the antiplatelet effect of clopidogrel: A randomized clinical crossover trial in patients after myocardial infarction evaluating the clopidogrel-PPIs drug interaction. Eur J Gastroenterol Hepatol. 2011; 23: 396-404.
- Kanmanthareddy A, Buddam A, Belbase R, Anugula D, Balmuri A, Kaushik M, et al. CRT-100.66 Platelet activity in clopidogrel users with and without omeprazole: Meta-analysis of pharmacodynamic function. JACC Cardiovasc Interv. 2017; 10: S21.
- Gilard M, Arnaud B, Cornily JC, Le Gal G, Lacut K, Le Calvez G, et al. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin. The randomized, double-blind OCLA (Omeprazole CLopidogrel Aspirin) study. J Am Coll Cardiol. 2008; 51: 256-260.
- Kreutz RP, Stanek EJ, Aubert R, Yao J, Breall JA, Desta Z, et al. Impact of proton pump inhibitors on the effectiveness of clopidogrel after coronary stent placement: The clopidogrel Medco outcomes study. Pharmacotherapy. 2010; 30: 787-796.
- US Food and Drug Administration. Information for healthcare professionals: Update to the labeling of Clopidogrel Bisulfate (marketed as Plavix) to alert healthcare professionals about a drug interaction with omeprazole (marketed as Prilosec and Prilosec OTC). 2010.
- European Medicines Agency. Interaction between clopidogrel and protonpump inhibitors. CHMP updates warning for clopidogrel-containing medicines. public statement. 2010.
- Charlot M, Ahlehoff O, Norgaard ML, Jørgensen CH, Sørensen R, Abildstrøm SZ, et al. Proton-pump inhibitors are associated with increased cardiovascular risk independent of clopidogrel use: A nationwide cohort study. Ann Intern Med. 2010; 153: 378-386.
- Ray WA, Murray KT, Griffin MR, Chung CP, Smalley WE, Hall K, et al. Outcomes with concurrent use of clopidogrel and proton-pump inhibitors: A cohort study. Ann Intern Med. 2010; 152: 337-345.
- Harjai KJ, Shenoy C, Orshaw P, Usmani S, Boura J, Mehta RH. Clinical outcomes in patients with the concomitant use of clopidogrel and proton pump inhibitors after percutaneous coronary intervention: an analysis from the Guthrie Health Off-Label Stent (GHOST) investigators. Circulation. 2011; 4: 162-170.
- Simon T, Steg PG, Gilard M, Blanchard D, Bonello L, Hanssen M, et al. Clinical events as a function of proton pump inhibitor use, clopidogrel use, and cytochrome P450 2C19 genotype in a large nationwide cohort of acute myocardial infarction: Results from the french registry of acute ST-Elevation and Non-ST-Elevation myocardial infarction (FAST-MI) registry. Circulation. 2011; 123: 474-482.
- Zairis MN, Tsiaousis GZ, Patsourakos NG, Georgilas AT, Kontos CF, Adamopoulou EN, et al. The impact of treatment with omeprazole on the effectiveness of clopidogrel drug therapy during the first year after successful coronary stenting. Can J Cardiol. 2010; 26: e54-57.
- Mandurino-Mirizzi A, Leonardi S, Melloni C. Concomitant use of proton pump inhibitors and dual antiplatelet therapy for cardiovascular outcomes. Minerva Endocrinol. 2017; 42: 228-237.
- Bhatt DL, Cryer BL, Contant CF, Cohen M, Lanas A, Schnitzer TJ, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010; 363: 1909-1917.
- Vaduganathan M, Cannon CP, Cryer BL, Liu Y, Hsieh WH, Doros G, et al. Efficacy and safety of proton-pump inhibitors in high-risk cardiovascular subsets of the COGENT Trial. Am J Med. 2016; 129: 1002-1005.
- Melloni C, Washam JB, Jones WS, Halim SA, Hasselblad V, Mayer SB, et al. Conflicting results between randomized trials and observational studies on the impact of proton pump inhibitors on cardiovascular events when coadministered with dual antiplatelet therapy: Systematic review. Circ Cardiovasc Qual Outcomes. 2015; 8: 47-55.
- Sherwood MW, Melloni C, Schuyler Jones W, Washam JB, Hasselblad V, Dolor RJ. Individual proton pump inhibitors and outcomes in patients with coronary artery disease on dual antiplatelet therapy: A systematic review. J Am Heart Assoc. 2015; 4.
- Serbin MA, Guzauskas GF, Veenstra DL. Clopidogrel-proton pump inhibitor drug-drug interaction and risk of adverse clinical outcomes among PCItreated ACS patients: A meta-analysis. J Manag Care Spec Pharm. 2016; 22: 939-947.
- Farhat N, Fortin Y, Haddad N, Birkett N, Mattison DR, Momoli F, et al. Systematic review and meta-analysis of adverse cardiovascular events associated with proton pump inhibitors used alone or in combination with antiplatelet agents. Vol. 49, Critical Reviews in Toxicology. Taylor and Francis Ltd. 2019; 215-261.
- Juurlink DN, Gomes T, Ko DT, Szmitko PE, Austin PC, Tu J V, et al. A population-based study of the drug interaction between proton pump inhibitors and clopidogrel. CMAJ. 2009; 180: 713-718.
- Hudzik B, Szkodzinski J, Danikiewicz A, Wilczek K, Romanowski W, Lekston A, et al. Effect of omeprazole on the concentration of interleukin-6 and transforming growth factor-β1 in patients receiving dual antiplatelet therapy after percutaneous coronary intervention. Eur Cytokine Netw. 2010; 21: 257- 263.
- Aubert R, Epstein RS, Teagarden J, Xia F, Yao J, Desta Z, et al. Abstract 3998: Proton pump inhibitors effect on clopidogrel effectiveness: The clopidrogel MEDCO outcomes study. Circulation. 2008; 118-S815.
- Steg PG, James SK, Atar D, Badano LP, Lundqvist CB, Borger MA, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Vol. 33, European Heart Journal. 2012; 2569-2619.
- Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2018; 39: 119- 177.
- Hsiao FY, Tsai YW, Huang WF, Wen YW, Chen PF, Chang PY, et al. A comparison of aspirin and clopidogrel with or without proton pump inhibitors for the secondary prevention of cardiovascular events in patients at high risk for gastrointestinal bleeding. Clin Ther. 2009; 31: 2038-2047.
- Urban P, Gershlick AH, Guagliumi G, Guyon P, Lotan C, Schofer J, et al. Safety of coronary sirolimus-eluting stents in daily clinical practice. Circulation. 2006; 113: 1434-1441.
- Saleh A, Hammoudeh A, Tabbalat R, Al-Haddad I, Al-Mousa E, Jarrah M, et al. Incidence and prognosis of stent thrombosis following percutaneous coronary intervention in Middle Eastern patients: The First Jordanian Percutaneous Coronary Intervention Registry (JoPCR1) From the. AnnSAudiMed.net Med. 2016; 36: 17-22.
- Buchanan GL, Basavarajaiah S, Chieffo A. Stent Thrombosis: incidence, predictors and new technologies. Thrombosis. 2012; 1-12.
- Plakht Y, Gilutz H, Shiyovich A. Ethnical disparities in temporal trends of acute myocardial infarction (AMI) throughout a decade in Israel. Soroka acute myocardial infarction (SAMI-II) project. Int J Cardiol. 2016; 214: 469-476.
- Plakht Y, Gilutz H, Shiyovich A, Zahger D, Weitzman S. Gender and ethnic disparities in outcome following acute myocardial infarction among bedouins and jews in Southern Israel. Eur J Public Health. 2011; 21: 74-80.
- Tamir O, Peleg R, Dreiher J, Abu-Hammad T, Abu Rabia Y, Abu Rashid M, et al. Cardiovascular risk factors in the Bedouin population: Management and compliance. Isr Med Assoc J. 2007; 9: 652-655.
- Plakht Y, Abu Tailakh M, Barabi T, Shiyovich A. Ethnic disparities in emergency department utilization patterns in southern Israel: A populationbased study. Intern Emerg Med. 2012; 7: 547-555.
- Chaudhary R, Sukhi A, Chaudhary R, Jindal M, Vyas A, Rout A, et al. Gender differences in thrombogenicity among patients with angina and nonobstructive coronary artery disease. J Thromb Thrombolysis. 2019; 48: 373- 381.
- Taueti VR. Sex-differences in the coronary artery system. In: Kerkhof P, Miller V, editors. Sex-specific analysis of cardiovascular function . Springer Nature, Switzerland. 2018; 257-278.
- Kashour T, Al-Tannir M, Bahamid R. Changing prescription pattern of Omeprazole among patients receiving clopidogrel. Int Heart J. 2014; 55: 93- 95.
- Guerin A, Mody R, Carter V, Ayas C, Patel H, Lasch K, et al. Changes in practice patterns of clopidogrel in combination with proton pump inhibitors after an fda safety communication. PLoS One. 2016; 11: e0145504.
- Choe JC, Cha KS, Ahn J, Park JS, Lee HW, Oh JH, et al. Comparison of prescription rates and clinical outcomes in acute coronary syndrome patients who underwent percutaneous coronary intervention using different P2Y12 inhibitors in a large observational study. Int J Cardiol. 2019.