
Review Article
Austin J Pharmacol Ther. 2021; 9(2).1133.
Lactobacillus rhamnosus Interferes with Candida albicans Adherence and Biofilm Formation: A Potential Alternative Treatment of Candidiasis
Ribeiro FC1, Iglesias MC2, Barros PP1, Santos SSF2, Jorge AOC1 and Leão MVP2,3*
¹Department of Biosciences and Oral Diagnosis, Sao Paulo State University/UNESP, Brazil
²Bioscience Basic Institute, University of Taubate, Bom Conselho, Taubate, SP, Brazil
³School of Medical Sciences of São José dos Campos, Brazil
*Corresponding author: Leão MVP, School of Medical Sciences of São José dos Campos, Humanitas, São José dos Campos, Brazil
Received: March 19, 2021; Accepted: April 24, 2021; Published: May 01, 2021
Abstract
The objective of the present study was to evaluate the ability of Lactobacillus rhamnosus, on different preparations (living lactobacilli, dead by heat lactobacilli and supernatant of lactobacilli suspension), to interfere with Candida albicans adherence to ephitelial cells and biofilm formation. The results showed a reduction of 66.2% in the number of Candida cells adhered to epithelial cells, when the suspension of living L. rhamnosus was used. On the same way, this suspension reduced the in vitro biofim formation by C. albicans. In conclusion, the suspension with living cells of L. rhamnosus was able to reduce the ability of C. albicans to adhere on ephitelial cells and to form biofilm, suggesting a potential use of this probiotic bacteria as a therapeutic agent in candidiasis.
Keywords: Biofilm; Candida; Lactobacillus; Adherence; Probiotic
Introduction
With large utilization of antifungal to control Candida infections, several species have become resistanto drugs, especially those of the azole class. This resistance profile changes with the specie and the strain due to the different mechanisms of resistance and also through the exposition time and drug concentration [1-3].
On the attempt to find new approaches of candidiasis treatment or improve the already existing ones, studies are being done in order to develop alternative methods to reduce fungal infections, or coadjuvant therapies to induce better effects [4–6].
In literature, it has been reported that different Lactobacillus strains, with probiotic properties, are able to interfere with C. albicans colonization and/or infection [7-9]. Lactobacillus can inhibit Candida virulence factors, as germ tube, yeast adherence and hyphae and biofim formation, leaving this yeast more susceptible to immune system action [10-13]. Lactobacillus can also change the sensitivity profile of C. albicans to antifungal, making them more susceptible to the treatment [14].
In this context, the present work aimed to study the ability of Lactobacillus rhamnosus LL0011 or only its products to inhibit C. albicans adherence to epithelial cells and biofilm formation.
Materials and Methods
Microorganisms
Lactobacillus rhamnosus LL0011 (Cefar Diagnóstica, São Paulo, Brasil) was plated on agar Man-Rogosa-Shape (MRS-Oxoid, Basingstroke, Hampshire, England) and cultivated on 37°C in 5% of CO2 for 48 hours. After this time, three preparations were obtained: SpL - living lactobacilli cells, constituted of 107 cells/mL of sterile saline, standardized in spectrophotometer at 530 nm; SpLA - dead by heat lactobacilli (SpLautoclaved by 15 min); SnLA - supernatant of SpLA.
C. albicans ATCC 18804 was grown in Sabouraud dextrose Agar (Difco, Detroit, USA), incubated at 37°C for 24h.
Adherence to oral epithelial cells assay
Epithelial cells from oral mucosa were obtained by four volunteers (same sanguine type, O group of the ABO system), through slight scraping of the mucosa, using disposable and sterilized wooden spatula. The obtained cells were placed in a sterilized tube with 2 mL of PBS, obtaining an ephitelial cells pool that were washed three times with sterilized PBS on centrifugation on 1800 X g by 5 minutes each. After the washing, the deposit was resuspended until the obtaining of 105 cells per mL, counted on Neubauer chamber. After the padronization of epithelial cells (described above) in the same tube was added C. albicans suspension of 106 cells/mL of sterile saline, standardized in spectrophotometer at 530 nm, and the different preparations of L. rhamnosus (SpL, SpLA, SnLA) or saline (negative control). The tubes were incubated for 4 hrs at 37°C with 5% of CO2. After 4 hrs the cells were washed and a total of one hundred cells were counted for each experiment.
Biofilm assay
To the formation of the biofilm was utilized 96 wells plate. In each plate were pipetted 200 μL of suspension of C. albicans prepared by YNB, the plate was incubated in agitation of 37°C by 120 minutes to the adherence initial phase. Completing this period, the suspensions were removed from the wells, which were washed on 200 μL of sterile saline solution. Afterwards, 100 μL de YNB improved with 100 mM of glucose were added to the wells plus 100 μL of each suspension of L. rhamnosus (SpL, SpLA ou SnLA) or saline solution (control). The plate was incubed to 37°C for 48 hours on agitation, changing the broth each 24 hours.
After 48 hours the biofilms were washed three times with saline solution, and detached using an ultrasonic homogenizer (Sonics Vibra-Cell VCX 130) with the potency of 50 W by 30s. From this solution, serial dilutions were obtained, plated in agar Sabouraud dextrose and incubed at 37°C for 48 hours, for counting of CFU/mL of C. albicans.
Results
In the adherence assay, it was observed that in the presence of living L. rhamnosus (SpL) there as a significant decrease (66.2%) in the adherence of C. albicans when compared with control (saline). Similar, but lower, results were observed when the SpLA was used, with 24.54% of reduction. However, the suspension containing only the supernatant of L. rhamnosus wasn’t able to inhibit C. albicans adherence (Figure 1).
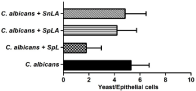
Figure 1: The counting of adhered C. albicans on ephitelial cells, on the
absence or presence of different suspensions of L. rhamnosus (SpL, SpLA
e SnLA). Kruskal-Wallis test and post Dunn multiple comparison test was
used. Candida vs. Candida + SnLA – p = 0.5380; Candida vs. Candida +
SpLA – p = 0.0002; Candida vs. Candida + SpL – p < 0.0001. (SpL - living lactobacilli cells; SpLA - dead by heat lactobacilli (autoclaved by 15 min);
SnLA - supernatant of lactobacilli suspension dead by heat).
The biofilm results showed that when the SpL was used, a significative reduction (p=0,036) in the CFU/mL of C. albicans from the biofilm was observed. The other suspensions also have a slight reduction on C. albicans biofim formation, however with no statistical significance when compared to the control (Figure 2).
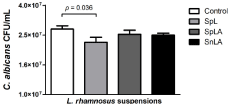
Figure 2: C. albicans CFU/mL counts from biofilms formed on the absence
or presence of different suspensions of L. rhamnosus (SpL, SpLA e SnLA) .
Student’s t test was used to compare the control group with the experimental
groups. Control vs. SnLA – p = 0.1321; Control vs. SpLA – p = 0.3349;
Control vs. SpL – p= 0.0365 (Control - C. albicans alone; SpL - living
lactobacilli cells; SpLA - dead by heat lactobacilli (autoclaved by 15 min);
SnLA - supernatant of lactobacilli suspension dead by heat).
Discussion
In this study we evaluated the anti-Candida potential of three different L. rhamnosus LL0011 suspensions against C. albicans on epithelial cells adherence and biofilm formation inhibition. Our data showed that presence of the L. rhamnosus, dead or alive, interfered on the adherence of C. albicans to the ephitelial cell of oral mucosa, meanwhile when the SnLA was used, we could not note a reduction on C. albicans adherence. This data suggests that whole cell of L. rhamnosus or its estrutural molecules, but not its metabolites, are able to inhibit the C. albicans adherence. In the literature, some studies have been stablished the effects of Lactobacillus on pathogenic microorganism adhesion, especially on yeast of the genus Candida, and the mechanisms involved are related to exclusion, competition for receptors sites and displacement of adhesion [15-17]. It seems that some molecules presented on Lactobacillus cells, as well as biosurfactants, have the property of changing the surface tension of the medium displaying an anti-adhesive effect [8,18].
Many probiotics used on dairy products are composed of live lactobacilli. Their development presents a challenge for industrial production, since, the industry need a suitable technology and parameters that involve the viability and the stability of the microorganisms (stress tolerance during processing and storage of the product) [19]. In this study, the suspension containing L. rhamnosus dead by heat also showed an antagonist effects on C. albicans adherence and this characteristic is extremely interesting for its use in probiotic products. Since the microorganisms are dead, the product becomes more stable and viable, simplifying various industrial processes generating lower costs for its production, and bringing more benefits to its consumers.
The formation of biofilm is one of the most important virulence factors of C. albicans, since this factor is intimately related to the pathogenicity, providing bigger resistance to the host immune system and the action of antifungal. Our results showed that only the suspension containing the live L. rhamnosus was able to significantly reduce the C. albicans biofilm formation. The heat killing and the supernatant free-cells suspensions of L. rhamnosus presented a slight reduction on the biofilm; however they do not show statistical difference.
The C. albicans biofilm inhibition can occur on different phases of the biofilm formation, as adherence, initial colonization or on the maturation phase. This inhibition seems to differ depending on Lactobacillus strains used, once some species have better results on initial colonization phase and others on the other phases of the biofilm formation [20-22]. In the present work, since the adherence phase was on absence of lactobacilli, the results point to a mechanism of action of L. rhamnosus involving destructuring of biofilm or by the consumption of nutrients [22].
The first step in the pathogenesis of C. albicans is its ability to adhere on biotic (e.g. tissues) and abiotic surfaces (e.g. catheters), allowing the colonization in a specific niche and starting the infection process [17]. The results obtained on the present work show a significant inhibition of C. albicans both on adherence to epithelial cells and abiotic surfaces. This is a very promising result, which leads the possibility that L. rhamnosus can be used as a therapy to inhibit infections caused by C. albicans both in mucous membranes and from devices that allow biofilm formation.
Conclusion
Thus, the present study demonstrates that the suspension of living L. rhamnosus was able to inhibit the adherence of C. albicans ephitelial cells from oral mucosa and also capable to inhibiting and reducing the C. albicans growing on biofilm. Our study opens the perspective that L. rhamnosus LL0011 can be an interestingly strain to be used in future therapeutics studies against C. albicans.
References
- Pristov KE, Ghannoum MA. Resistance of Candida to azoles and echinocandins worldwide. Clin Microbiol Infect. 2019; 25: 792-798.
- Teo JQM, Lee SJY, Tan AL, Lim RSM, Cai Y, Lim TP, et al. Molecular mechanisms of azole resistance in Candida bloodstream isolates. BMC Infect Dis. 2019; 19: 1-4.
- Prasad R, Nair R, Banerjee A. Multidrug transporters of Candida species in clinical azole resistance. Fungal Genet Biol. 2019; 132: 103252.
- Scorzoni L, de Menezes RT, Pereira TC, Oliveira PS, Ribeiro F de C, Santos EL de S, et al. Antifungal and anti-biofilm effect of the calcium channel blocker verapamil on non-albicans candida species. An Acad Bras Cienc. 2020; 92: 1-14.
- Rossoni RD, de Barros PP, Mendonça I do C, Medina RP, Silva DHS, Fuchs BB, et al. The Postbiotic Activity of Lactobacillus paracasei 28.4 Against Candida auris. Front Cell Infect Microbiol. 2020; 10: 397.
- de Barros PP, Rossoni RD, Ribeiro F de C, Silva MP, de Souza CM, Jorge AOC, et al. Two sporulated Bacillus enhance immunity in Galleria mellonella protecting against Candida albicans. Microb Pathog. 2019; 132: 335-42.
- Santos RB, Scorzoni L, Namba AM, Rossoni RD, Jorge AOC, Junqueira JC. Lactobacillus species increase the survival of Galleria mellonella infected with Candida albicans and non-albicans Candida clinical isolates. Med Mycol. 2019; 57: 391-394.
- Santos C, Ramos França Y, Duarte Lima Campos C, Quaresma Bomfim MR, Oliveira Melo B, Assunção Holanda R, et al. Antifungal and antivirulence activity of vaginal lactobacillus spp. Products against candida vaginal isolates. Pathogens. 2019; 8: 150.
- de Barros PP, Scorzoni L, Ribeiro F de C, Fugisaki LR de O, Fuchs BB, Mylonakis E, et al. Lactobacillus paracasei 28.4 reduces in vitro hyphae formation of Candida albicans and prevents the filamentation in an experimental model of Caenorhabditis elegans. Microb Pathog. 2018; 117: 80-87.
- Vilela SFG, Barbosa JO, Rossoni RD, Santos JD, Prata MCA, Anbinder AL, et al. Lactobacillus acidophilus ATCC 4356 inhibits biofilm formation by C. Albicans and attenuates the experimental candidiasis in Galleria mellonella. Virulence. 2015; 6: 29-39.
- de Oliveira FE, Rossoni RD, de Barros PP, Begnini BE, Junqueira JC, Jorge AOC, et al. Immunomodulatory effects and anti-Candida activity of lactobacilli in macrophages and in invertebrate model of Galleria mellonella. Microb Pathog. 2017; 110: 603-611.
- Matsuda Y, Cho O, Sugita T. Culture supernatants of lactobacillus gasseri and l . Crispatus inhibit candida albicans biofilm formation and adhesion to hela cells. Mycopathologia. 2018; 183: 691-700.
- Allonsius CN, Vandenheuvel D, Oerlemans EFM, Petrova MI, Donders GGG, Cos P, et al. Inhibition of Candida albicans morphogenesis by chitinase from Lactobacillus rhamnosus GG. Sci Rep. 2019; 9: 1-12.
- De Oliveira JR, De Aguiar Almeida RB, Das Graças Figueiredo Vilela P, De Oliveira FE, Da Rocha RF, Jorge AOC, et al. Control of microorganisms of oral health interest with Arctium lappa L. (burdock) extract non-cytotoxic to cell culture of macrophages (RAW 264.7). Arch Oral Biol. 2014; 59: 808-814.
- Verdenelli MC, Coman MM, Cecchini C, Silvi S, Orpianesi C, Cresci A. Evaluation of antipathogenic activity and adherence properties of human Lactobacillus strains for vaginal formulations. J Appl Microbiol. 2014; 116: 1297-1307.
- Poupet C, Saraoui T, Veisseire P, Bonnet M, Dausset C, Gachinat M, et al. Lactobacillus rhamnosus Lcr35 as an effective treatment for preventing Candida albicans infection in the invertebrate model Caenorhabditis elegans: First mechanistic insights. PLoS One. 2019; 14: e0216184.
- Ribeiro FC, Rossoni RD, de Barros PP, Santos JD, Fugisaki LRO, Leão MPV, et al. Action mechanisms of probiotics on Candida spp. and candidiasis prevention: an update. J Appl Microbiol. 2020; 129: 175-185.
- De Gregorio PR, Parolin C, Abruzzo A, Luppi B, Protti M, Mercolini L, et al. Biosurfactant from vaginal Lactobacillus crispatus BC1 as a promising agent to interfere with Candida adhesion. Microb Cell Fact. 2020; 19: 1-16.
- Sarao LK, Arora M. Probiotics, prebiotics, and microencapsulation: A review. Crit Rev Food Sci Nutr. 2017; 57: 344-371.
- Matsubara VH, Wang Y, Bandara HMHN, Mayer MPA, Samaranayake LP. Probiotic lactobacilli inhibit early stages of Candida albicans biofilm development by reducing their growth, cell adhesion, and filamentation. Appl Microbiol Biotechnol. 2016; 100: 6415-6426.
- Son S, Jeon H, Yang S, Lee N, Paik H. In vitro characterization of Lactobacillus brevis KU15006 , an isolate from kimchi , reveals anti-adhesion activity against foodborne pathogens and antidiabetic properties. Microb Pathog. 2017; 112: 135-141.
- de Camargo Ribeiro F, Junqueira JC, dos Santos JD, de Barros PP, Rossoni RD, Shukla S, et al. Development of probiotic formulations for oral candidiasis prevention: Gellan Gum as a Carrier to Deliver Lactobacillus paracasei 28.4. Antimicrob Agents Chemother. 2020; 64: e02323-19.