
Review Article
Austin J Pharmacol Ther. 2021; 9(3).1136.
Targeting the Gut Dysbiosis as a Treatment for Alcoholic Liver Disease
Shaikh GM1 and Gupta GL1,2*
¹Shobhaben Pratapbhai Patel School of Pharmacy & Technology Management, SVKM'S NMIMS, Mumbai, India
²School of Pharmacy & Technology Management, SVKM'S NMIMS, Shirpur Campus, Shirpur, India
*Corresponding author: Girdhari Lal Gupta, Department of Pharmacology, School of Pharmacy & Technology Management, SVKM’S NMIMS, Shirpur Campus, Shirpur-425405, India
Received: May 03, 2021; Accepted: May 28, 2021; Published: June 04, 2021
Abstract
Alcohol Use Disorders (AUD) originates due to heavy and uncontrolled drinking of alcohol. It is one of the most prevalent mental disorders, which predominantly affects men globally. In the review article alcohol use disorders and several risk factors like gender, drinking habit, genetic differences, and obesity Hepatitis C virus has been described for the provocation of intestinal dysbiosis. Alcoholic Liver Disease (ALD) is a spectrum of diseases from steatohepatitis to Hepatocellular Carcinoma (HCC). Due to dysbiosis, there are microbial changes also taking place in the liver and it further worsens the conditions. Treatment involves treating gut dysbiosis and altered balance of the micro-organism. The treatment strategy of ALD may also involve a non-dietary approach or dietary approach or by microbiota modulation.
Keywords: AUD; Alcohol; Microbial dysbiosis; ALD; Treatment
Abbreviations
51Cr-EDTA: 51Cr-Ethylenediamine Tetraacetic Acid; ACC: Anterior Cingulate Cortex; ADH: Alcohol Dehydrogenase; ALD: Alcoholic Liver Disease; ALDH: Aldehyde Dehydrogenase; ALDH2: Aldehyde Dehydrogenase-2; ALT: Alanine Aminotransferase; AST: Aspartate Transaminase; AUD: Alcohol Use Disorders; BCAA: Branched-Chain Amino Acid; BMI: Body Mass Index; FMT: Fecal Matter Transfer; GIT: Gastro Intestinal Tract; HCC: Hepato-Cellular Carcinoma; HCV: Hepatitis C Virus; HE: Hepatic Encephalopathy; HSD17B13: Hydroxysteroid 17-Beta Dehydrogenase 13; IL- 17: Interleukin-17; IL-6: Interleukin-6; LOLA: L-Ornithine-LAspartate; LPS: Lipo-Polysaccharides; MBOAT7: Membrane-Bound O-Acyltransferase Domain- Containing 7; MDA: Malondialdehyde; MHE: Minimal Hepatic Encephalopathy; NAFLD: Non-Alcoholic Fatty Liver Disease; NASH: Non-Alcoholic Steatohepatitis; NF- κB: Nuclear Factor Kappa-light chain enhancer of activated B cells; NHANES III: The Third National Health and Nutrition Examination Survey; NIAAA: National Institute on Alcohol Abuse and Alcoholism; PEG: Polyethylene Glycol; PGN: Peptidoglycan; PNPLA-3: Patatin-Like Phospholipase Domain-Containing Protein 3; PPAR-a: Peroxisome Proliferator Activated Receptor-a; Reg3b: Regenerating Islet-Derived Protein 3-beta; Reg3g: Regenerating Islet- Derived Protein 3-gamma; ROS: Reactive Oxygen Species; SCFA: Short Chain Fatty Acids; TM6SF2: Transmembrane 6 Superfamily Member 2; TNFa: Tumor Necrosis Factor-a
Introduction
Alcohol Use Disorders (AUD) is characterized by heavy alcohol use coupled with the loss of control over its intake. It is one of the most prevalent mental disorders, which predominantly affects men globally. A person suffering from AUD is unable to control the consumption of alcohol and thereby affects their health, family, friends, and social lives. Therefore, it results in compulsive use of alcohol [1,2]. AUD is also accompanied by liver cirrhosis and death [3]. AUD is also coupled with a high mortality rate and it was found that in the European Union, in 2004 AUD was accounted for 62% of alcohol use [4]. In Nordic Countries, it was found that people with AUD were having a shorter life span by 24-28 years than in the general population [5]. Due to the difference in socioeconomic status, individuals with low socioeconomic status are at a higher risk of AUD than high socioeconomic status [6].
Risk Factors for AUD
People with a heavy intake of alcohol are more likely to develop AUD which is associated with steatohepatitis, fibrosis, and cirrhosis. But, some patients do not develop cirrhosis even after chronic intake of alcohol [7]. These are dependent on various factors which are discussed below.
Gender
Women consume less alcohol than men and therefore have a lower chance of developing AUD [8]. Longitudinal studies have discovered that men have a higher prevalence of AUD than women [9]. Women are more likely than men to develop hepatotoxicity due to lower levels of gastric Alcohol Dehydrogenase (ADH). This is most likely due to higher levels of alcohol in the bloodstream increasing alcohol bioavailability. This has an impact on hormonal activity [10,11]. Estrogen receptors are found in both parenchymal and nonparenchymal cells. Huge amounts of alcohol can also increase the expression of estrogen receptors [12]. Alcohol levels are also affected by hormone levels in the body. It was discovered that oestrogen therapy raises the risk of alcoholic-induced steatohepatitis, while ovariectomy reduces it. Estrogen therapy also stimulates kupffer cells, which raises TNF levels. Its inflammatory properties aid in increased alcohol permeation via the intestine, resulting in apoptosis [13]. It is also reported that male mice have higher levels of hepatoprotective betaine-homocysteine methyltransferase [14,15]. The change in the ratios of anti-inflammatory ω-3 and pro-inflammatory ω-6 are also responsible for AUD development. A report stated that in female drinkers, levels of pro-inflammatory were increased, whereas, in male drinkers, levels of anti-inflammatory were increased [16]. All these above studies suggest that differences in the hormone, hepatoprotective agent, proinflammatory conditions may be a responsible difference in male and female Alcoholic Liver Disease (ALD) prevalence.
Drinking habit
A study conducted that there is a shift in the drinking pattern of heavy drinkers and binge drinkers [17]. According to several surveys, binge drinking refers to an episode where men tend to have five or more drinks or women tend to have four or more drinks. The Centers for Diseases Control in 2010 conducted a survey and estimated that one in every six adults is engaged in binge drinking. In this survey, it was also found that 50% of college students found themselves in binge drinking [18]. Binge drinking is an important risk factor for young adults for alcohol dependence and its abuse [19]. In the previous section, we have seen that women are prone to deleterious effects of heavy drinking. Therefore, younger women are likely to binge drinking. It is also supported by the study which suggests that hormone levels of female mice contribute to binge drinking [20].
Genetic differences
AUD is dependent on environmental as well as host factors. It was found that Hispanics are more prone to AUD whereas monozygotic twins have a high probability of cirrhosis as compared to dizygotic twins [21]. Aldehyde Dehydrogenase-2 (ALDH-2) is responsible for the conversion of Acetaldehyde to Acetic acid. A study reported that inactivate form of ALDH-2 (E487K) is responsible for the flush reaction, and it was expected that 40% of East Asian shows this effect [22]. The PNPLA-3 is a well-known risk factor of Non-Alcoholic Steatohepatitis (NASH) is associated with the development of AUD, which ultimately leads to cirrhosis [23]. A study from two cohorts from Europe found that TM6SF2 and MBOAT7 were responsible for cirrhosis [24]. Further, a study on hydroxysteroid 17-Beta Dehydrogenase (HSD17B13) found that it is capable of reducing alcohol-induced cirrhosis [25].
Obesity
According to the World Health Organization, a person with a BMI of more than 25kg/m2 and 30kg/m2 is said to be overweight and obese. During the initial diagnosis of steatosis (an early stage of AUD), there are high levels of triglycerides present in hepatocytes and histologically it was found that 90% of alcoholics suffered from the fatty liver [26]. The reports from The Third National Health and Nutrition Examination Survey (NHANES III) found that patients with AUD that 44.5% obesity prevalence and high liver-related mortality [27]. Individuals who consume a lot of alcohol and are overweight have their liver enzymes elevated synergistically. This effect is responsible for an 8.9-fold increase in serum Alanine Aminotransferase (ALT) and a 21-fold increase in serum Aspartate Transaminase (AST) [28].
Hepatitis C virus
When compared to non-drinkers, 1574 Hepatitis C Virus (HCV) patients with chronic liver disease who consumed more than 50g of alcohol a day had a higher risk of developing fibrosis [29]. HCV patients who drink alcohol are found to be 2-3 times more likely than non-drinkers to develop AUD [30].
Gastrointestinal Flora and their Useful Functions
The Gastro-Intestinal Tract (GIT) has 1014 microorganisms, which is approximately said to be equal to the total number of cells present in the body [31]. This microbial colony resides in the outer layer of the gut lumen of mucus and it is expected to have a total mass of 2kg. The microbiome contains 150 times more genes than the human genome [32]. It has been proved that these gut microflorae act as beneficial factors. They supply essential nutrients like vitamins. They also help in metabolizing indigestible food. They also act as a protective shield against the colonization of pathogens. They also actively help in enhancing immunity. As a result, they are also in charge of converting various metabolites that are likely to be absorbed into the bloodstream and eventually enter the brain and liver through cellular pathways [33].
However, there is a presence of physical barriers between systemic circulation and lumen that prevents penetration of bacteria along with the nutrients. The intestinal barrier is made of enterocytes, which are held by tight junctions and adherens junction. The enterocytes are covered by mucus layers which act as a physical barrier and protect epithelium against harmful agents and bacteria. This mucus barrier has two separate layers. The inner mucus layer is in direct contact with the epithelial cell and contains no bacterial colonies, while the outer mucus layer is easily washable and contains bacterial colonies [34].
The mucus lining of the intestine is made up of secreted and membrane-bound mucins, which are formed by goblet cells and are responsible for protecting the mucus layer from pathogens and preserving its viscosity. Reg3b and Reg3g, which are produced by regenerating islets, are also secreted by Paneth cells in the mucus layer. They are responsible for maintaining the intestinal homeostasis balance and also exert anti-microbial activity [35]. The intestine also shows the presence of lamina propria which plays an important role in providing immunity against the invading bacteria [36].
Intestinal Dysbiosis due to Alcohol
Intestinal dysbiosis is a disorder in which the microbial equilibrium in the gut is disrupted. Excessive alcohol consumption is a significant underlying factor in dysbiosis. Microorganisms play a significant role in an individual's innate immunity. But due to excess alcohol, there are various changes taking place into the gut and thereby are responsible for tissue inflammation. The mucus lining holds different microbial colonies, however, due to excess intake of alcohol, the mucus lining decreases, and thereby the tight junctions began to lose their integrity. Owing to hampered gut permeability, the microbial colonies can easily permeate into the portal circulation with their metabolic by-products. Accompanied with microbes, Peptidoglycan (PGN) and Lipopolysaccharides (LPS) are also leaked out. They result in the activation of various inflammatory chemicals namely IL-1, IL-6, and TNF-a (Figure 1). These chemicals get concentrated into the nearby tissue especially in the liver and result in inflammation [37]. The altered permeability of the gut can be determined using permeability enhancers like mannitol, PEG, and 51Cr-EDTA. Keshavarzian et al. found that individuals that were consuming higher amounts of alcohol were subjected to higher levels of above permeability enhancers in urine [38].
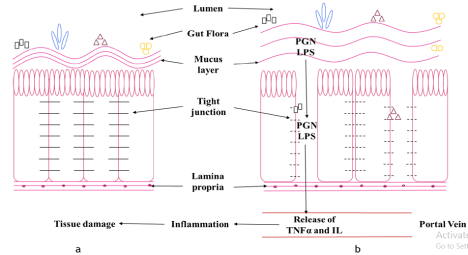
Figure 1: (a) Normal and (b) Altered intestinal functioning.
The above Figure 1 is representative of normal and altered intestinal functioning when a person is consuming a high amount of alcohol. Figure 1a, shows the
presence of normal functioning that is in absence of alcohol. It consists of the outermost layer of the lumen. It shows the presence of a mucus layer to which
different microbial gut floras are attached. The intestinal epithelial cells are held together with the help of tight junctions. The base consists of lamina propria which
is responsible for providing immunity. Due to the presence of a thick mucus membrane and tight junctions, the gut flora is unable to permeate through the cell and
thereby helping the gut in the various processes like digestion. Figure 1b, shows altered intestinal functioning due to excess alcohol. Due to excess alcohol intake,
the viscosity of the mucus layer decreases, and also there is a loss of integrity functioning of the tight junction. These results in the permeation of gut microbiota into
the portal vein. Accompanied with them, metabolic by-products, LPS, and PGN are also permeated. The presence of LPS and PGN is responsible for the activation
of TNFa and IL which are inflammatory markers. They get accumulated in the nearby tissue and resulting in tissue inflammation.
Mutlu et al. have found that the bacterial composition of the gut is highly affected by high alcohol intake. They made use of the Tsukamoto-French model. The rats where administered ethanol intra-gastrically for three weeks. This resulted in the development of steatosis coupled with inflammation and mild fibrosis [39]. Yan et al. have used a mouse model concluded that there were changes in the bacterial composition of the gut. They found that there was a decrease in Firmicutes whereas Bacteroidetes were found to increase. Also, it was reported that there was a decrease in beneficial bacteria like Lactobacillus, Pediococcus, Leuconostoc, and Lactococcus as compared to a control group [40]. Grander et al have found that there was the suppression of a good bacteria Akkermansia muciniphila which uses mucin from the gut as a carbon and nitrogen source. This bacteria helps in promoting the thickening of the mucus and also helps in maintaining the barrier functioning of the gut [41]. The gut also consists of commensal fungi names Mycobiome. Also, other bacterial species like Candida spp., Saccharomyces cerevisiae, and Malassezia spp. Due to their dysbiosis, they are also responsible for organ damage but their mechanism is unknown [42].
Alcoholic Liver Disease
Alcoholic Liver Disease (ALD) involves a spectrum of diseases right from steatosis to Hepatocellular Carcinoma (HCC). When an individual consumes high amounts of alcohol, he has a greater potential of developing ALD. It was found that 35% of heavy drinkers tend to develop ALD [43]. The spectrum of ALD includes steatosis, steatohepatitis, fibrosis, cirrhosis, and HCC [26].
Steatosis
90% of heavy drinkers develop steatosis due to the deposition of fats into hepatocytes. This deposition can be macrovesicular or microvesicular. Macrovesicular is due to the deposition of a large fat droplet into a hepatocyte whereas Microvesicular is due to deposition of many small fat droplets into a hepatocyte [44]. Due to steatosis, various enzyme-like AST, ALT, and γ-glutaryl transferase are also found to be elevated [45].
Mechanism of alcohol-induced Steatosis: Ethanol is converted into acetaldehyde by the action of ADH. Acetaldehyde increases the activity of the anterior cingulate cortex (ACC) which is responsible for increased alcohol consumption [46]. Ethanol acts on the adipose tissue and thereby decreases the level of Adiponectin. This decrease results in increased activation of TNF-a and a decrease in PPAR-a. Adiponectin and PPAR-a are responsible for β-oxidation of fatty acids. Decreased levels of Adiponectin and PPAR-a result in a decrease in β-oxidation. This decrease coupled with increase ACC activity results in elevation of fat synthesis and increased fat accumulation into the liver giving rise to steatosis (Figure 2).
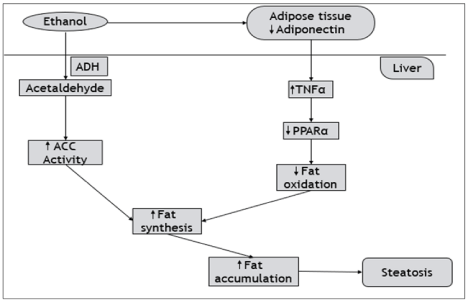
Figure 2: Schematic illustration of Alcohol-induced steatosis.
Figure 2 represents the schematic of how the excess of alcohol is responsible for inducing steatosis. Due to the high intake of alcohol, it acts on adipose tissue and
thereby decreases the levels of Adiponectin. In the liver, there are increased TNF-a and decreased PPAR-a levels. They are responsible for β-oxidation of fatty
acid. O the other hand, ethanol is converted into acetaldehyde by the action of ADH and acts on ACC which is responsible for alcohol consumption pattern. Due
to increased ACC and decreased β-oxidation of fatty acid, there is increased fat synthesis which results in a high amount of fat deposition into the liver causing
steatosis.
Steatohepatitis and fibrosis
It is a condition characterized by fatty liver coupled with inflammation in the liver. Not all individuals suffering from steatosis develop steatohepatitis. Only 20-40% of the population experiences steatohepatitis [47]. Because of the reduced-oxidation of fatty acids caused by ethanol intake, fats accumulate in the liver, resulting in steatosis. Furthermore, ROS and free radicals are generated. As a result of their action on lipids, MDA and 4-hydroxy nonenal are generated as by-products. They are in charge of growing NF-κB counts and activating stellate cells. NF-κB cells are effective at releasing cytokines, while stellate cells increase the number of neutrophils in the body, which causes liver inflammation. When this inflammation becomes more serious, it leads to fibrosis of the living tissue [48].
Cirrhosis
Cirrhosis occurs in just 8-20 % of people with steatohepatitis. When fibrosis has progressed to a greater degree, hepatocytes die, resulting in cirrhosis of the liver. The cirrhotic liver is characterized by vascular narrowing, ascites, and esophageal varices [49].
Hepatocellular carcinoma
Ethanol is converted into acetaldehyde by the action of ADH. This acetaldehyde is further metabolized by ALDH into acetate. It was found that acetaldehyde produced is a procarcinogen. Thereby, individuals with less level of ALDH enzymes are more prone to Hepatocellular Carcinoma (HCC). When ethanol is converted into acetaldehyde by the action of ADH, it results in the activation of the CYP2E1 enzyme. CYP2E1 metabolizes ethanol to hydroxyethyl radical. This radical acts on the fats and causes lipid peroxidation. It produces 4-hydroxy nonenal as a byproduct which has a good binding affinity to purines and pyrimidines. On binding, it gets bound to DNA to form exocyclic etheno-DNA adducts and results in uncontrolled cell proliferation which ultimately results in HCC [22].
Microbial changes in the liver
Non-Alcoholic Fatty Liver Disease (NAFLD) and NASH have a global prevalence of 25% of liver diseases and important causes of liver transplant [50]. There are two hit mechanisms. Primary hit involves various stages of liver damage. It consists of lipid abnormalities in the liver, accumulation in the liver, obesity, adipokine abnormalities, and insulin resistance. Secondary hit consists of oxidative stress leading to mitochondrial dysfunction, lipid peroxidation, retention of inflammatory cells, and cytokine-mediated recruitment [51]. The person with such conditions consumes alcohol was found to show increased firmicutes to bacteroidetes ratio which results in increased carbohydrate metabolism and increased alcohol concentration [52]. Also, when gut dysbiosis takes place in combination with NAFLD and NASH, it further damages the liver by increasing more gut permeability, oxidative stress, and increased lipogenesis [53].
Patients with ALD also suffer from gut dysbiosis. Thereby, causes various abnormal conditions. There was an increase in the Enterobacteriaceae family. They are responsible for the secretion of pro-inflammatory. Increased Enterobacteriaceae results in increased release of the pro-inflammatory and damaging liver via increased production of TNF-a and IL-1β [54]. Moreover, reduction in Lactobacillus, Bacteroides (good bacteria of the gut), and decreased Lachnospiracea and Ruminococcaceae levels are responsible for the synthesis of Short-Chain Fatty Acids (SCFA). Decreased levels result in decreased synthesis of SCFA and thereby poor gut barrier functioning (Figure 3) [40,55,56]. Dysbiosis in cirrhotic patients due to high alcohol consumption further worsens the liver condition [57]. It was found that the oral microflora was found in the feces. It was due to transformation in salivary microbiota, low levels of gastric acid, and periodontitis [58].
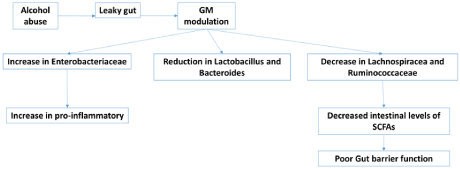
Figure 3: Alcohol induced gut dysbiosis.
Binge alcohol intake alters gut microbiota and increase Enterobacteriaceae family’s bacteria which is responsible for the secretion of pro-inflammatory cytokines like
TNF-a and IL-1β. Moreover, reduction in Lactobacillus, Bacteroides (good bacteria of the gut), and decreased Lachnospiracea and Ruminococcaceae levels are
responsible for the synthesis of Short-Chain Fatty Acids (SCFA). Decreased levels result in decreased synthesis of SCFA and thereby poor gut barrier functioning.
Chronic HCV is progressive liver fibrosis and cirrhosis. It is developed by 20%-30% of untreated fibrosis and cirrhosis patients after 20 to 30 years. Out of this, 1% to 4% develops HCC [59]. It was found that there was an increase in Prevotella and Faecalibacterium whereas the decrease in Acinetobacter, Veillonella and Phascolarctobacterium [60]. During the end-stage ALD that is HCC, due to leaky gut, endotoxemia, and immuno-modulation, this condition further gets complexed [61]. It was reported that there is an increase in E. coli and LPS serum levels whereas reduced levels of Lactobacillus, Bifidobacterium, and Enterococcus were also reported [62]. Hepatic Encephalopathy (HE) is an end-stage liver disease that occurs mostly due to higher levels of ammonia, amino acid metabolites like indoles, oxindoles, and endotoxins. The levels of Klebsiella and Proteus were also found to be increased [63].
Treatment
Treatment can be in three ways that are using the non-dietary approach, using a dietary approach, or by microbiota modulation in the gut.
Non-Dietary approach
In this approach, diet is not altered. Patients are given a normal diet. It consists of administering disaccharides to the patient [64]. Disaccharides include lactulose and lactitol which act by inducing the cathartic effect, reducing intestinal transit time, reducing the content of toxic compounds, and reducing levels of ammonia. Lactulose is partially metabolized by the gut microbiota and results in the production of acetate and lactate. This causes a reduction in pH inducing an acidic atmosphere into the gut. This results in the conversion of ammonia to ammonium and thereby reduces ammonia absorption into the body [65]. Bothe et al reported that lactulose shows a dose-dependent effect. In the study conducted by his group, 4g per day administration of lactulose was found to have a positive effect for increasing lactate production and decrease ammonia production [66].
Dietary approach
Diet plays an important role; thereby modulating diet can be beneficial in treating dysbiosis. Therefore, a proper diet helps in the modulation of nitrogen metabolism those results in interrupting chains of reactions. A proposed diet includes appropriate protein intake, increased fiber intake, and the use of foods with prebiotic and probiotic effects [67]. The dietary approach can be implemented by the administration of protein, branched-chain amino acids, l-ornithine-l-aspartate, vitamin, and micro-nutrient.
Protein
Protein intake is extremely important in patients with gut and liver conditions. As the catabolism of protein is responsible for the increased production of ammonia. The patient should also avoid fasting for longer than 3-6 hr. It is reported that 50g of carbohydrate in evening snacks can help in substrate utilization and nitrogen production [68]. An appropriate protein intake should consist of 1.2- 1.5 g per kg per day. Vegetable protein should be preferred which helps in ammonia detoxification and has high fiber content which will help in reducing the transit time in the gut [69].
Branched-chain amino acid
Branched-Chain Amino Acids (BCAA) is used to prevent excessive protein catabolism and to reduce ammonia levels in patients. Commonly used BCAA are valine, leucine, and isoleucine which are indeed essential amino acids [70]. These essential amino acids are required for the amidation of glutamine and ammonia detoxification. In cirrhosis patients, there are decreased BCAA levels, and this affects ammonia detoxification. Therefore, administering BCAA helps in increasing ammonia detoxification and helps in liver repairment [71].
L-ornithine-L-Aspartate
L-Ornithine-L-Aspartate (LOLA) is a mixture of 2 endogenous amino acids. It is capable of fixing ammonia in the form of urea or glutamine [69]. It also activates glutamine production and promotes liver regeneration. Also, LOLA takes part in the urea cycle which results in increased ammonia detoxification [64].
Vitamin
Vitamin deficiencies are seen with a patient suffering from Cirrhosis/HE. It is due to malabsorption and altered hepatic functioning. It also affects cognitive function. Such patients are administered with vitamin B complex [64].
Zinc
Cirrhosis patients suffer from reduced levels of micronutrients, out of which zinc levels have decreased to a drastic level. Glutamine synthetase and ornithine transcarbamylase are responsible for ammonia detoxification and zinc-dependent enzyme. Therefore, a zinc-rich diet and supplements can provide beneficial effects [72].
Microbiota modulation
As such, microbiota plays a vital role in healthy individuals. Dysbiosis results in inflammation and liver disorder. Therefore, microbiota modulation in the gut will help in controlling the inflammation of the gut as well as in the liver [73]. It can be achieved by prebiotic, probiotic, probiotic yogurt, and fecal matter transplant.
Prebiotic and probiotic
Prebiotic is the food substrate that selectively is used by the host micro-organism. They result in the alteration in the composition of the gut microbiota and thereby affecting their activity. Probiotics are live micro-organism that is to be ingested in adequate amount. They help in increasing the count of good bacteria. Synbiotic refers to administering the combination of prebiotic and probiotics [73]. They help in reducing the pH of the gut by reducing the pathogenic bacteria and thereby beneficial in reducing ammonia. Also, they reduce gut dysbiosis and inflammation [74].
Prebiotic include lactulose, lactitol, fructooligosaccharides, and galactooligosaccharides. It was found that combinations of probiotics and fructooligosaccharides were effective in treating Minimal Hepatic Encephalopathy (MHE). It also helps in improving neuropsychiatric function when compared both to placebo and lactulose [75]. Synbiotic preparation of probiotics and the aforesaid fermentable fibers would reverse the conditions of MHE in 50% of patients. It was found that fermentable fibers alone capable to be benefit [76]. Dalal et al. have compared prebiotic and probiotic. They found that probiotics helped in improving the recovery time. Also, there were decreased plasma ammonia concentrations with no reported mortality [77,78].
Probiotic yogurt
Bajaj et al. have studied the effect of probiotic yogurt in cirrhosis patients. A randomized trial was conducted for 60 days. Individuals were administered 350g per day of prebiotic yogurt for 60 days. It was found to have reversed MHE and useful in reducing the levels of E. coli [79].
Fecal matter transfer
Fecal Matter Transfer (FMT) is a process of transplantation of fecal material, which contains bacteria from a healthy individual, to a recipient. It is an effective approach for the treatment of microbiota dysbiosis and ulcerative colitis. It acts by establishing strains of beneficial bacteria and aids in the production of antimicrobial components [80]. Ferrere et al. have conducted a study on FMT using mice. They took feces from healthy alcohol-resistant mice and it was transplanted to alcohol-sensitive mice. This process was repeated thrice a week for three weeks. It was found that FMT had prevented dysbiosis of microbiota and hepatic steatohepatitis [81].
Conclusion and Future Perspective
Microbiota dysbiosis is caused by excessive alcohol consumption which further alters membrane permeability. Steatosis, steatohepatitis, fibrosis, cirrhosis, and HCC are all diseases associated with ALD. Non-dietary, dietary approach and/or microbiota modulation in the gut can be a good strategy in controlling dysbiosis and further complications.
Acknowledgment
Authors are grateful to SVKM’S NMIMS University, Mumbai, India for providing the necessary facilities for writing this review article. Nikita Patil Samant's contribution in the formatting of the manuscript is also acknowledged.
References
- Carvalho AF, Heilig M, Perez A, Probst C, Rehm J. Alcohol use disorders. Lancet. 2019; 394: 781-792.
- Hammer JH, Parent MC, Spiker DA. World Health Organization. Global status report on alcohol and health. 2018; 65.
- Rehm J, Lachenmeier DW, Llopis EJ, Imtiaz S, Anderson P. Evidence of reducing ethanol content in beverages to reduce harmful use of alcohol. Lancet Gastroenterol Hepatol. 2016; 1: 78-83.
- Schwarzinger M, Thiébaut SP, Baillot S, Mallet V, Rehm J. Alcohol use disorders and associated chronic disease - A national retrospective cohort study from France. BMC Public Health. 2017; 18: 1-9.
- Westman J, Wahlbeck K, Laursen TM, Gissler M, Nordentoft M, Hällgren J, et al. Mortality and life expectancy of people with alcohol use disorder in Denmark, Finland and Sweden. Acta Psychiatr Scand. 2015; 131: 297-306.
- Probst C, Roerecke M, Behrendt S, Rehm J. Gender differences in socioeconomic inequality of alcohol-attributable mortality: A systematic review and meta-analysis. Drug Alcohol Rev. 2015; 34: 267-277.
- Savolainen V, Perola M, Lalu K, Penttilä A, Virtanen I, Karhunen PJ. Early perivenular fibrogenesis - precirrhotic lesions among moderate alcohol consumers and chronic alcoholics. J Hepatol. 1995; 23: 524-531.
- Nolen-Hoeksema S. Gender differences in risk factors and consequences for alcohol use and problems. Clin Psychol Rev. 2004; 24: 981-1010.
- Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017; 74: 911-923.
- Poynard1 T, Mathurin P, Lai C-L, Guyader D, Poupon R, Tainturier M-H, et al. A comparison of fibrosis progression in chronic liver diseases Thierry. J Hepatol. 2003; 38: 257-265.
- Seitz HK, Egerer G, Simanowski UA, Waldherr R, Eckey R, Agarwal DP, et al. Human gastric alcohol dehydrogenase activity: Effect of age, sex, and alcoholism. Gut. 1993; 34: 1433-1437.
- Colantoni A, Emanuele MA, Kovacs EJ, Villa E, Van Thiel DH. Hepatic estrogen receptors and alcohol intake. Mol Cell Endocrinol. 2002; 193: 101- 104.
- Yin M, Ikejima K, Wheeler MD, Bradford BU, Seabra V, Forman DT, et al. Estrogen is involved in early alcohol-induced liver injury in a rat enteral feeding model. Hepatology. 2000; 31: 117-123.
- Donohue TM, Curry-McCoy TV, Nanji AA, Kharbanda KK, Osna NA, Radio SJ, et al. Lysosomal leakage and lack of adaptation of hepatoprotective enzyme contribute to enhanced susceptibility to ethanol-induced liver injury in female rats. Alcohol Clin Exp Res. 2007; 31: 1944-1952.
- Tadic SD, Elm MS, Li HS, Van Londen GJ, Subbotin VM, Whitcomb DC, et al. Sex differences in hepatic gene expression in a rat model of ethanol-induced liver injury. J Appl Physiol. 2002; 93: 1057-1068.
- Vatsalya V, Song M, Schwandt ML, Cave MC, Barve SS, George DT, et al. Effects of Sex, Drinking History, and Omega-3 and Omega-6 Fatty Acids Dysregulation on the Onset of Liver Injury in Very Heavy Drinking Alcohol- Dependent Patients. Alcohol Clin Exp Res. 2016; 40: 2085-2093.
- Kerr WC, Mulia N, Zemore SE. US Trends in Light, Moderate, and Heavy Drinking Episodes from 2000 to 2010. Alcohol Clin Exp Res. 2014; 38: 2496- 2501.
- Llerena S, Arias-Loste MT, Puente A, Cabezas J, Crespo J, Fábrega E. Binge drinking: Burden of liver disease and beyond. World J Hepatol. 2015; 7: 2703- 2715.
- Chassin L, Pitts SC, Prost J. Binge drinking trajectories from adolescence to emerging adulthood in a high-risk sample: Predictors and substance abuse outcomes. J Consult Clin Psychol. 2002; 70: 67-78.
- Satta R, Hilderbrand ER, Lasek AW. Ovarian Hormones Contribute to High Levels of Binge-Like Drinking by Female Mice. Alcohol Clin Exp Res. 2018; 42: 286-294.
- Stinson FS, Grant BF, Dufour MC. The critical dimension of ethnicity in liver cirrhosis mortality statistics. Alcohol Clin Exp Res. 2001; 25: 1181-1187.
- Jin S, Chen J, Chen L, Histen G, Lin Z, Gross S, et al. ALDH2(E487K) mutation increases protein turnover and promotes murine hepatocarcinogenesis. Proc Natl Acad Sci USA. 2015; 112: 9088-9093.
- Tian C, Stokowski RP, Kershenobich D, Ballinger DG, Hinds DA. Variant in PNPLA3 is associated with alcoholic liver disease. Nat Genet. 2010; 42: 21- 23.
- Buch S, Stickel F, Trépo E, Way M, Herrmann A, Nischalke HD, et al. A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nat Genet. 2015; 47: 1443-1448.
- Abul-Husn NS, Cheng X, Li AH, Xin Y, Schurmann C, Stevis P, et al. A protein-truncating HSD17B13 variant and protection from chronic liver disease. N Engl J Med. 2018; 378: 1096-1106.
- Gao B, Bataller R. Alcoholic liver disease: Pathogenesis and new therapeutic targets. Gastroenterology. 2011; 141: 1572-1585.
- Stepanova M, Rafiq N, Younossi ZM. Components of metabolic syndrome are independent predictors of mortality in patients with chronic liver disease: A population-based study. Gut. 2010; 59: 1410-1415.
- Loomba R, Bettencourt R, Barrett-Connor E. Synergistic association between alcohol intake and body mass index with serum alanine and aspartate aminotransferase levels in older adults: The Rancho Bernardo Study. Aliment Pharmacol Ther. 2009; 30: 1137-1149.
- Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet (London, England). 1997; 349: 825-832.
- Iida-Ueno A, Enomoto M, Tamori A, Kawada N. Hepatitis B virus infection and alcohol consumption. World J Gastroenterol. 2017; 23: 2651-2659.
- Sender R, Fuchs S, Milo R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell. 2016; 164: 337-340.
- Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010; 464: 59-65.
- Stärkel P, Leclercq S, de Timary P, Schnabl B. Intestinal dysbiosis and permeability: The yin and yang in alcohol dependence and alcoholic liver disease. Clin Sci. 2018; 132: 199-212.
- Matsuo K, Akamatsu T, Katsuyama T. Histochemistry of the surface. Technology. 1997; 40: 782-789.
- Dessein R, Gironella M, Vignal C, Peyrin-Biroulet L, Sokol H, Secher T, et al. Toll-like receptor 2 is critical for induction of Reg3b expression and intestinal clearance of Yersinia pseudotuberculosis. Gut. 2009; 58: 771-776.
- Gallo RL, Hooper LV. Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol. 2012; 12: 503-516.
- Leclercq S, Cani PD, Neyrinck AM, Stärkel P, Jamar F, Mikolajczak M, et al. Role of intestinal permeability and inflammation in the biological and behavioral control of alcohol-dependent subjects. Brain Behav Immun. 2012; 26: 911-918.
- Keshavarzian A, Holmes EW, Patel M, Iber F, Fields JZ, Pethkar S. Leaky gut in alcoholic cirrhosis: A possible mechanism for alcohol- induced liver damage. Am J Gastroenterol. 1999; 94: 200-207.
- Mutlu E, Keshavarzian A, Engen P, Forsyth CB, Sikaroodi M, Gillevet P. Intestinal dysbiosis: A possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcohol Clin Exp Res. 2009; 33: 1836- 1846.
- Yan AW, Fouts DE, Brandl J, Stärkel P, Torralba M, Schott E, et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011; 53: 96-105.
- Grander C, Adolph TE, Wieser V, Lowe P, Wrzosek L, Gyongyosi B, et al. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut. 2018; 67: 892-902.
- Wang ZK, Yang YS, Stefka AT, Sun G, Peng LH. Review article: Fungal microbiota and digestive diseases. Aliment Pharmacol Ther. 2014; 39: 751- 766.
- Ohashi K, Pimienta M, Seki E. Alcoholic liver disease: A current molecular and clinical perspective. Liver Res. 2018; 2: 161-172.
- You M, Arteel GE. Effect of ethanol on lipid metabolism. J Hepatol. 2019; 70: 237-248.
- Yeh MM, Brunt EM. Pathological features of fatty liver disease. Gastroenterology. 2014; 147: 754-764.
- Rasineni K, Casey CA. Molecular mechanism of alcoholic fatty liver. Indian J Pharmacol. 2012; 44: 299-303.
- Perumpail BJ, Khan MA, Yoo ER, Cholankeril G, Kim D, Ahmed A, et al. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. 2017; 23: 8263-8276.
- DAY CP, JAMES OFW. Steatohepatitis: A Tale of Two ‘“Hits”’? Gastroenterology. 1995; 114: 842-845.
- Seitz HK, Bataller R, Cortez-pinto H, Gao B, Gual A, Lackner C, et al. Alcoholic liver disease. Nat Rev Dis Prim. 2018; 4: 1-22.
- Younossi ZM, Marchesini G, Pinto-Cortez H PS. Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis: Implications for Liver Transplantation. Transplantation. 2019; 103: 22-27.
- Géraldine Gentric, Vanessa Maillet, Paradis V, L’Hermitte DCA, Panasyuk G, Fromenty B, et al. Oxidative stress promotes pathologic polyploidization in nonalcoholic fatty liver disease. J Clin Invest. 2015; 125: 981-992.
- Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology. 2013; 57: 601-609.
- Poeta M, Pierri L, Vajro P. Gut-Liver Axis Derangement in Non-Alcoholic Fatty Liver Disease. Children. 2017; 4: 66.
- Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B, et al. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. 2018; 15: 397-411.
- Mutlu EA, Gillevet PM, Rangwala H, Sikaroodi M, Naqvi A, Engen PA, et al. Colonic microbiome is altered in alcoholism. Am J Physiol - Gastrointest Liver Physiol. 2012; 302: 966-978.
- Bajaj JS, Kakiyama G, Zhao D, Takei H, Fagan A, Hylemon P, et al. Continued Alcohol Misuse in Human Cirrhosis is Associated with an Impaired Gut–Liver Axis. Alcohol Clin Exp Res. 2017; 41: 1857-1865.
- Vlachogiannakos J, Viazis N, Vasianopoulou P, Vafiadis I, Karamanolis DG, Ladas SD. hepatic encephalopathy hepatorenal syndrome rifaximin spontaneous bacterial peritonitis variceal bleeding. J Gastroenterol Hepatol. 2013; 28: 450-455.
- Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, et al. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014; 513: 59-64.
- Lee MH, Yang HI, Yuan Y, L’Italien G, Chen CJ. Epidemiology and natural history of hepatitis C virus infection. World J Gastroenterol. 2014; 20: 9270- 9280.
- Aly AM, Adel A, El-Gendy AO, Essam TM, Aziz RK. Gut microbiome alterations in patients with stage 4 hepatitis C. Gut Pathog. 2016; 8: 1-12.
- Wan MLY, El-Nezami H. Targeting gut microbiota in hepatocellular carcinoma: probiotics as a novel therapy. HepatoBiliary Surg Nutr. 2018; 7: 11-20.
- Grat M, Wronka KM, Krasnodebski M, Masior, Lewandowski Z, Kosinska I, et al. Profile of Gut Microbiota Associated With the Presence of Hepatocellular Cancer in Patients With Liver Cirrhosis. Transplant Proc. 2016; 48: 1687- 1691.
- Häussinger D, Schliess F. Pathogenetic mechanisms of hepatic encephalopathy. Gut. 2008; 57: 1156-1165.
- Campion D, Giovo I, Ponzo P, Saracco GM, Balzola F, Alessandria C. Dietary approach and gut microbiota modulation for chronic hepatic encephalopathy in cirrhosis. World J Hepatol. 2019; 11: 489-512.
- Van Leeuwen PA, CL VB, PB S. New mode of action for lactulose. Lancet. 1988; 331: 55-56.
- Bothe MK, Maathuis AJH, Bellmann S, van der Vossen JMBM, Berressem D, Koehler A, et al. Dose-dependent prebiotic effect of lactulose in a computercontrolled in vitro model of the human large intestine. Nutrients. 2017; 9.
- Merli M, Iebba V, Giusto M. What is new about diet in hepatic encephalopathy? Metab Brain Dis. 2016; 31: 1289-1294.
- Pearlman M, Akpotaire O. Diet and the Role of Food in Common Gastrointestinal Diseases. Med Clin North Am. 2019; 103: 101-110.
- Hirschfield GM, Beuers U, Corpechot C, Invernizzi P, Jones D, Marzioni M, et al. Clinical Practice Guidelines EASL Clinical Practice Guidelines : The diagnosis and management of patients with primary biliary cholangitis q Clinical Practice Guidelines. J Hepatol. 2017; 67: 145-172.
- Fischer JE, Baldessarini RJ. False Neurotransmitters and Hepatic Failure. Lancet. 1971; 298: 75-80.
- Kawaguchi T, Taniguchi E, Sata M. Effects of oral branched-chain amino acids on hepatic encephalopathy and outcome in patients with liver cirrhosis. Nutr Clin Pract. 2013; 28: 580-588.
- Chavez-Tapia NC, Cesar-Arce A, Barrientos-Gutiérrez T, Villegas-López FA, Méndez-Sanchez N, Uribe M. A systematic review and meta-analysis of the use of oral zinc in the treatment of hepatic encephalopathy. Nutr J. 2013; 12: 1-6.
- Tsai YL, Lin TL, Chang CJ, Wu TR, Lai WF, Lu CC, et al. Probiotics, prebiotics and amelioration of diseases. J Biomed Sci. 2019; 26: 1-8.
- Liu Q, Duan ZP, Ha DK, Bengmark S, Kurtovic J, Riordan SM. Synbiotic Modulation of Gut Flora: Effect on Minimal Hepatic Encephalopathy in Patients with Cirrhosis. Hepatology. 2004; 39: 1441-1449.
- Malaguarnera M, Gargante MP, Malaguarnera G, Salmeri M, Mastrojeni S, Rampello L, et al. Bifidobacterium combined with fructo-oligosaccharide versus lactulose in the treatment of patients with hepatic encephalopathy. Eur J Gastroenterol Hepatol. 2010; 22: 199-206.
- Liu X, Cao S, Zhang X. Modulation of Gut Microbiota-Brain Axis by Probiotics, Prebiotics, and Diet. J Agric Food Chem. 2015; 63: 7885-7895.
- Dalal R, Rg M, Sm R, Ac W. Probiotics for people with hepatic encephalopathy (Review) Summary Of Findings For The Main Comparison. Cochrane Database Syst Rev. 2017: 1-109.
- Agrawal A, Sharma BC, Sharma P, Sarin SK. Secondary prophylaxis of hepatic encephalopathy in cirrhosis: An open-label, randomized controlled trial of lactulose, probiotics, and no therapy. Am J Gastroenterol. 2012; 107: 1043-1050.
- Bajaj JS, Saeian K, Christensen KM, Hafeezullah M, Varma RR, Franco J, et al. Probiotic yogurt for the treatment of minimal hepatic encephalopathy. Am J Gastroenterol. 2008; 103: 1707-1715.
- Borody TJ, Campbell J. Fecal Microbiota Transplantation. Gastroenterol Clin NA. 2012; 41: 781-803.
- Ferrere G, Wrzosek L, Cailleux F, Turpin W, Spatz M, Ciocan D, et al. Fecal microbiota manipulation prevents dysbiosis and alcohol-induced liver injury in mice. J Hepatol. 2016; 66: 806-815.