
Research Article
Phys Med Rehabil Int. 2023; 10(1): 1208.
Vibroacoustic Therapy in Parkinsonian Patients. A Complementary Approach to Improve Rehabilitation Outcome
Dolciotti C1, Della Ragione R2, Gabrielli G3, Stampacchia G4 and Bongioanni P4*
1Spinal Cord Injuries Section, Azienda Ospedaliero-Universitaria Pisana, Italy
2Neuro Care Livorno onlus, Italy
3Neuro Care onlus, Italy
4Spinal Cord Injuries Section, Azienda Ospedaliero-Universitaria Pisana, Italy
*Corresponding author: Bongioanni P Spinal Cord Injuries Section, Azienda Ospedaliero-Universitaria Pisana, Via Paradisa, 2 - 56124 - Pisa, Italy
Received: December 30, 2022; Accepted: January 25, 2023; Published: January 31, 2023
Abstract
Background: Parkinson’s Disease (PD) is a chronic progressive neurodegenerative disorder with motor and non-motor symptoms. Rehabilitation represents an adjuvant intervention to pharmacological and surgical therapy. Our study aim was to investigate the effectiveness of the Vibroacoustic Therapy (VAT) according to the Metodo Magic Music combined with conventional Training (Tr) versus Tr alone in improving motor symptoms and postural control in PD patients.
Methods: Twenty-four patients with idiopathic PD were enrolled. They were randomly assigned to the Tr-VAT or the Tr group. Total intervention time was 40 min (Tr) and 60 min (Tr-VAT) 3 times a week for 3 months. Both groups carried out at baseline and follow-up clininetric scales and tests to assess clinical outcome. Speed of postural transitions and gait parameters (endurance and comfortable and fast speed) have been measured.
Results: Follow-up evaluation showed in PD patients from the Tr-VAT group as compared to those from the Tr group an improvement of postural control assessed with Berg Balance Scale (p = 0.054) and Timed-Up and Go test (TUG) test (p = 0.058). Moreover, significant (p < 0.05) differences between groups were found for comfortable gait speed (p = 0.004) and speed of postural changes - from right side to supine (p = 0.03), from supine to right and left side (p = 0.01), from supine to prone (p = 0.002) and supine to sitting (p = 0.005), respectively.
Conclusions: Our study demonstrated the effectiveness of VAT as complementary treatment with conventional rehabilitation approaches, by improving mobility, agility and motor flexibility in PD patients.
Keywords: Parkinson’s Disease; Rehabilitation; Complementary Treatment; Vibroacoustic Therapy; Training; Metodo Magic Music
Abbreviations: 3-MWT: 3-min Walking Test; ABI: Acoustic-Based Interventions; BBS: Berg Balance Scale; BT: Balance Training; CGS: Comfortable Gait Speed test; FGS: Fast Gait Speed test; H&Y: Hoehn &Yahr; L.E.D.: L-DOPA Equivalent Dose; M.M.M.: Metodo Magic Music; PD: Parkinson’s Disease; QoL: Quality of Life; RAS: Rhytmic Auditory Stimulation; Tr: Training; TUG: Timed-Up and Go test; TW: Treadmill Walking; UPDRS-III: Unified Parkinson’s Disease Rating Scale - Section III; VAT: Vibroacoustic Therapy; WBV: Whole Body Vibration
Introduction
Parkinson’s Disease (PD) is a chronic progressive neurodegenerative disorder, first described by James Parkinson in 1817 as “shaking palsy”. It is accompanied by both motor and non-motor symptoms, due to loss of striatal dopaminergic and non-dopaminergic neurons [1]. PD symptoms include tremor, bradykinesia, rigidity, postural instability, freezing of gait and motor coordination dysfunction. The disease is characterized by non-motor symptoms too, such as hyposmia, sleep disorders, depression and/or anxiety, bowel dysfunction (frequently constipation), progressive cognitive decline and dementia. Patients often complain of other disturbances, such as early muscle fatigue and pain [2].
The “gold standard” pharmacologic PD treatment is represented by L-DOPA. Whereas dopaminergic medications can effectively manage motor symptoms, drug resistance and L-DOPA-induced side effects following long-term use are common problems [3]. L-DOPA introduction has changed PD management: however, it soon became apparent that pharmacological treatment offered mainly a symptomatic relief and poorly affected the underlying pathophysiology. On the other hand, chronic use of drugs was associated with several dose-dependent effects [3]. Current therapeutic management seeks to delay long-term complications of drug therapy for as long as possible, introducing also complementary interventions.
Rehabilitation
Research has suggested that a regular physical activity can also be added to drug therapy to limit disability and improve Quality of Life (QoL) of PD patients [4]. In the recent years, novel treatment approaches have demonstrated benefits for both motor and non-motor symptoms. Growing number of evidence suggests that endurance exercise (particularly, high-intensity exercise) modifies disease severity in de-novo patients with early onset or mild/moderate disease [5]. As intensive aerobic exercise, in the latest decade, treadmill training has been frequently used in rehabilitation protocols and described as improving gait parameters in PD patients: especially, clinical evidence shows that a personalized high-intensity Treadmill Walking (TW) program not only improves gait parameters, but also may favorably influence non-motor symptoms and QoL [6].
On the other hand, more recent combined rehabilitation protocols, integrating aerobic exercise with task-oriented circuit training and postural training, seem improving not only gait and balance performance, but also patients’ QoL, because of a positively translation into the functional daily ability [7,8].
Furthermore, one should not neglect also the positive impact of short-term and long-term effects provided by recent innovative intervention approaches, such as the application of Whole Body Vibration (WBV) [9] and the Acoustic-Based Interventions (ABI) implemented as Rhytmic Auditory Stimulation (RAS) or the Vibroacustic Therapy (VAT) [10].
Regarding WBV, a 2014 literature review [11] reported mixed results in its favour for improving postural stability: anyway, it mostly suggests a benefit in mobility and balance, but not significantly higher than the more conventional active intervention approaches. A recent survey shows that, despite the favorable WBV effects on mobility and postural stability in PD patients, only few studies have an appropriate methodological quality [9].
ABI may be effective in promoting not only wellness, but also in maintaining physical and cognitive health, as long as possible, in PD patients. In this regard, clinical evidence highlights the potential effect of music, as complementary treatment, in relieving non-motor symptoms, such as depression, anxiety, neuropathic pain and stress: advanced studies of brain imaging gave information regarding the mechanisms of brain response to music stimulation [12]. Furthermore, other studies provided evidence of a close relationship between auditory neurons, basal ganglia and cerebellar areas [13]. Currently, preliminary studies of ABI with RAS and VAT performed by patients with extra pyramidal motor symptoms provided interesting results, supported also by brain imaging findings and neurophysiological studies [10].
Vibroacoustic Therapy (VAT)
We are focusing more specifically on the VAT, because of its integration in our rehabilitative program, with a novel method and protocol, in comparison to previous clinical experiences. VAT is a treatment method using sinusoidal low-frequency sounds in 30-120 Hz range, complemented by music for therapeutic purposes [14]. The origins of VAT come from the studies of Skille: he used in an experimental protocol deep vibration in subjects suffering from various disabilities [15].
VAT exploits frequencies within the hearing range, but at a specific pitch where the vibrating effect of the tone can be felt as a sensed vibration in the body: any sound vibration works on the principle of sympathetic resonance, where an object has a resonant frequency at which it vibrates in sympathy with the sound [16]. Currently, VAT is indicated for use in spastic cerebral paralysis and in other disorders of the central nervous system, as it helps to enhance rehabilitation intervention and improve motor skills and brain functions, connectivity, through oscillatory coherence [17].
Given the effectiveness of intensive training and use of acoustic and sound vibrations as drug therapy-associated treatment in improving motor symptoms in PD patients, our main purpose was to test a complementary rehabilitation program, combining aerobic exercise and postural task with VAT. To this aim, we have studied changes of clinical outcomes overall, by evaluating gait performances, static and dynamic balance parameters and execution speed of postural transitions in PD patients receiving two different interventions (with and without VAT).
Patients and Methods
Study Design
The present study was randomized, observational-interventional and prospective. Patients were randomly sorted into two groups, the former as control one - referred as Training (Tr), and the latter one - referred as Training-VAT (Tr-VAT) group, depending on the type of intervention (Figure 1). Experimental design included two phases, the baseline (t0) for enrollment and randomization procedures, and the follow-up (t1), spaced by at least 3 months.
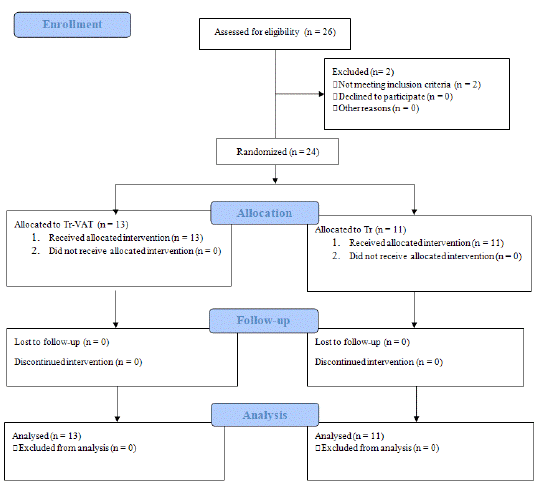
Figure 1: Study design.CONSORT 2010 Flow Diagram (Pseudo-randomized controlled parallel group trial)
Patients
Twenty-six PD patients were enrolled, after neurological assessment and neuropsychological screening. To meet inclusion criteria, a Mini-Mental State Examination score ≥24.3/30 and a mild diseases severity (corresponding to a modified Hoehn & Yahr (H&Y) classification stage ≤2.5) were required.
All patients signed an informed consent approved by the Ethics Committee of the Azienda Ospedaliero-Universitaria Pisana. All procedures of the present study were carried on according to the Helsinki Declaration and Guidelines of good clinical practice.
Population sample consisted of 19 men (aged 69.89±6.09 yrs) and 5 women (aged 61.8±8.63 yrs): two patients, after the randomization phase, declined to participate, due to inter current illness. Main aspects of clinical history, including H&Y stage, years of disease, clinical phenotype and pharmacologic treatment of the total sample, are shown in (Table 1).
Patient
Age
Gender
H&Y
Year of disease
Clinical phenotype
L.E.D. (mg)
Intervention
P 1
57
M
2.5
10
B, R, T
640
Tr-VAT
P 2
65
M
2
7
B, R
304
Tr-VAT
P 3
58
F
2.5
8
B, R
319
Tr-VAT
P 4
63
F
2
11
B, R, T
420
Tr-VAT
P 5
67
M
2.5
10
B, R
659
Tr-VAT
P 6
48
F
2
10
B, T
420
Tr-VAT
P 7
72
M
2
12
B, R
500
Tr-VAT
P 8
55
M
2.5
12
B, R, T
532
Tr-VAT
P 9
76
M
2.5
12
B, R
800
Tr-VAT
P 10
74
M
2.5
11
B, R
620
Tr-VAT
P 11
56
M
2
8
B, R
566
Tr-VAT
P 12
72
M
2.5
13
B, R
800
Tr-VAT
P 13
74
F
2.5
13
B, R, T
732
Tr-VAT
P 14
69
M
1.5
6
B, R
666
Tr
P 15
79
M
2,5
13
B, R
900
Tr
P 16
76
M
2.5
14
B, R
700
Tr
P 17
74
M
2.5
11
B, R
739
Tr
P 18
74
M
1,5
7
B, R
340
Tr
P 19
73
M
2.5
11
B, R
840
Tr
P 20
76
M
2.5
12
B, R
520
Tr
P 21
69
M
2
6
B, R
541
Tr
P 22
66
F
2
5
R
421
Tr
P 23
69
M
2.5
12
B, R
760
Tr
P 24
65
M
2
7
B, R
499
Tr
B: Bradikynesia; R: Rigidity; T: Tremor; COMT: Catechol-O-Methyl-Transferasee; R: Rasagiline; Tr-VAT: Training-Vibroacustic Therapy; Tr: Training; L.E.D.: L-Dopa Equivalent Dose.
Table 1: Population description.
Methods
Interventions: After randomization, 13 patients (9 men and 4 women, aged 65.13 ± 8.18 yrs, with a disease duration of 10.53 ± 1.86 yrs, H&Y stage of 2.30 ± 0.24 and L-DOPA equivalent dose (L.E.D.) of 562.46 ± 159.75 mg) were allocated in the Tr-VAT group; and 11 patients (10 men and 1 woman, aged 71.81 ± 4.28 yrs, with a disease duration of 9.45 ± 3.11 yrs, H&Y stage of 2.18 ± 0.38 and L.E.D. of 629.64 ± 169.74 mg) in the Tr group. Both groups performed 36 sessions for 12 weeks. All patients were trained always in the ON-phase, 60 min after having taken dopaminergic drugs.
First intervention type: Training (Tr): The Tr protocol (3 times a week) consisted in 45-min sessions of Balance Training (BT) followed by TW.
Fifteen-min BT sessions included a set of 20balance tasks (10 performed on hard and 10 on soft surface): 81-min stepping exercises, intervalled by a 5-s rest time in bipedal stance; 12 10-s exercises performed in tandem standing, alternatively with both legs, intervalled by a 5-s rest time in unipedal stance. In order to correct trunk rotation, patients performed postural task in front of a mirror as visual feedback.
Regarding the 30-min TW sessions, a treadmill TOORX EVO was used, with a progressive speed calibrated between 1.4 and 2.5 km/h, regulating the cadence by auditory cueing (provided by a metronome).
Second intervention type: Training-Vibroacoustic Therapy (Tr-VAT): Patients underwent Tr and VAT provided by AcusticA®, a specifically built device, a wooden chaise longue, according to the so-called “Metodo Magic Music”(M.M.M.) based on the patent “Harmonic Vibro-Massage Unit” [18]. (Figure 2) shows a picture of AcusticA® equipped with a CD player, functioning as a resonance chamber (like a musical instrument stimulating the whole body according to the sound frequencies from musical tracks). It has been built in order to provide a large sound-surface, on which patients can lay down. In such a way patient’s body is pervaded by vibrations emitted by specific melodies, composed by a music therapist, according to the M.M.M. specifically, the transduction technology used in AcusticA® covers sound frequencies in the range of 15 Hz to 20 kHz, with a transduction force of 31 N. In such a way, VAT is able to stimulate a physiological response and directly act on muscles and the nervous system to induce a relaxation and modify the subject’s reactivity, as observed in previous pilot studies, that have been conducted with healthy subjects [19,20].
Figure 2: Vibroacoustic device AcusticA®
Twenty-min sessions were carried out. For each session, a specific CD music was administered, alternating 4 different tracks with specific vibrotactile force: the so-called stimulating ones, S1 (20 Hz-15 kHz) and S2 (15 Hz-18 kHz), and relaxing ones, R1 (20 Hz-18 kHz) and R2 (20 Hz-20 kHz).
Clinical Assessment
At baseline (t0) and after training (t1), we performed the following rating scales and clinical tests to assess motor and postural control functions of patients: the Unified Parkinson’s Disease Rating Scale - Section III (UPDRS-III), the Berg Balance Scale (BBS) and the Timed Up and Go test (TUG).
To evaluate gait performance two main parameters were considered: the speed and the length of the distance covered. Patients performed the 3-min WalkingTest (3-MWT); furthermore, we asked them to walk for a 10-m distance, a first time at a comfortable speed(Comfortable Gait Speed test, CGS) and a second time at a fast speed (Fast Gait Speed test, FGS).
To assess agility of movement and speed of postural change execution, we measured the time spent on executing the transition from right and left side to supine and vice versa (Right side to Supine, Rs-to-Sup; Supine to Right side, Sup-to-Rs; Left side to Supine, Ls-to-Sup; Supine to Left side, Sup-to-Ls), from supine to prone (Sup-to-Pron) and, finally, from supine to sitting (Sup-to-Sit).
Statistical Analysis
For all parameters variance analysis was carried out, by means of two-way ANOVA test, considering two conditions, intervention (type) effect and time (pre-post intervention) effect, respectively, in independent subjects’ samples. Post-hoc analysis was performed by using Turkey-Kramer’s test. Our analysis was focused on the intervention-time interaction effect. Each parameter, whose value was found statistically significant (p< 0.05) was plotted in graphics. We used the software MATLAB 2021.
Results
Rating Scales and Clinical Tests
Data analysis concerning UPDRS-III showed that motor symptoms improved significantly (p<0.001) after 3 months in both patients’ groups, regardless of the intervention type. No significant differences in UPDRS-III scores were found between the intervention groups (Table 2): 28.61 ± 5.07 (Tr-VAT) vs 29.18 ± 3.37 (Tr) - p = 0.76.
ClinicalAssessment
Tr-VAT
meanTr-VAT
SDTr
meanTr
SDDifference
p-Value
UPDRS-III (ts)
At t031.76
5.24
31.54
5.31
0.22
0.922
UPDRS-III (ts)
At t128.61
5.07
29.18
3.37
-0.57
0.765
BBS (ts)
Att054.23
2.00
51.27
5.72
2.96
0.109
BBS (ts)
Att155.15
1.09
53.61
1.61
1.79
0.005
TUG-t (s)
Att010.16
3.19
12.11
4.9
- 1.95
0.229
TUG-t (s)
Att18.58
2.56
10.62
2.15
- 2.04
0.058
3-MWT (m)
Att0189.23
36.38
177.27
33.66
11.96
0.435
3-MWT (m)
Att1211.34
43.12
188.54
34.43
22.84
0.176
CGS (s)
Att09.11
1.4
10.58
1.82
-1.46
0.048
CGS (s)
Att17.89
1.2
9.97
1.84
-2.08
0.004
FGS (s)
Att07.55
1.56
8.04
1.69
-0.49
0.484
FGS (s)
Att16.92
1.37
7.57
1.56
-0.88
0.175
Rs/Sup (s)
Att03.24
1.61
3.19
1.66
-1.04
0.279
Rs/Sup (s)
Att12.25
1.11
3.2
1.78
-2.02
0.029
Sup/Rs (s)
Att03.84
1.89
4.95
3.43
-1.13
0.282
Sup/Rs (s)
Att12.40
1.12
4.9
2.81
-2.54
0.014
Ls/Sup (s)
Att03.04
1.37
3.0
1.48
0.04
0.949
Ls/Sup (s)
Att12.11
0.84
3.13
1.94
-1.02
0.069
Sup/Ls (s)
Att03.89
1.73
5.02
3.91
-1.13
0.282
Sup/Ls (s)
Att12.43
1.03
4.96
4.17
-2.54
0.014
Sup/Pron (s)
At t06.61
2.11
11.30
4.65
-5.15
0.037
Sup/Pron (s)
At t13.50
1.57
10.92
4.74
-7.43
0.002
Sup/Sit (s)
At t05.49
1.41
6.20
1.26
-0.71
0.424
Sup/Sit (s)
At t13.07
0.80
6.64
1.53
-3.27
0.0005
Tr: Training; VAT: Vibro Acoustic Therapy; SD: Standard Deviation; UPDRS-III: Unified Parkinson’s Disease III Section; BBS: Berg Balance Scale; TUG-t: Timed Up mand Go test; ts: Total Score; s: Second.; 3-MWT: 3-Minutes Walk Test; CGS: Confortable Gait Speed; FGS: Fast Gait Speed; m: meter; Rs/Sup: Right side to Supine; Sup/Rs: Supine to Right side: Ls/Sup: Left side to Supine; Sup/Ls: Supine to Left side; Sup/Pron: Supine to Prone; Sup/Sit: Supine to Sitting.
Table 2: Clinical data and parameters.
Follow-up evaluation showed a significant (p < 0.05) improvement of postural control with both intervention types, as demonstrated by results of BBS and TUG test.BBS scores were higher in Tr-VAT group in respect to Tr group (55.15 ± 1.09 vs 53.61 ± 1,61) with a p-value at the upper limit (p=0.054). Similarly, a difference (p = 0.058) between groups was found for the TUG test, confirming here too a better performance in Tr-VAT group than in Tr group (8.58 ± 2.56 vs 10.62 ± 2.15).
All these data are reported in (Table 2), while values of single patients are plotted in (Figure 3).
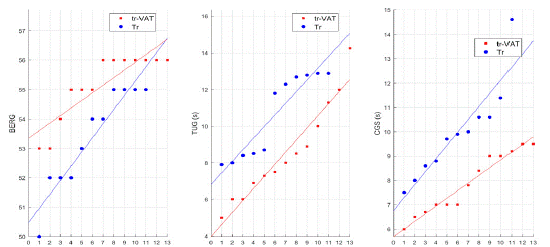
Figure 3: Distribution of patients’ values from Tr-VAT (red) and Tr (blue) groups according to different assessments.
BERG: Berg Balance Scale (BBS); TUG: Timed Up and Go Test; CGS: Comfortable Gait Speed; s: second; Tr-VAT: Training-Vibro Acoustic Therapy; Tr: Training
Gait Parameters
A significant (p<0.05) improvement for 3-MWT in both Tr group (p=0.03) and Tr-VAT group (p=0.01) has been found at follow-up. Also, a very significant (p < 0.01) increase of speed during walking has been reported (both in CGS and FGS).
Post-hoc analysis confirmed a very significant (p<0.01) improvement of CGS values, in patients who performed also VAT, since they walked the 10-m distance in less time: 7.89 ± 1.2 s vs 9.07 ± 1.84 s - p=0.004.
Although the other variables, relative to a forced performance and endurance, seem to have a larger positive trend in patients of the Tr-VAT group, we have not found significative differences for FGS (6.92 ± 1.37 vs 7.57 ± 1.56 s, in Tr-VAT and Tr group, respectively) and 3-MWT (211.34 ± 43 vs 188.54 ± 34.43 m in Tr-VAT and Tr group, respectively). These results are reported in Table 2, whereas values of single patients are plotted in (Figure 3).
Speed of Postural Changes
Both intervention types have been shown to improve speed and agility in postural changing: particulary, very significant (p < 0.01) values for Ls-to-Sups, and Sup-to Rs and Ls in both Tr and Tr-VAT groups have been found. For Sup-to-Pron and Sup-to-Sit parameters, also significant improvements have been reported in both Tr group (Sup-to-to-Prom: p=0.02; Sup-to-Sit: p=0.01) and Tr-VAT group (Sup-to-Pron: p=0.01; Sup-to-Sit: p= 0.02).
Analysis of these variables has also demonstrated better performances in patients from the Tr-VAT group than in those of Tr group. Indeed, the differences (in term of time (s) spent on transition) were statistically (p < 0.05) significant for Rs-to-Sup, Sup-to-Rs, Sup-to-Ls, Sup-to-Pron, and Sup-to-Sit. All data are reported in (Table 2) and values of single patients are plotted in (Figures 4 and 5).
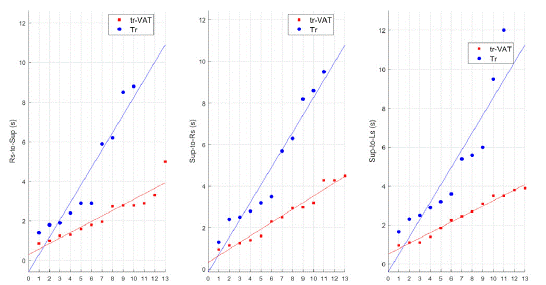
Figure 4: Distribution of patients’ values from Tr-VAT (red) and Tr (blue) groups according to different assessments.
Rs-to-Sup: Right side to Supine; Sup-to-Rs: Supine to Right side; Sup-to-Ls: Supine to Left side; s: second; Tr-VAT: Training-Vibro Acoustic Therapy; Tr: Training
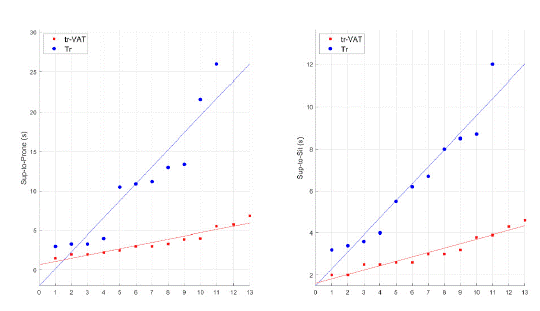
Figure 5: Distribution of patients’ values from Tr-VAT (red) and Tr (blue) groups according to different assessments.
Sup-to-Prone: Supine to Prone; Sup-to-Sit, Supine to Sitting; s: second; Tr-VAT: Training-Vibro Acoustic Therapy; Tr: Training
Discussion
The main aim of this study was to demonstrate the effectiveness of VAT on reducing PD motor symptoms, as a complementary intervention associated with conventional rehabilitation approaches.
Our results agree with other studies concerning the effectiveness of rehabilitation programs on motor symptoms of Parkinsonian patients. Physical activity, as ameliorative intervention on gait disability and postural instability, was referred in several clinical trials, although it remains relatively debated, because of the low level of homogeneity and standardization of rehabilitation protocols [21].
Because of this, a growing interest arouse for new approaches of intervention, focused on VAT [22,23]. The main results of the present study provided some evidence for efficacy and benefit of physical training associated with VAT. In addition to static and dynamic balance, also mobility, agility and flexibility significantly improved in the Tr-VAT group patients as compared to those of the Tr group.
Improvement of Primary Motor Symptoms
Although the small sample size of patients enrolled in our study, nonetheless we obtained a clinically significant response - according to the “minimal clinically important difference” of UPDRS-III motor scores, set as a change of 5 points [24]. In Tr-VAT group, 11 out of 13 patients (84.6%) were regarded as well responders, while in Tr-group, only 6 out of 11 patients (54.5%) achieved this goal. In particular, in most patients from Tr-VAT group, an improvement of rigidity and bradykinesia has been found.
These findings agree with results of other studies, showing the effectiveness of vibration therapy administered through different methods. In this regard, a previous study reported an improvement of the primary motor symptoms in PD patients after whole body sound waves vibrations, delivered through physioacoustic chair, using a frequency range between 27-113 Hz [22]. More recently, a randomized controlled study also demonstrated that physioacoustic method could improve both rigidity and dyskinesia in PD patients by using a 40-Hz frequency [25].
Improvement of Static and Dynamic Balance
Differences between groups were found for TUG test and BBS scores, with better performances in Tr-VAT group than in Tr group. We hypothesize that VAT with balance training, conditioned by sensory (visual feed-back) and sensitive (proprioceptive and vibrotactile) stimuli, might promote a strategy of postural learning, improving balance control.
It is well known that proprioceptive impairment is a common feature in PD patients [26], partially restored by pharmacological treatment. Clinical evidence from a previous study, focused on proprioceptive recovery in PD patients, demonstrated that proprioception is trainable and improves with a somatosensory-focused training, which enhances visuo-spatial capability and accuracy, particularly in mild/moderate PD patients, promoting finally a process of motor learning [27].
On the other hand, in PD a dysregulation of motor and postural learning has been reported: certain aspects of learning, especially automatic responses to feedback are faulty, resulting in a deficit on feed forward systems of movement and/or postural learning and control [27].
Other experimental findings confirm the impaired postural learning process in PD patients, particularly while performing balance training with required flexibility and quick adaptation [28]: PD patients exhibit a greater asymmetry while performing challenging postural tasks, with transition from bipedal to tandem standing and vice versa, as compared to healthy age-matched controls, suggesting impaired mechanisms of adaptation and consolidation of postural control [28]. Disease severity and duration impact on motor and postural learning. Because of this, it is thought that PD patients could require a prolonged training period to achieve motor and postural learning and also additional sensory and sensitive stimuli in order to facilitate such learning [29]. Moreover, research studies showed that training should ensure enough repetition to allow adequate time for feed forward learning [30]. Challenging balance training program seems to have positive long-term effects. Indeed, challenging postural task, required also in activities of daily life, can exacerbate the postural body adjustments [31].
Actually, we suppose that VAT carried out with balance training (as in our Tr-VAT group) may play a role in further enhancing postural control of PD patients. A recent retrospective study shows that the PD patients who performed standardized focal vibration training, assessed through UPDRS-III motor section and BBS scales, reduced their rate of fall and improved ability in daily activities, suggesting the impact of focal vibration in enhancing the effects of postural training, carrying out also a proprioceptive stimulus [32]. On the other hand, the impact of WBV method, as complementary treatment of balance instability and overall motor disability, is debated. A recent metanalysis of clinical trials [33] focused on WBV shows that there are no clear symptoms reducing effects provided by this approach. Compared to WBV, our VAT method allows for the effective delivery of vibration to the entire body; moreover, in previous studies with WBV the frequencies used were either randomized or set very low (<20 Hz), thus producing a minimal clinical benefit.
Speed of Gait and Postural Transition
Reduction of severity of primary symptoms has improved significantly speed of postural transition in Tr-VAT group. Regarding gait parameters, TW program seems to enhance the endurance and speed in both groups. Only for CGS was found a statistically significant difference, with higher speed in Tr-VAT group than in Tr group. Previous studies showed the effectiveness of TW training, proposing also some criteria for its standardization, such as lasting of a single session and week frequency of interventions [34]. Although few studies are randomized, or assessed by a follow-up evaluation, there is evidence that TW can improve dynamic balance and asymmetry of gait, reducing symptoms, such as freezing of gait and rate of fall [34].
In our study VAT and TW seem to be more effective than RAS alone during training session: the significative improvement of comfortable speed during gaitin Tr-VAT group could reflect an individual adaptive modulation of motor skills and a reduction of motor inhibition [35]. RAS intervention is characterized, traditionally, by fixed frequencies, whereas in VAT by the M.M.M. we used a wide range of frequencies. Despite several previous studies promoting RAS intervention, a recent study suggests that using RAS at fixed frequencies might be an inappropriate strategy in respect to frequencies adapted to individual characteristics of gait [36].
The Mechanism Underlying the Effects of Vibroacustic Therapy
Mechanisms behind our approach with VAT as well as those of other methods of vibration therapy, are not fully understood.
One could speculate that the effects of VAT according to the M.M.M. on motor symptoms of PD patients are due to both relaxation of body muscles and modulation of neuron networks activity [37].
It was suggested that increased muscle activity and the resulting relaxation phase depend on a “resonance like-phenomenon”, provided by vibration therapy. Using electromyography and accelerometers an experimental study evaluated muscle response to sinusoidal frequencies, delivered as WBV, with a range of frequency beetwen10 and 54 Hz. It was found that muscle activity and acceleration amplitude increased with frequency. Assuming the hypothesis that muscle activation is proportional to muscle displacement, the authors suggested also that treatment optimization, which is linked to customize (no fixed) level of frequency, could be obtained by monitoring local acceleration [38]. VAT frequencies, delivered from our device, as well as other methods [22] could promote entrainment of natural and physiological frequencies in resonance with external frequencies, resulting in increased blood circulation and decreased tension of muscles. In addition, experimental features from healthy controls [19,20] showed that our VAT can induce an overall relaxation effect. Physiological response to VAT, monitored by wearable sensors detecting heart rate variability and galvanic skin response, showed a reduction of stress level and enhanced relaxation, obtained from a reduction of sympathetic/vagal ratio.
On the other hand, a recent research has focused on potential benefits of vibration therapy, conceived as an exercise modality. Experimental evidence suggests that acute “vibration exercise” seems to elicit a specific warm-up effect, so that vibration training appears to improve muscle power [39]. In this regard, a study [40] showed that vibration therapy, compared to traditional training, does not require a long time of muscle recovery and, moreover, leads to less feeling of fatigue, as confirmed by the measuring of oxygen consumption.
Conclusions
The present study demonstrated the effectives of VAT, based on entire body administration of harmonic vibrations, according to the so-called M.M.M., associated with TW and BT, resulting an improvement of mobility, agility, flexibility and balance control in PD patients.
Further studies are needed to assess long-term effects of Tr-VAT intervention in a larger population of PD patients. Moreover, we would like to check benefit of VAT according to the M.M.M., administered without training, as an alternative approach of rehabilitation for PD patients with reduced compliance for aerobic exercise and high risk of fall.
References
- Surmeier DJJ. Determinants of dopaminergic neuron loss in Parkinson’s disease. FEBS J. 2018; 285: 3657-3668.
- Pfeiffer RF. Non-motor symptoms in Parkinson’s disease. Parkinsonism & Relat Disord. 2016; 22: S119-122.
- Voon V, Napier TC, Frank MJ, Sgambato-Faure V, Grace AA, Rodriguez-Oroz M, et al. Impulse control disorders and levodopa-induced dyskinesias in Parkinson’s disease: an update. Lancet Neurol. 2017; 16: 238-250.
- Shalash A, Roushdy T, Essam M, Fathy M, Dawood NL, Abushady EM, et al. Mental health, physical activity, and quality of life in Parkinson’s disease during COVID-19 pandemic. Mov Dis. 2020; 35: 1097-1099.
- HandleryR, Stewart JC, Pellegrini C, Monroe C, Hainline G, Flach A, et al. Physical activity in de novo Parkinson disease: daily step recommendation and effects of treadmill exercise on physical activity. Phys Ther. 2021; 101: pzab174.
- Schenkman M, Moore CG, Kohrt WM, Hall DA, Delitto A, Comella CL, et al. Effect of high-intensity treadmill exercise on motor symptoms in patients with de novo Parkinson disease: a phase 2 randomized clinical trial. JAMA Neurol. 2018; 75: 219-226.
- Soke F, Guclu-Gunduz A, Kocer B, Fidan I, Keskinoglu P. Task-oriented circuit training combined with aerobic training improves motor performance and balance in people with Parkinson’s disease. Acta Neurol Belg. 2019; 121: 535-543.
- De Freitas TB, Leite PHW, Donà F, Pompeu JE, Swarowsky A, Torriani-Pasin C. The effects of dual task gait and balance training in Parkinson’s disease: a systematic review. Physiother Theory Pract. 2020; 36: 1088-1096.
- Alashram AR, Padua E, Annino G. Effects of whole-body vibration on motor impairments with neurological disorders: a systematic review. Am JPhys Med Rehab. 2019; 98: 1084-1098.
- Leuk JSP, Low LLN, Teo W-P. An overview of acoustic-based interventions to improve motor symptoms in Parkinson’s disease. Front Aging Neurosci. 2020; 12: 243.
- Sharififar S, Coronado RA, Romero S, Azari H, Thigpen M. The effects of whole body vibration on mobility and balance in Parkinson disease: a systematic review. Iran J Med Sci. 2014; 39: 318-326.
- Maggioni E, Arienti F, Minella S, Mameli F, Borellini L, Nigro M, et al. Effective connectivity during rest and music listening: an EEG study on Parkinson’s disease. Front Aging Neurosci. 2021; 13: 657221.
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev. 2000; 31: 236-250.
- Campbell EA, Kantor J, Kantorovà L, Svobodovà Z, Wosh T. Tactile low frequency vibration in dementia management: a scoping review protocol. Int J Environ Res Public Health. 2021; 18: 1904.
- Skille O. Vibroacustic therapy. Music Therapy. 1989; 8: 61-77.
- Campbell EA, Hynynen J, Burger B, Vainionpaa A, Ala-Ruona E. Vibroacoustic treatment to improve functioning and ability to work: a multidisciplinary approach to chronic pain rehabilitation. Disabil Rehab. 2021; 43: 2055-2070.
- Bartel LR, Chen R, Alain C, Ross B. Vibroacustic stimulation and brain oscillation: from basic research to clinical practice. Music & Medicine. 2017; 9: 153-166.
- Patent “Harmonic Vibro-Massage Unit” [https://patentimages.storage.googleapis.com/08/29/6b/248e71a2297132/EP1827351B1.pdf]
- Del Mastro F, Di Martino F, Dolciotti C. Physiological impact of vibroacoustic therapy on stress and emotions through wearable sensors. IEEE Int Conf on Pervasive Computing and Communication Workshops (Percom Workshops). 2018; 1-6.
- Cavallo F, Rovini E, Dolciotti C, Radi L, Della Ragione R, Bongioanni P, et al. Physiological response to vibro-acoustic stimulation in healthy subjects: a preliminary study. Ann Int Conf IEEE Eng Med Biol Society. 2020; 5921-5924.
- Kim Y, Lai B, Mehta T, Thirumalai M, Padalabalanarayanan S, Rimmer JH, et al. Exercise training guidelines for multiple sclerosis, stroke and Parkinson disease: rapid review and synthesis. Am J Phys Med Rehabil. 2019; 98: 613-621.
- King LK, Almeida QJ, Ahonen H. Short-term effects of vibration therapy on motor impairments in Parkinson’s disease. Neuro Rehab. 2009; 25: 297-306.
- Calabrò RS, Naro A, Filoni S, Pullia M, Billeri L, Tomasello P, et al. Walking to your right music: a randomized controlled trial on the novel use of treadmill plus music in Parkinson’s disease. Neuroeng Rehab. 2019; 16: 68.
- Sanchez-Ferro A, Matarazzo M, Martinez-Martìn P, Martinez-Avila JC, De la Camara AG, Giancardo L, et al. Minimal clinically important difference for UPDRS-III in daily practice. Mov Disord - Clin Pract. 2018; 5: 448-450.
- Mosabbir A, Almeida QJ, Ahonen H. The effects of long-term 40-Hz physioacoustic vibrations on motor impairments in Parkinson’s disease: a double-blinded control trial. Healthcare. 2020; 8: 113.
- Peterka M, Odorfer T, Schwab M, Volkmann J, Zeller D. LSVT-BIG therapy in Parkinson’s disease: physiological evidence for proprioceptive recalibration. BMC Neurology. 2020; 20: 276.
- Marinelli L, Quartarone A, Hallett M, Frazzitta G, Ghilardi MF. The many facets of motor learning and their relevance for Parkinson’s disease. Clin Neurophysiol. 2017; 128: 1127-1141.
- Beretta VS, Bluken Gobbi LT, Lirani-Silva E, Simeli L, Orcioli-Silva D, Barbieri FA. Challenging postural tasks increase asymmetry in patients with Parkinson’s disease. Plos One. 2015; 10: e0137722.
- Ossmy O, Mansano L, Frenkel-Toledo S, Kagan E, Koren S, Gilron R, et al. Motor learning in hemi-Parkinson using VR-manipulated sensory feedback. Disabil Rehab Assist Technol. 2022: 17: 349-361.
- Oates AR, Van Ooteghem K, Frank JS, Patla AE, Horak FB. Adaptation of gait termination on a slippery interface in Parkinson’s disease. Gait & Posture. 2013; 37: 516-520.
- Conradsson D, Löfgren N, Nero H, Hagströmer M, Ståhle A, Lökk J, et al. The effects of highly challenging balance training in elderly with Parkinson’s disease. A randomized controlled trial. Neurorehab Neural Repair. 2015; 29: 827-836.
- Serio F, Minosa C, De Luca M, Conte P, Albani G, Peppe A. Focal vibration training (Equistasi®) to improve posture stability. A retrospective study in Parkinson’s Disease. Sensors. 2019; 19: 2101.
- Dincher A, Schwarz M, Wyda G. Analysis of the effects of whole-body vibration in Parkinson disease - Systematic review and meta-analysis. PM & R. 2019; 1: 640-653.
- Mehrholz J, Kugler J, Storch A, Pohl M, Hirsch K, Elsner B. Treadmill training for patients with Parkinson disease. An abridged version of Cochrane Review. Eur J Phys Rehab Med. 2016; 52: 704-713.
- Cerasa A, Donzuso G, Morelli M, Mangone G, Salsone M, Passamonti L, et al. The motor inhibition system in Parkinson’s disease with levodopa-induced dyskinesias. Mov Dis. 2015; 30: 1912-1920.
- Minino R, Troisi Lopez E, Sorrentino P, Rucco R, Lardone A, Pesoli M, et al. The effects of different frequencies of rhythmic acoustic stimulation on gait stability in healthy elderly individuals: a pilot study. Sci Report. 2021; 11: 19530.
- Salimpoor VN, Benovoy M, Larcher K, Dagher A, Zatorre RJ. Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nature Neurosci. 2011; 14: 257-262.
- Fratini A, La Gatta A, Bifulco P, Romano M, Cesarelli M. Muscle motion and EMG activity in vibration treatment. Med Eng Physics. 2009; 31: 1166-1172.Rittweger J. Vibration as an exercise modality: how it may work, and what its potential might be. Eur J Appl Physiol. 2010; 108: 877-904.
- Corbianco S, Cavallini G, Baldereschi G, Carboncini MC, Fiamingo FL, Bongioanni P, et al. Whole body vibration and treadmill training in Parkinson’s disease rehabilitation: effect on energy cost and recovery phases. Neurol Sci. 2018; 39: 2159-2168.