
Research Article
Phys Med Rehabil Int. 2023; 10(3): 1220.
Combination of Electrical Stimulation and Motor Control Exercise for Chronic Nonspecific Low Back Pain: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Xiaoyu G1#; Wuwei S2#; Huihui L3; Wenyu J1; Jiacheng Y1; Xiang W1*; Hongsheng Z1*
¹Shi’s Center of Orthopedics and Traumatology (Institute of Traumatology), Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, China
²Huamu Community Health Service Centre of Shanghai Pudong New District, China
³Department of Orthopedics and Traumatology, Chongming Branch of Xinhua Hospital Affiliated to Shanghai Jiaotong University School of Medicine, China
*Corresponding author: Xiang W Shi’s Center of Orthopedics and Traumatology (Institute of Traumatology, Shuguang Hospital), Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, 185 Pu’an Road, Huangpu District, Shanghai 200021, China.
Hongsheng Z, Shi’s Center of Orthopedics and Traumatology (Institute of Traumatology, Shuguang Hospital), Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, 185 Pu’an Road, Huangpu District, Shanghai 200021, China. Email: w8x@163.com; zhanhongsheng@shutcm.edu.cn
#These authors contributed equally to this work.
Received: September 28, 2023 Accepted: October 26, 2023 Published: November 02, 2023 Phys Med
Abstract
Objective: To evaluate the efficacy of the combination of electrical stimulation and motor control exercise for chronic nonspecific low back pain compared to other treatments.
Data Sources: A systematic search was conducted in PubMed, EMBASE, Web of Science, Cochrane Central Register of Controlled Trials (CENTRAL) and the Cumulative Index to Nursing & Allied Health Literature (CINAHL) from the earliest record to 30 April 2022.
Methods: We included randomized controlled trials that investigated the effect of combination of electrical stimulation and motor control exercise compared to control groups for patients with chronic nonspecific low back pain. The meta-analysis is performed to compare the pain and disability outcome measures. The protocol for this systematic review was prospectively registered on PROSPERO (registration number: CRD42022324850).
Results: Ten articles enrolling 665 participants were included in the meta-analysis. The meta-analysis found low quality evidence (six studies) that the pain relief differed significantly between the combination of Electrical Stimulation with Motor Control Exercise (ES+MCE) and non-ES controls (sham, placebo or without ES) (Standardized Mean Difference (SMD)=-0.25, 95% Confidence Interval (CI) with lower and upper limits, -0.47 to -0.028, the p value (P)=0.028). For disability outcome, low quality of evidence (five studies) indicated that it differed not significantly between ES+MCE and non-ES controls (SMD=-0.20, 95% CI,-0.43 to 0.04, P=0. 0.10). Our results found out that the combination of electrical stimulation and motor control exercise had a beneficial effect for pain relief but no significant difference in disability outcome.
Conclusion: Our study supported the combination of electrical stimulation and motor control exercise to alleviate the chronic nonspecific low back pain. No significant difference for the disability outcome was observed in our study.
Keywords: Chronic nonspecific low back pain; Electrical stimulation; Motor control exercise; Randomized controlled trail; Meta-analysis
Abbreviations: LBP: Low Back Pain; CNSLBP: Chronic Nonspecific Low Back Pain; MCE: Motor Control Exercise; ES: Electrical Stimulation; NMES: Neuromuscular Electrical Stimulation; CENTRAL: Cochrane Central Register of Controlled Trials; CINAHL: The Cumulative Index to Nursing & Allied Health Literature; RCT: Randomized Controlled Trial; TENS: Transcutaneous Electrical Nerve Stimulation; PENS: Percutaneous Electrical Nerve Stimulation; GRADE: Grading of Recommendations, Assessment, Development, and Evaluation; SMD: Standardized Mean Difference; CI: Confidence Interval; VAS: Visual Analogue Scale; NPRS: Numerical Pain Rating Scale; Seven studies provided disability outcomes with MODQ: Modified Oswestry Disability Questionnaire; ODI: Oswestry Disability Index; ODQ: Oswestry Disability Questionnaire; RMDQ: Roland Morris Disability Questionnaire; NHP: Nottingham Health Profile; IO: Internal Oblique; EO: External Oblique; IG: Intervention Group; CG: Control Group; NA: Not Available; MODQ: Modified Oswestry Disability Questionnaire; tDCS: Transcranial Direct Current Stimulation; CSE: Core Stabilization Exercise; US: Ultrasound; IFC: Interferential Current; HMP: Hot Moist Pack; TMT: Trunk Muscle Training
Introduction
Low Back Pain (LBP) is a worldwide problem, which is defined as pain, muscle tension or stiffness localized below the costal margin and above the inferior gluteal folds, with or without sciatica (pain travelling down the leg from the lower back) [1]. According to the 2019 Global Burden of Disease Study, LBP is the leading health condition contributing to the need for rehabilitation services in 134 of the 204 countries analyzed, with 568 million people (505–640) and 64 million (45–85) lived with disability [2]. According to pain duration, low back pain is classified as acute (<6 weeks), subacute (6~12 weeks) or chronic (>12 weeks). Almost everyone has a brief, acute episode of LBP during their lifetime. 5~10% of the acute low back pain persist and develop into a chronic condition with fluctuating or persisting pain [3]. Many factors and risks contribute to pathogenesis of LBP, and patients with LBP are classified into four broad categories: those with a visceral disorder, a specific spinal disease, radicular syndromes or nonspecific low back pain [1]. The nonspecific low back pain probably develops from the interaction of biologic, psychological, and social factors, and accounts for approximately 80 to 90% of all cases of low back pain [4].
One of the pathophysiological theories is the spinal instability introduced by Panjabi [5]. In his theory, spinal stability seems to be a result of coordination among three major systems: active, passive, and neural. And lumbar segmental instability contributes to an increased range of motion, leading to the painful condition like Chronic Nonspecific Low Back Pain (CNSLBP). Muscles attributes to the spinal stability, which are divided into two types, the global and the local. The former provides general stability of the trunk, and the latter provides segmental stability [6]. Evidence advice that lumbar multifidus and transversus abdominis, two deep trunk muscles control the lumbar intervertebral movement [7]. Recent years, increasing attention has been paid to the preferential retraining of the local stabilizing muscles of the spine [8].
Considering the pathophysiological theory of CNSLBP is hard to define, the multidisciplinary treatment gets increasing attention. Exercise therapy is recommended as the main treatment and Motor Control Exercise (MCE) targets on activating of the deep trunk muscles to retrain the optimal control and coordination of the spine. MCE reduces the activity of superficial muscles, and pre-activates of the deep trunk muscles, with progression toward more complex static, dynamic, and functional task [9,10]. The motor control decreases pain and increases multifidus and transversus abdominis muscles thickness and lumbar mobility in patients with chronic LBP [8], but its benefit is not universal or complete. One of the theories is that the deep trunk muscles lack of immediate activation. To date, in patients with LBP, exercises targeting the lumbar multifidus, have failed to induce immediate changes in multifidus [11]. Electrical Stimulation (ES) is suggested as an adjunct to patients suffering the pain. It’s common that studies chose ES to enhance the effect of exercises [12,13]. ES entails several types. One modality widely studied is transcutaneous electrical nerve stimulation, which can alleviate the short-term pain. And Neuromuscular Electrical Stimulation (NMES) is effective in improving range of motion, reducing muscle spasm, and reducing pain in patients with LBP [14]. ES, NMES, in particular, provides feedback to the patient enabling reproducible muscle recruitment and activation. It also helps to retrain the transverse abdominis and multifidus while performing exercises [15], and is used to reduce immediate pain and provides another treatment for kinesophobia [16]. The trials that combined ES and MCE are increasing [16-18]. In order to see whether it could help to improve the condition of CNSLBP, we conducted this systematic review and meta-analysis.
Methods
Search Strategy
This review conformed to PRISMA guidelines [19] (Supplementary Table 1). The protocol for this systematic review was prospectively registered on PROSPERO (CRD42022324850). A systematic search was conducted in PubMed, EMBASE, Web of Science, Cochrane Central Register of Controlled Trials (CENTRAL) and the Cumulative Index to Nursing & Allied Health Literature (CINAHL) from the earliest record to 30 April 2022. We also searched additional records identified through other sources such as citation tracking and article reviewing. Search strategies followed the recommendations of the Cochrane Back and Neck Group [20]. We chose the key words mainly according to the Medical Subject Headings and entry items of it. To avoid missing the key words, we expanded it with relevant works. Specific subject subheadings and word truncations were used according to each database, with no language limitation. Detailed search terms were described in the Supplementary (Supplementary 2). Duplicate citations were eliminated after the preliminary search results were obtained. To identify the final studies that would be included in the meta-analysis, two independent reviewers (XYG, WWS) examined the title and abstract of each article for eligibility. Full articles were obtained and reviewed by two independent reviewers (XYG, WWS) on the basis of a standardized inclusion and exclusion criteria form. In the case of disagreement regarding whether a study meet all of the inclusion criteria and none of the exclusion criteria, a third reviewer (HHL) joined and identified until a common consensus was reached.
Study
Country
Participation
Duration
Outcome time of outcome assessing
Outcome parameter
Adverse event
Attrition
Population
Sex (female)
Age
BMI
Akhtar, M. W., et al. (2017)28
Pakistan
120
NA
IG:46.39±7.43
CG:45.50±6.61IG:24.15±2.38
CG:24.82±3.026 weeks
Baseline
2 weeks during the treatment
4 weeks during the treatment
immediately post-treatmentVAS
NA
10%
Alrwaily, M., et al. (2018)30
USA
30
63%
IG: 33.40±9.0
CG:38.33±11.3IG: 26.47±2.9
CG:25.89±3.86 weeks
Baseline
immediately post-treatmentMODQ
NPRS
Fear-Avoidance Beliefs Questionnaire
paraspinal muscle strength0
13%
Atli, E., et al. (2021)12
Turkey
36
63.9%
IG:35.3±15.4
CG:33.3±11.4IG:23.3±4.2
CG:24.3±3.84 weeks
Baseline
immediately post-treatment
4 weeks follow-upOswestry Disability Index
VAS
Nottingham Health Profile
ultrasonography of the transversus abdominis, internal oblique and external oblique musclesNA
16.7%(4weeks)
25%(8weeks)Durmus, D., et al. (2009)25
Turkey
68
100%
IG(ES): 49.00±7.87
IG(US): 48.31±8.95
CG:47.05±12.46IG(ES):30.50±5.37
IG(US):28.89± 3.98
CG:28.50 ± 1.846 weeks
Baseline
3 weeks during the treatment
immediately post-treatmentVAS
The Oswestry Disability Questionnaire
Pain disability index
modified Schober test, lumbar Schober test, and finger tip to floor distance
The 6-min walk distance test
Trunk flexor muscle strength
extensor muscle strength
Muscle duration
the MOS item short from health survey
Beck Depression InventoryNA
13.20%
Embaby, E. A., et al. (2021)24
Egypt
45
100%
IG(IFPCs):21.73±2.25
IG(RC):21.37±2.03
CG:23.64±4.22IG(IFPCs):24.42±1.88
IG(RC):24.26±3.08
CG:25.08±3.786 weeks
Baseline
immediately post-treatmentmultifidus activation
NPRSNA
0
Franco, K. M., et al. (2016)17
Brazil
148
72.3%
IG: 43.9±15.5
CG:43.6±14.1IG: 26.7±4.8
CG:26.8±5.56 weeks
Baseline
immediately post-treatment
6 months follow-upPain Numerical Rating Scale,
pressure pain threshold
Roland Morris Disability Questionnaire
Tampa Scale for Kinesophobia
Patient-Specific Functional Scale
Global Perceived Effect ScaleNA
2%
Hicks, G. E., et al. (2016)29
USA
64
48.4%
IG:70.7±6.8
CG:69.5±7.0IG:28.8±6.9
CG:30.0±4.512 weeks
Baseline
immediately post-treatment
3 months follow-upTimed Get Up-and-Go (TUG)
Gait Speed
MODQ
NPRS
Tampa Scale of Kinesophobia
Global Rating of Functional ImprovementOne participant tripped and fell.
10.90%
Kim, T. H., et al. (2014)26
South Korea
53
100%
CG:29.7±3.9
IG:28.6±3.2NA
8 weeks
baseline
immediately post-treatment
2 months follow-upVAS at rest and movement
pressure pain
active range of pain-free motion and trunk proprioceptionNA
28.40%
Mane, N. P., et al. (2019)27
India
66
NA
30-60
NA
2 weeks
baseline
immediately post-treatmentElectromyography of transversus abdominis and mulitifidus
NPRS
MODQNA
0
Straudi, S., et al. (2018)31
Italy
35
74.3%
IG:54.3±12.4
CG:56±12.9NA
4 weeks
baseline
immediately post-treatment
1 month follow-upVAS
Roland Morris Disability Questionnaire
EuroQuol-5 Dimension and Patient Health Questionnaire-9NA
11.40%
NA: Not Available, IG: Intervention Group, CG: Control Group, VAS: Visual Analog Scale, USA: the United States of America; NPRS: Numerical Pain, Rating Scale; MODQ: Modified Oswestry Disability Questionnaire, ES: Electrical Stimulation; US: Ultrasound; IFPCs: Low-Frequency Pulsed Currents; RC: Russian Current
Table 1: Characteristics of Included Studies in the Meta-Analysis (Basic Information).
Selection Criteria
1. Studies were considered for including in this meta-analysis if they met all of the following inclusion crThe study was a Randomized Controlled Trial (RCT). Participators were patients with CNSLBP. According to the latest review, CNSLBP is defined as pain below the costal margin and above the inferior gluteal folds, with or without leg pain, which lasts for twelve weeks and excludes the specific disorders of spinal and no spinal origin. The former one includes the hip conditions, diseases of the pelvic organs (prostatitis and endometriosis), and vascular (aortic aneurysm) or systematic disorders, the latter one includes herniated disk, spinal stenosis, fracture, tumor, infection, and axial spondylarthritis [4].The interventions involved ES and MCE. There were various modalities of ES, like transcutaneous electrical nerve stimulation, percutaneous electrical nerve stimulation and Neuromuscular Electrical Stimulation (NMES). We took consideration of the intervention, which was described as MCE, specific trunk muscle stabilization exercise [21] or the exercise treatment aimed to activate, train or restore the function of specific muscles of the spine, like multifidus and transversus abdominis [22].The control group could be negative or active, for example, placebo, and exercises only or electrical stimulation only.
2. Studies were excluded if they did not provide enough information concerning inclusion criteria or numerical data regarding the degree of pain or disability.
Data Extraction
Two independent reviewers (XYG, WWS) extracted all data for data synthesis and a third reviewer (HHL) resolved the disagreements if necessary. The reviewers collected all the characteristics of the included studies, classified them and presented their main characteristics. More specifically, the following information was extracted: basic information of study (study’s name, first author, year of publication), population characteristics (patient population source or setting, study inclusion criteria, duration of CNSLBP episode, age and sex of patients), intervention characteristics (description of electrical stimulation, types of exercise therapy, duration of treatment sessions, intervention delivery type, and co-interventions), the control group characteristics (description of control group) and outcome measures (the primary outcome and secondary outcome). Besides, we extracted mean scores, Standard Deviations (SD) and sample sizes from the studies for further analysis. We contacted corresponding authors for trials in which data for key study characteristics or primary outcomes were missing or incomplete. If they didn’t reply in one month, we would use the other related data for the meta-analysis or exclude the study.
Risk of Bias and Quality Assessment
We used the Updated Cochrane Back and Neck Group [20] expanded 13-item criteria to assess the risk bias of the included studies, which were selection bias (random sequence generation, allocation concealment, group similarity at baseline), performance bias(blinding of participants, blinding of therapists, cointerventions, compliance), attrition bias (incomplete outcome data, intention to treat analysis), detection (or measurement) bias (blinding of outcome assessors, timing of outcome assessments), reporting bias(selective reporting) and other bias(like ‘industry-sponsored trials’). The risk of bias was classified as ‘Low risk’, ‘High risk’ or ‘Unclear’.
The quality assessment of each outcome was in accordance with the Grading of Recommendati ons, Assessment, Development, and Evaluation (GRADE) [23]. Five domains were conducted in the GRADE, involving consideration of 1) Study design and risk of bias, 2) Inconsistency, 3) Indirectness, 4) Imprecision and 5) Other factors (reporting bias, publication bias). We rated the quality of the evidence as ‘High’, ‘Moderate’, ‘Low’, ‘Very Low’. And RCTs were recommended of ‘High’ quality, and downgraded to ‘Moderate’, ‘Low’ or ‘Very Low’ quality evidence (Supplementary Table 2A, Supplementary Table 2B). Risk of bias of studied included in the meta-analysis and quality assessment of each outcome were performed by two independent reviewers (XYG, WWS) and any uncertainties were resolved by discussion or arbitration by a third review author (HHL).
study
intervention group
control group
ES
MCE
other intervention
types
duration
location
stimulation
types
duration
movements
Akhtar, M. W., et al. (2017)28
1.CSE Group + TENS + therapeutic ultrasound
1.Routine Physical Therapy + TENS + therapeutic ultrasound
TENS
10 minutes
lumbar spine
Continuous mode
CSE
Last 40 minutes with
5-10 minutes rest interval, twice a week at home for up to six weeksHamstring stretching
Calf stretching
Hip flexors stretching
Back extensors stretching
Abdominal curl-up exercise in supine
Back extensors exercise in prone
Hip extensors exercises in pronetherapeutic ultrasound
(3MH for 10 minutes at 50% intensity)Alrwaily, M., et al. (2018)30
1.Stabilization exercises program supplemented with NMES
1.Stabilization exercises program only
NMES
20 minutes at first
the lumbar paraspinal muscles bilaterally
pulse frequency of 75 pulses per second, a pulse duration of 250s,
with a 4-s ramp up and ramp down time, and a 6-s stimulation period at the maximum amplitude, followed by a 50 s rest period to minimize fatigueStabilization Exercises
20 minutes
abdominal bracing exercises,
side support exercises
quadruped exercisesNA
Atli, E., et al. (2021)12
1.NMES simultaneously applied with CSE
1.sham NMES and CSE
NMES
35 minutes
paravertebral muscles bilaterally at
the level of L4-5 spinousthe first 2 minutes (warming up phase), frequency is 6Hz. Contraction phase includes consecutive cycles of contractile frequency of 40Hz for 6 seconds and the rest frequency of 4Hz for 12 seconds, lasting a total of 30 minutes. The last 3 minutes (recovery phase) frequency is 3Hz. The
ramp up time is 2 seconds and the ramp down time is one second.CSE
10 times of 12 sessions
abdominal bracing exercises in supine, prone and quadruped positions, bridge, crunch and plank
NA
Durmus, D., et al. (2009)25
1.ES program and exercises
2.US treatment and exercises1.only exercises
ES
15 minutes
the second lumbar vertebrum to fourth lumbar vertebrum levels over the erector spinae muscles bulks motor points
The symmetric biphasic wave was applied with the frequency of 50 Hz and 50 ms of phase time. The intensity of the current was arranged separately one by one for each patient until apparent muscle contraction was established (60–130 mA). The stimulation
was applied as 10 s of contraction and 10 s of relaxationexercises
45 minutes,3 days a week
1. Motion, Flexibility, and back strengthening exercises of the cervical, thoracic, and lumbar spine; stretching of the erector spine muscle, hamstring muscles, pelvic muscles, and abdominal muscles: (1) pelvic tilt, (2) knee to chest, (3) lower abdominal exercises, (4) cat and camel, (5) back extension exercises.
2. Special exercises to correct mobility of the spine and hip joints, activate the stabilizing muscles of the spine, and increase flexibility of the lower limb muscles.
3. Functional exercises to improve postural control, dynamic body balance, and coordination.
4. Progressive relaxation exercises to normalize muscle tension.US: using Enraf Nonius Sonoplus that operated at 1 MHz frequency and 1 W/cm2 intensity and a transducer head with an area of 5 cm, an ERA of 4 cm, and a BNR of 1:5. Slow circular movements were applied by the transducer head over the paravertebral low back region. The treatment duration was 10 minutes.
Embaby, E. A., et al. (2021)24
1. LFPCs + lumbar stabilization exercises
2. RC + lumbar stabilization exercises1.only lumbar stabilization exercises
NMES
20 minutes at first
located bilaterally at an interelectrode
spacing of approximately 2 cm on a cross-line drawn on L4-5 interspinousLFPCs: a constant current and asymmetrical biphasic waveform and Biphasic symmetrical pulses of 200 μs with an interpulse delay of 100 μs were employed;50 Hz; contraction for 500 μs and relaxation for 19 ms
RC: 2500-Hz alternating current, applied in 50-Hz rectangular bursts with a burst duty cycle of 50%. The burst duration is 10 ms at 50 Hz. The other is same as the LFPCs.lumbar stabilization exercises
15–20 minutes,2 times per day
abdominal bracing exercises
side support exercises
quadruped exercisesNA
Franco, K. M., et al. (2016)17
1.IFC Active + Pilates
1.IFC Placebo + Pilates
IFC
30 minutes in first two weeks
at the site of pain
IFC was applied with bipolar (pre-modulated) application with two channels under medium-frequency alternating currents, the current's amplitude was increased until the participant reported a strong but comfortable tingling sensation, and this procedure was repeated every five minutes.
Pilates
additional 40 minutes in the last four weeks
Initially the patient was trained to contract isometrically the powerhouse muscles (e.g. transversus abdominis, multifidus, and pelvic floor muscles) during exhalation and then was instructed to hold this contraction during all exercises to stabilize the spine The level of difficulty and the patient’s preferences in relation to the exercises were individualized. Ten repetitions of each exercise were performed.
NA
Hicks, G. E., et al. (2016)29
1.TMT+NMES
1.Passive Control Intervention
NMES
15 minutes, twice
weeklylumbar paraspinals bilaterally (L2-L5)
deliver a 2500Hz, alternating current, modulated at 50 bursts per second. The stimulus amplitude was increased to a minimum level that resulted in a full, sustained, isometric contraction of the lumbar paraspinals (no evidence of muscle fasciculations on visual
inspection) and then further increased to the subject’s maximum tolerance. Stimulus on/off, time settings were10 seconds on and 60 seconds off. Each participant received 15 electrically stimulated contractions of the lumbar paraspinal muscles per treatment session.TMT
30 minutes, twice weekly
Abdominal Bracing in supine
Bracing in hook-lying with leg movements
Bracing in hook-lying with bridging
Abdominal Bracing in standing
Single arm or leg lifts-standing with elbows leaning on counter
Opposite arm and leg lifts standing with elbows on counter
Single arm or leg lifts-prone over bolster
Opposite arm and leg lifts-prone over bolster
Same exercises performed in quadruped
Curl ups
Remedial side bridge against wall
Remedial side bridge with leg lift on floor/bed
Horizontal side bridgePassive control group lasts 40-45 minutes, including 20 minutes of moist heat to their lower back, 7 minutes of sub-therapeutic ultrasound (continuous pulse, 25 watts/cm2) to the lumbar paraspinal region, 8 minutes of light effleurage massage to the thoracolumbar region and 5 minutes of upper extremity stretching exercises focused on the shoulder girdle and upper back
Kim, T. H., et al. (2014)26
1.CES+ standard treatment
1.the standard treatment
TENS
20 minutes
NA
NA
CSE
30minutes, five times per week
The CORE program consists of 14 exercises (head to toe prep, tongue stretch, belly blaster, belly breath, the cobra, butterfly/heel beats comba, three part pelvic stabilizers, four-part progressive hamstring stretch, double knee to chest, lying spinal twist, belly breath, cross extension, the cobra, belly breath final) and indicates specific trunk muscle activation.
15minute hot-pack
treatmentMane, N. P., et al. (2019)27
1.Motor control training, HMP and TENS
1.Core muscle training, HMP and TENS
TENS
NA
NA
NA
MCE
NA
First week: 8 reps: (1) Activation of transversus abdominis. (2) Activation of multifidus
Second week: 15 reps, 5-10 sec hold:(1) Strengthening of transversus abdominis. (2) Strengthening of Multifidus.HMP
Straudi, S., et al. (2018)31
1.Real-tDCS+ group exercises
1.Sham-tDCS+ group exercises
tDCS
20 minutes
The location of the motor cortex was estimated using the international 10–20 electroencephalogram system and placing the center of the electrode pad at the third or fourth cervical vertebra.
This continuous stimulation lasted 20minutes, with an intensity of 2mA for five consecutive days
Group Exercise intervention
1 hour, 2~3 times a week
group exercises, including education, strengthening exercise, stretching exercise, relaxation techniques
NA
ES: Electrical Stimulation; MCE: Motor Control Exercise; CSE: Core Stabilization Exercise; TENS: Transcutaneous Electrical Nerve Stimulation; NMES: Neuromuscular Electrical Stimulation; NA: Not Available, L4-5: The Fourth Lumbar vertebrum and Fifth Lumbar Vertebrum; US: Ultrasound; LFPCs: Low-Frequency Pulsed Currents; RC: Russian Current; IFC: Interferential Current; TMT: Trunk Muscle Training; HMP: Hot Moist Pack, tDCS: Transcranial Direct Current Stimulation
Table 2: Summary of Included Studies in the Meta-Analysis (Intervention).
Outcome Measures and Statistical Analysis
Meta-analysis was performed to compare pain and disability outcome measures between combination of ES and MCE (ES+MCE) and other control groups. Control groups were summarized into four groups, which were non-ES controls (sham, placebo or without ES), ES controls (other types of ES), exercise controls (other types of exercises), and non-exercise controls (sham, placebo or without exercises). All data analyses were performed using STATA SE-64 version software. If the outcomes used different scales, like 10 cm or 100 mm VAS or 0~10 point numerical pain rating scale of pain measure, they were converted into a same one. A Standardized Mean Difference (SMD) with a corresponding 95% Confidence Interval (CI, lower and upper limits) was calculated for the outcomes of studies combined. The heterogeneity decided the effects model we chose. A Χ2-based test of heterogeneity was performed and determined by the inconsistency index (I2) and Q statistics. Thus, a random effects model was used if the I2 statistic was greater than 50% and a fixed model was used when the I2 statistic was smaller than 50%. We conducted a sensitivity analysis for the outcomes to explore the effects of the included articles with high heterogeneity by leave-one-out approach, and planned to assess publication by funnel plot if more than 10 studies are required as Cochrane recommended [23]. Subgroup analysis was performed to evaluate treatment efficacy according different types of ES and intervention duration (=4 or >4 weeks). We paid attention to muscle in the treatment of CNSLBP, so during the subdividing of the studies, the interventions groups, which applied ES into muscular fiber, was subdivided into the NMES group. The others were subdivided into the non-NMES group.
Results
Search Methods
The database search retrieved 424 records of trials and 2 additional records were identified through citation tracking. Among which, 134 duplicates were removed and 263 trails were excluded after screening the title and abstract.
Seventeen trails were excluded in the full-text reviewing and eventually 10 trails were included the systematic review and meta-analysis. Figure 1 presented a flow diagram of study selection.
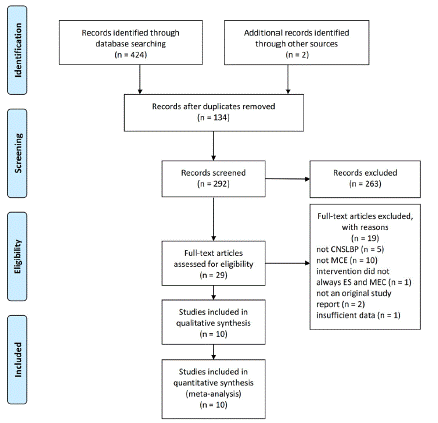
Figure 1: PRISMA flow diagram.
Included Studies and Study Characteristics
In total, 665 participants were enrolled in ten trials. The percentage of female in the included studies ranged from 48.4% to 100%. Three studies (24-26) targeted the situation of CNSLBP of female because low back pain had a 20% increased risk in female compared with male [1]. Two studies [27,28] did not provide the sex of populations. Other studies [12,17,29-31] recruited the patients included females and males. The studies were performed in Asia (Pakistan, Turkey, South Korea, India), America (USA, Brazil), Africa (Egypt), Europe (Italy). The study periods varied widely, with the shortest being 2 weeks and the longest 12 weeks. But most of the studies consisted of 4~6weeks intervention duration. Only one study [29] reported adverse event, which was one participant tripped and fell with no correlation of the intervention. Other studies showed that the patients had great tolerance of ES+MCE. Table 1, 2 described the characteristics of the included studies.
Risk of Bias and Quality Assessment
Figure 2A, B showed the potential risks of bias for individual studies. All studies had bias in one or more categories. The most significant bias came from the performance category, especially in the blinding of therapists because of the application of intervention. And two studies [25,27] did not provide enough information to assess all biases. The heterogeneity of the studies didn’t reduce after sensitivity analysis. Quality assessment of pain scores and disability outcomes were conducted as GRADE recommended and presented in Table 3, 4.
Study (quality of evidence)
Measurement
Populations
Baseline
During the intervention
Immediately after the intervention
Follow-up
Mean changes from baseline
ES+MCE Versus non-ES controls(low)
Alrwaily, M., et al. (2018)30
NPRS
15 vs 15
4.20(1.9) vs 4.44(1.8)
NA
2.34(1.5) vs 2.07(1.1)
NA
NA
Atli, E., et al. (2021)12
VAS
4 weeks: 15 vs 15
8 weeks: 14 vs 136.16(1.67) vs 5.41(1.28)
NA
1.79 (1.68) vs 1.79(1.03)
1.78(1.75) vs 1.85(1.4)
4 weeks: -4.37(0.01) vs -3.62(0.25)
8 weeks: -4.38 (0.08) vs -3.56 (0.12)Durmus, D., et al. (2009)25, a
VAS
20 vs 20
5.05(1.65)vs 4.80(1.1)
NA
1.20(1.13) vs 2.30(0.875)
NA
NA
Franco, K. M., et al. (2016)17
NPRS
74 vs 74
6.5(1.8) vs 6.7(1.6)
NA
2.2(2.1) vs 2.5(2.4)
4.4(2.8) vs 4.3(2.6)
6weeks: 4.3(3.4~5.1) vs 4.3(3.5~5.0)
6 months: 2.2(1.2~3.2) vs 2.4(1.7~3.2)Embaby, E. A., et al. (2021)24
NPRS
15(LFPCs) vs 16(RC) vs 14
7.13(1.3)(LFPCs) vs 7.12(1.15)(RC) vs 7.28(1.59)
NA
2.53(0.99)(LFPCs) vs 2.5(1.26)(RC) vs 5.5(1.16)
NA
NA
Straudi, S., et al. (2018)31, b
VAS
18 vs 17
55.7(18.3) vs 50.3(24.4)
T1:38.8(23.4) vs 41.5(24.2)
NA
NA
T1: –16.9(20.4) vs –8.8(29.2)
T2: –21.2(30.7) vs –7.2(32.5)
T3: –27.7(30.4) vs –2.2(30.1)ES+MCE Versus ES controls(low)
Embaby, E. A., et al. (2021)24
NPRS
15(LFPCs) vs 16(RC)
7.13(1.3) (LFPCs) vs 7.12(1.15) (RC)
NA
2.53(0.99) (LFPCs) vs 2.5(1.26) (RC)
NA
NA
ES+MCE Versus exercise controls (very low)
Akhtar, M. W., et al. (2017)28
VAS
53 vs 55
5.77 (1.08) vs 5.40(1.24)
NA
2.69 (0.93) vs3.69(0.79)
NA
3.08vs 1.71(reduction)
Mane, N. P., et al. (2019)27
NPRS
33 vs 33
4.09(1.04) vs 4.00 (0.93)
NA
0.90 (0.76) vs 0.90(0.84)
NA
NA
ES+MCE Versus non-exercise controls(low)
Hicks, G. E., et al. (2016)29
NPRS
26vs31
5.7 (1.7) vs6.2 (2.1)
NA
3.7 (2.1) vs3.9 (2.6)
3.2 (2.3) vs3.5 (1.9)
NA
Kim, T. H., et al. (2014)26
VAS
27vs26
56.1(7.9) vs 54.9(9.8)
NA
20.6(8.1) vs 49.1(11.1)
26.7 (8.9) vs 52.6(10.2)
NA
ES: Electrical Stimulation; MCE: Motor Control Exercise; NPRS: Neuromuscular Electrical Stimulation; NA: Not Available; VAS: Visual Analog Scale; LFPCs: Low-Frequency Pulsed Currents; RC: Russian Current
The score for pain is presented as the mean (SD) useless otherwise noted.
aDurmus, D., et al. (2009) The original data of VAS did not provide in the study, The data presented was converted by the data provided in the pain score of quality of life, depression of the patients.
bStraudi, S., et al. (2018) T1~3 presented baseline, immediately post-treatment, 1 month follow-up.
Table 3: Summary of Pain Scores.
Study
Measurement
Populations
Baseline
During the intervention
Immediately after the intervention
Follow-up
Mean changes from baseline
ES+MCE Versus non-ES controls(low)
Alrwaily, M., et al. (2018)30
MODQ
15 vs 15
30.52(7.8) vs 30.80(10.2)
NA
14.49(10.2) vs 12.81(5.2)
NA
NA
Atli, E., et al. (2021)12
ODI
4 weeks: 15 vs 15
8 weeks: 14 vs 1322.8(8.61) vs 26(15.95)
8(6.63) vs 10(5.01)
8.86(5.96) vs 8.92(6.2)
4 weeks: -14.8(1.98) vs -16 (10.94)
8 weeks: -13.94(2.65) vs -17.08 (9.75)Durmus, D., et al. (2009)25
ODQ
20 vs 20
28.40(5.30) vs 26.40(5.94)
NA
6.8(2.52) vs 8.40(3.99)
NA
NA
Franco, K. M., et al. (2016)17, a
RMDQ
74 vs 74
11.9(5.0) vs 12.1(4.4)
NA
4.2(4.1) vs 4.8(5.1)
6.3(5.6) vs 6.8(6.2)
6weeks:7.5(6.0~9.1) vs 7.5(6.0~9.1)
6months:5.7(4.1~7.4) vs 5.4 (3.7~7.1)Straudi, S., et al. (2018)31, b
RMDQ
18 vs 17
9.2 (4.1) vs 10.2 (4.7)
NA
NA
NA
T1:3.1(2.9) vs –2.7(3.8)
T2: –3.8(3.7) vs –3.5(4.2)
T3: –4.7(3.9) vs –3.5(4.4)ES+MCE Versus ES controls
none
ES+MCE Versus exercise controls (low)
Mane, N. P., et al. (2019)27
MODQ
33 vs 33
30.48(10.92) vs 24.72(11.64)
NA
2.78(2.95) vs 3.39(3.58)
NA
NA
ES+MCE Versus non-exercise controls(moderate)
Hicks, G. E., et al. (2016)29
MODQ
26vs31
34.9(11.1) vs 33.2(8.9)
NA
22.(11.1) vs 25.6(11.4)
23.1(14.4) vs 27.8(10.1)
NA
ES: Electrical Stimulation; MCE: Motor Control Exercise; MODQ: Modified Oswestry Disability Questionnaire; NA: Not Available, ODI: Oswestry Disability Index, ODQ: Oswestry Disability Questionnaire, RMDQ: Roland Morris Disability Questionnaire
The score for disability is presented as the mean (SD) useless otherwise noted.
a Franco, K. M., et al. (2016) changes from baseline presented as mean (95% CI)
b Straudi, S., et al. (2018) T1~3 presented baseline, immediately post-treatment, 1 month follow-up.
Table 4: Summary of Disability Outcomes.
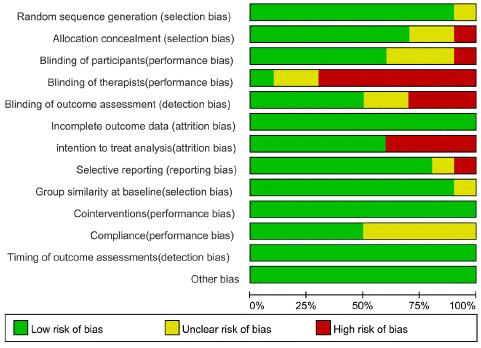
Figure 2A: Risk of bias graph.
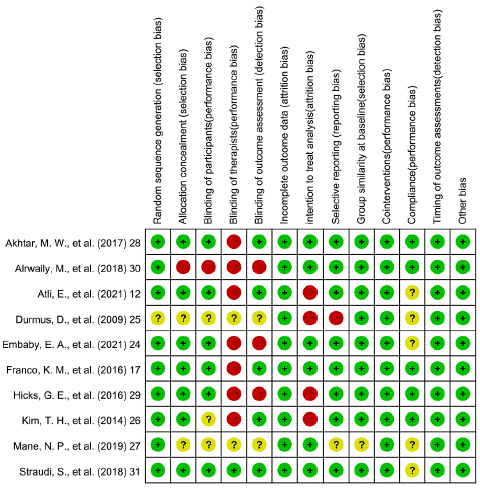
Figure 2B: Risk of bias summary.
Outcomes
All included studies measured pain intensity with avisual analogue scale or numerical pain rating scale. Seven studies provided disability outcomes with Modified Oswestry Disability Questionnaire, Oswestry Disability Index, Oswestry Disability Questionnaire and Roland Morris Disability Questionnaire. Subgroup analysis and sensitive analysis were only performed in the group of ES+MCE Versus non-ES controls because other subgroups merely consisted of one or two studies. Moreover, no funnel plot was conducted for no groups have more than ten studies. Pain relief and disability outcomes in patients were summarized in Table 3, 4 separately. And Figure 3 presented the meta-analysis of pain outcome while Figure 4 presented disability outcome.
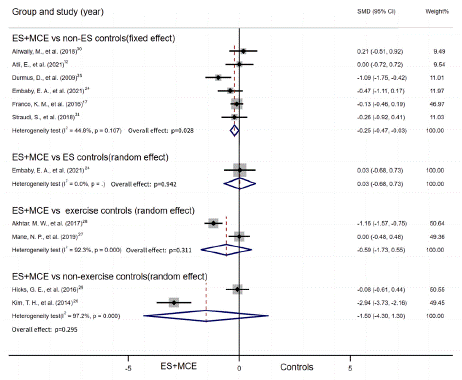
Figure 3: Summary of pain outcome.
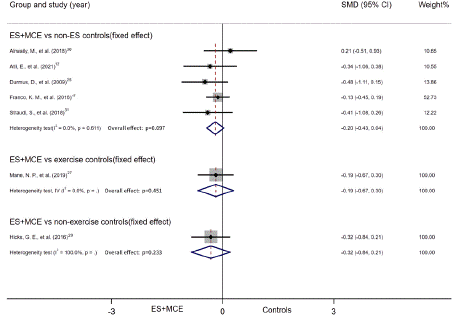
Figure 4: Summary of disability outcome.
Pain Intensity
ES+MCE Versus non-ES control: Six studies reported numerical data (mean and SD) for pain scores after the intervention for patients who received ES+MCE or the control group without ES and were included in the meta-analysis. There was evidence of heterogeneity among the studies (I2=44.8%); therefore, a fixed effect model of analysis was used. The total SMD indicated that pain relief did differ significantly between the two groups (SMD = -0.25, 95% CI,-0.47 to -0.028, P=0.028), while this result was of low quality. The quality of evidence was low and differed insignificantly in pain relief of the NMES group (SMD = -0.35, 95% CI, -0.91 to 0.21, P = 0.218) or non-NMES group (SMD = -0.16, 95% CI, 0.45 to 0.1, P=0.289) vs non-ES controls (Figure 5A). The pain relief in subgroup of duration had no significant difference between the groups with duration less than 4 weeks (SMD = -0.14, 95% CI, -0.63 to 0.35, P=0.577) and the control groups, as well as those with duration over 4 weeks (SMD = -0.35, 95% CI, -0.83 to 0.12, P=0.146) and their control groups (Figure 5B).
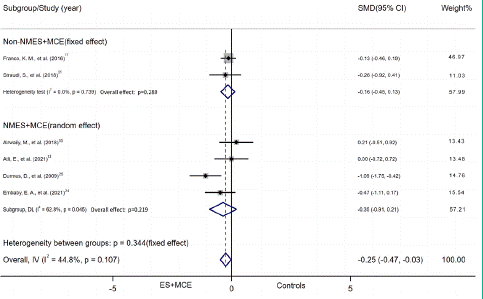
Figure 5A: Subgroup of pain outcome by intervention.
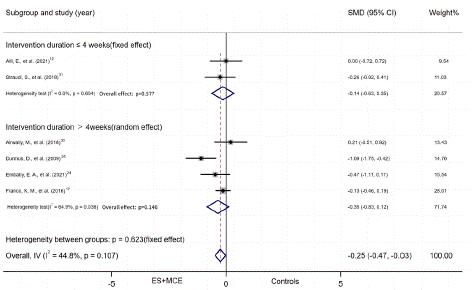
Figure 5B: Subgroup of pain outcome by duration.
ES+MCE Versus ES controls: Only one study reported numerical data (mean and SD) for pain scores between ES+MCE and ES controls, however, the quality of which is low,and the result indicated that the pain relief differed significantly between ES+MCE and ES controls (SMD = 0.03, 95% CI, -0.68 to 0.73, P=0.94).
ES+MCE Versus exercise controls: Two studies were included in this group, which compared the pain scores after the intervention of ES+MCE to exercise controls. Random effect model (I2=92.3%) was adapted. The result with very low quality found no significant difference in pain relief between the two groups (SMD = -0.59, 95% CI, -1.73 to 0.55, P=0.31).
ES+MCE Versus non-exercise controls: Two studies were included in this group, which compared the pain scores after intervention of ES+MCE with non-exercise controls. We adapted the random effect model (I2=92.3%). A low quality of evidence indicated no significant difference in pain relief between the two groups (SMD = -1.50, 95% CI, -4.30 to 1.30, P=0.30).
Disability
ES+MCE Versus non-ES controls: Five studies reported numerical data (mean and SD) for disability outcome between patients who received ES+MCE or the control group without ES. There was no evidence of heterogeneity among the studies (I2=0%). Thus, we adapted fixed effect model. And the quality of the result was low. The 95% CI indicated that the disability outcome showed no significant difference between the 2 groups (SMD = -0.20, 95% CI, -0.43 to 0.04, P=0.097). Random effect model was used only in the subgroup of NMES+MCE vs non-ES controls (I2=62.8%). There was no significant difference in the subgroups. (NMES subgroup: SMD = -0.23, 95% CI, -0.63 to 0.17, P=0.257; Non-NMES subgroup: SMD = -0.18, 95% CI, -0.47 to 0.11, P=0.220; duration=4 weeks subgroup: SMD = -0.37, 95% CI, -0.87 to 0.12, P=0.283; duration 4weeks subgroup: SMD = -0.15, 95% CI, -0.41 to 0.12, P=0.134) (Figure 6A, B).
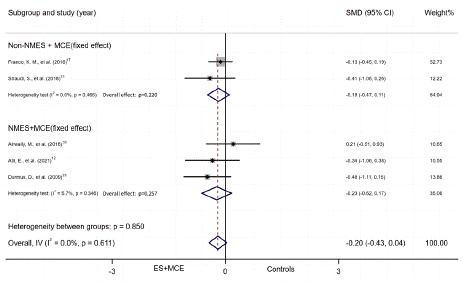
Figure 6A: Subgroup of disability outcome by intervention
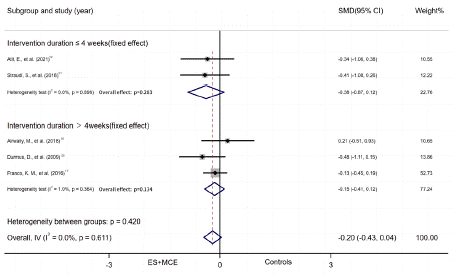
Figure 6B: Subgroup of disability outcome by duration.
ES+MCE Versus ES controls: No study was included in this group.
ES+MCE Versus exercise controls: Only 1 study was included in this group and the quality of evidence was low, which indicated that the disability outcome did not differ significantly between the ES+MCE and exercise controls (SMD =-0.19, 95% CI, -0.67 to 0.3, P=0.451).
ES+MCE Versus non-exercise controls: Only one study reported numerical data (mean and SD) of disability outcome. Moderate quality evidence indicated no significant difference in disability outcome between the ES+MCE and non-exercise controls (SMD = -0.32, 95% CI, -0.84 to 0.21, P=0.233).
Discussion
The meta-analysis evaluated the effect of ES+MCE for the treatment of CNSLBP. Ten studies enrolled 665 participants were included in this meta-analysis, which compared the ES+MCE to the non-ES controls, ES controls, non-exercise controls or exercise controls with pain intensity and disability outcome. The previous reviews were focused on one particular treatment, such as MCE [22], Yoga [32], transcutaneous electrical nerve stimulation [33] and so on. To our knowledge, this is the first systematic review to consider whether the combination of ES+MCE is effective to CNSLBP or not. Our study showed that ES+MCE had significant difference compared with non-ES controls in pian relief, but there was no significant difference in the disability outcome.
The pathoanatomical cause of CNSLBP cannot be determined, so the treatment focuses on reducing pain and its consequence [34]. Exercise therapy and behavioral therapy represent first-line options, with medications considered to be second-line options [4]. According to the model put up by Panjabi, deep trunk muscles contributed a lot to the lumbar stabilization [5] and multifidus muscle is responsible for more than two thirds of the spinal motion [35]. It was confirmed that compared with asymptomatic, healthy persons, patients with chronic low back pain had smaller multifidus muscle cross-sectional area in the fourth lumbar vertebrum to fifth lumbar vertebrum [36]. MCE was developed based on the principle that individuals with LBP had a lack of control of the trunk muscles. And MCE was recommended in two low back pain guidelines [34,37]. A meta-analysis of MCE to nonspecific LBP with at least 6 weeks’ duration or recurrent showed that MCE, alone or as a supplement to another therapy, was effective in reducing pain and disability [21]. But another Cochrane review of MCE found that it was not superior to other forms of exercises [22], which indicated the limited effect of MCE.
Our results found that a combination of ES and MCE was superior to MCE of pain relief, which was consistent with previous findings. The patients with high level of pain intensity or with kinesophobia can choose ES+MCE as a treatment. And the theories to interpret the pain-reducing effect varied from the different types of ES, like NMES for deep trunk muscle activation and stimulation of type II fiber [16] and transcutaneous electrical nerve stimulation and percutaneous electrical nerve stimulation for the release of endogenous opioids and the gate control theory of pain [14]. A meta-analysis of ES confirmed that it was significantly better than placebo/control during intervention [38]. But the subgroup analysis indicated NMES did not provide additional benefits of pain relief and the intervention duration was not of great significance either. Assessing the underlying mechanisms of pian reducing in ES could provide more effective evidence, and we hope future studies will pursue this as an avenue of research. The results of other control groups in pain relief were not conclusive because only one or two studies was included in this meta-analysis. More studies are yearned for.
As for the disability outcome, we thought the improvement of disability outcome for the combination of ES and MCE was accordance with the model of spinal instability. And ES has benefits in reducing pain could improve patients’ adherence to exercise program, though the result of this meta-analysis was not aligned with our hypothesis.
One possibility is that ES truly does not add any clinical benefits when combined with MCE. A meta-analysis of MCE indicated that MCE was probably more effective than a minimal intervention for reducing pain, but probably did not have an important effect on disability in patients with chronic LBP [22]. Though NMES was observed to provide benefits to multifidus contraction among chronic LBP [11], the subgroup analysis of NMES+ES did not make a significant difference. It indicated that the multidisciplinary treatments targeted on the musculoskeletal system were not enough to improve the disability outcome. Second possibility is that the improvement can be showed in follow-ups, considering of the fact that ES has better performance of disability outcome in long follow-ups. A randomized controlled trail suggested that electrical muscle stimulation can be an effective adjunctive treatment modality for LBP. The effect of this combined therapy seem to last beyond the duration of ES treatment [18]. A meta-analysis of twelve studies enrolling 700 patients over 8 countries indicated that transcutaneous electrical nerve stimulation did not provide improvement in disability when compared with control treatment, but it was more effective in improving functional disability within 6 weeks after the treatment [33]. Our study did not have enough RCTs to make a confirm conclusion of the effect of MCE+ES in follow-ups, which needed further explorations. This systematic review has certain limitations. Our analysis included a limited number of studies with a variance of interventions and a lack of follow-up analysis among the studies. It raises an important issue that more detailed subgroups analysis of ES and outcomes need more high-quality RCTs in the future studies, like the type and dosage of ES, morphology of deep trunk muscles, long-time intervention duration and follow-up observation of the combination of ES and MCE.
Conclusions
Our study supported combination of electrical stimulation and motor control exercise to alleviate the CNSLBP. No significant difference for the disability outcome was observed in our studies. The treatment of ES+MCE is a good option for CNSLBP with high level of pain intensity or with kinesophobia, but it is not suitable to improve the disability outcome.
Author Statements
Funding
The authors supported by Shanghai Clinical Research Center for Musculoskeletal Health (20MC1920600), National Natural Science Foundation of China (82074466, 82374481) and Clinical study of traditional Chinese manipulation in the treatment of Frozen Shoulder (22Y21920200).
Disclosure
We declared no known conflicts of interest or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to express our gratitude to all those who helped us during the writing of this study. A special acknowledgement should be shown to Miss Qian for her help to perfect the figures included in the systemic review.
References
- Vlaeyen JWS, Maher CG, Wiech K, Van Zundert J, Meloto CB, Diatchenko L, et al. Low back pain. Nat Rev Dis Primers. 2018; 4: 52.
- Cieza A, Causey K, Kamenov K, Hanson SW, Chatterji S, Vos T. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2021; 396: 2006-17.
- Ladouceur A, Rustamov N, Dubois JD, Tessier J, Lehmann A, Descarreaux M, et al. Inhibition of pain and pain-related brain activity by heterotopic noxious counter-stimulation and selective attention in chronic non-specific low back pain. Neuroscience. 2018; 387: 201-13.
- Chiarotto A, Koes BW. Nonspecific low back pain. N Engl J Med. 2022; 386: 1732-40.
- Panjabi MM. The stabilizing system of the spine. Part I. Function, dysfunction, adaptation, and enhancement. J Spinal Disord. 1992; 5: 383-9.
- Bergmark A. Stability of the lumbar spine. A study in mechanical engineering. Acta Orthop Scand Suppl. 1989; 230: 1-54.
- Shadani A, Mohseni Bandpei MA, Rahmani N, Bassampour SA. A comparison of the abdominal and lumbar multifidus muscle size in patients with lumbar spondylolisthesis and healthy patients at rest and during contraction using ultrasonography. J Manipulative Physiol Ther. 2018; 41: 691-7.
- Akbari A, Khorashadizadeh S, Abdi G. The effect of motor control exercise versus general exercise on lumbar local stabilizing muscles thickness: randomized controlled trial of patients with chronic low back pain. J Back Musculoskelet Rehabil. 2008; 21: 105-12.
- Foster N. Therapeutic exercise for spinal segmental stabilization in low back pain: scientific basis and clinical approach. Phys Ther Rev. 2000; 5: 247-8.
- Vikranth GR. Effectiveness of core stabilization exercises and motor control exercises in patients with low back ache. Int J Physiother. 2015; 2.
- Sions JM, Crippen DC, Hicks GE, Alroumi AM, Manal TJ, Pohlig RT. Exploring neuromuscular electrical stimulation intensity effects on multifidus muscle activity in adults with chronic low back pain: an ultrasound imaging-informed investigation. Clin Med Insights Arthritis Musculoskelet Disord. 2019; 12: 1179544119849570.
- Atli E, Karagözoglu Coskunsu D, Ozyemisci Taskiran O, Turan Z. Does neuromuscular electrical stimulation have an effect on disabiliy, pain, abdominal and lumbar muscle thickness in chronic low back pain? Gait Posture. 2020; 81: 13-4.
- Franco YR, Franco KF, Silva LA, Silva MO, Rodrigues MN, Liebano RE, et al. Does the use of interferential current prior to Pilates exercises accelerate improvement of chronic nonspecific low back pain? Pain Manag. 2018; 8: 465-74.
- Lepley LK, Wojtys EM, Palmieri-Smith RM. Combination of eccentric exercise and neuromuscular electrical stimulation to improve quadriceps function post-ACL reconstruction. Knee. 2015; 22: 270-7.
- Bilgin S, Temucin CM, Nurlu G, Kaya DO, Kose N, Gunduz AG. Effects of exercise and electrical stimulation on lumbar stabilization in asymptomatic subjects: a comparative study. J Back Musculoskelet Rehabil. 2013; 26: 261-6.
- Alrwaily M. A comparison between stabilization exercises and stabilization exercises supplemented with neuromuscular electrical stimulation in patients with chronic low back pain: A phase I randomized controlled trail. 2017.
- Franco KM, Franco YD, de Oliveira NB, Miyamoto GC, Santos MO, Liebano RE, et al. Is interferential current before Pilates exercises more effective than placebo in patients with chronic nonspecific low back pain?: A randomized controlled trial. Arch Phys Med Rehabil. 2017; 98: 320-8.
- Glaser JA, Baltz MA, Nietert PJ, Bensen CV. Electrical muscle stimulation as an adjunct to exercise therapy in the treatment of nonacute low back pain: A randomized trial. J Pain. 2001; 2: 295-300.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009; 62: e1-34.
- Furlan AD, Malmivaara A, Chou R, Maher CG, Deyo RA, Schoene M, et al. 2015 Updated method guideline for systematic reviews in the cochrane back and neck group. Spine (Phila Pa 1976). 2015; 40: 1660-73.
- Maher C, editor. The effect of motor control exercises versus graded activity in patients with chronic low back pain: a randomised controlled trial. 2011.
- Saragiotto BT, Maher CG, Yamato TP, Costa LO, Menezes Costa LC, Ostelo RW, et al. Motor control exercise for chronic non-specific low-back pain. Cochrane Database Syst Rev. 2016; 2016: CD012004.
- Higgins J, Green S. GSe, cochrane handbook for systematic reviews of interventions. Naunyn-Schmiedebergs Arch Exp Pathol Pharmakopsychiatr. 2011; 5: 38.
- Embaby EA, Elsayed WH, Ahmed RM. Abdel azeim AS. Comparative effectiveness of Russian current and low-frequency pulsed current in mechanical low back pain. Turk J Physiother Rehabil. 2021; 32: 12285-94.
- Durmus D, Durmaz Y, Canturk F. Effects of therapeutic ultrasound and electrical stimulation program on pain, trunk muscle strength, disability, walking performance, quality of life, and depression in patients with low back pain: a randomized-controlled trial. Rheumatol Int. 2010; 30: 901-10.
- Kim TH, Kim EH, Cho HY. The effects of the CORE programme on pain at rest, movement-induced and secondary pain, active range of motion, and proprioception in female office workers with chronic low back pain: a randomized controlled trial. Clin Rehabil. 2015; 29: 653-62.
- Mane NP, Varadharajulu G, Shinde S. Effect of motor control training on isolated lumbar stabilizer and core muscle training in chronic low back pain patients. Indian J Public Health Res Dev. 2019; 10: 37-42.
- Akhtar MW, Karimi H, Gilani SA. Effectiveness of core stabilization exercises and routine exercise therapy in management of pain in chronic nonspecific low back pain: a randomized controlled clinical trial. Pak J Med Sci. 2017; 33: 1002-6.
- Hicks GE, Sions JM, Velasco TO, Manal TJ. Trunk muscle training augmented with neuromuscular electrical stimulation appears to improve function in older adults with chronic low back pain: a randomized preliminary trial. Clin J Pain. 2016; 32: 898-906.
- Alrwaily M, Schneider M, Sowa G, Timko M, Whitney SL, Delitto A. Stabilization exercises combined with neuromuscular electrical stimulation for patients with chronic low back pain: a randomized controlled trial. Braz J Phys Ther. 2019; 23: 506-15.
- Straudi S, Buja S, Baroni A, Pavarelli C, Pranovi G, Fregni F, et al. The effects of transcranial direct current stimulation (tDCS) combined with group exercise treatment in subjects with chronic low back pain: a pilot randomized control trial. Clin Rehabil. 2018; 32: 1348-56.
- Wieland LS, Skoetz N, Pilkington K, Vempati R, D’Adamo CR, Berman BM. Yoga treatment for chronic non-specific low back pain. Cochrane Database Syst Rev. 2017; 1: CD010671.
- Wu LC, Weng PW, Chen CH, Huang YY, Tsuang YH, Chiang CJ. Literature review and meta-analysis of transcutaneous electrical nerve stimulation in treating chronic back pain. Reg Anesth Pain Med. 2018; 43: 425-33.
- Maher C, Underwood M, Buchbinder R. Non-specific low back pain. Lancet. 2017; 389: 736-47.
- Wilke HJ, Wolf S, Claes LE, Arand M, Wiesend A. Stability increase of the lumbar spine with different muscle groups. A biomechanical in vitro study. Spine. 1995; 20: 192-8.
- Hides J, Gilmore C, Stanton W, Bohlscheid E. Multifidus size and symmetry among chronic LBP and healthy asymptomatic subjects. Man Ther. 2008; 13: 43-9.
- Knezevic NN, Candido KD, Vlaeyen JWS, Van Zundert J, Cohen SP. Low back pain. Lancet. 2021; 398: 78-92.
- Resende L, Merriwether E, Rampazo ÉP, Dailey D, Embree J, Deberg J, et al. Meta-analysis of transcutaneous electrical nerve stimulation for relief of spinal pain. Eur J Pain. 2018; 22: 663-78.