
Research Article
Phys Med Rehabil Int. 2023; 10(3): 1221.
Functional Connectivity, Physical Activity, and Behavioral Abnormality in Patients with Vascular Cognitive Impairment
Ya-Ting Chang, MD, PhD1,2; Chun-Ting Liu, MD3,4; Shih-Wei Hsu, MD5; Chen-Chang Lee, PhD5; Pei-Ning Huang, BS1
1Department of Neurology, Kaohsiung Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Taiwan
2Department of Psychiatry, Osaka University Graduate School of Medicine, Japan
3Department of Chinese Medicine, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Taiwan
4Graduate Institute of Integrated Medicine, College of Chinese Medicine, China Medical University, Taiwan
5Department of Radiology, Kaohsiung Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Taiwan
*Corresponding author: Ya-Ting Chang Department of Neurology, Kaohsiung Chang Gung Memorial Hospital 123, Ta-Pei Road, Niaosung, Kaohsiung 83301, Taiwan. Tel: +886-7-731-7123 ext. 3389 Email: emily0606@cgmh.org.tw
Received: November 21, 2023 Accepted: December 19, 2023 Published: December 26, 2023
Abstract
Purpose of the research: Neuropsychiatric Symptoms (NPSs) can negatively impact the survival and quality of life in Vascular Cognitive Impairment (VCI) patients. Physical Activity (PA) has been shown to reduce NPSs in dementia patients, possibly by influencing synaptic plasticity. This study investigates the relationship between NPSs, Default Mode Network (DMN), and PA in patients with VCI.
Methods: The study included 42 VCI patients. Functional Connectivity (FC) within the DMN and neurobehavioral performance was assessed. NPSs were categorized. The severity of Hyperactivity and Behavioral Symptoms (HBS) was quantified using a Hyperactivity and Behavioral Composite Score (HBCS). Patients’ PA levels were measured using Fitbit Charge 2.
Principal results: After accounting for disease severity, increased FC between the left Posterior Cingulate Cortex (PCC) seed and the right inferior parietal gyrus was linked to more severe HBS. There was an inverse correlation between HBCS and average step counts per day (steps/d) as well as average distance per day (km/d). This suggests that higher levels of PA were associated with less severe HBS. HBCS was also inversely correlated with steps/d and km/d, reinforcing the idea that increased PA was linked to reduced symptom severity.
Major conclusions: The study concludes that increased FC within the DMN is associated with more severe HBS in VCI patients. Greater levels of PA (measured by step counts and distance) were associated with a reduction in the severity of HBS. This suggests that FC within the DMN may play a role in the modulation of HBS by PA in VCI patients.
Keywords: Actigraphy; Brain network; Cognition; Functional connectivity; Neuropsychiatric symptoms; Physical activity
Abbreviations: ACS: Affective Composite Scores; AD: Alzheimer’s Disease; HBCS: Hyperactivity and Behavioral Composite Scores; HBS: Hyperactivity and Behavioral Symptoms; CDR: Clinical Dementia Rating; CVVLT-10 min: free recall of number of items retrieved over four learning trials of a 9-word list 10 minutes after Chinese Version Verbal Learning Test (CVVLT); FC: Functional Connectivity; FDS: Forward Digital Span; FLAIR: Fluid-Attenuated-Inversion-Recovery; IADL: Instrumental Activities of Daily Living; IPG: Inferior Parietal Gyrus; IQR: Interquartile Range; MoCA: Montreal Cognitive Assessment; MMSE: Mini-Mental State Examination; MNI: Montreal Neurological Institute; MRI: Magnetic Resonance Imaging; rs-fMRI: Rest State Functional MRI; NPS: Neuropsychiatric Symptoms; PA: Physical Activity; PCC: Posterior Cingulate Cortex; PCS: Psychotic Composite Scores; PS: Psychotic Symptoms; PSD: Post-Stroke Depression; ROCF-copy: Modified Rey-Osterrieth Complex Figure copy; ROCF-recall: Modified Rey-Osterrieth Complex Figure recall; TMB: Trail Making Test B; VCI: Vascular Cognitive Impairment; VOSP: Visual Object and Space Perception; WATs: Wearable Activity Trackers
Introduction
Vascular Cognitive Impairment (VCI) is indeed a broad term that encompasses a range of cognitive disorders resulting from Cerebrovascular Diseases (CVDs) [1,2]. These diseases can lead to reduced blood flow to the brain due to blockages or damage to blood vessels. This reduced blood flow can result in cognitive decline [1,2]. It encompasses various conditions, including individuals who exhibit both the pathological changes associated with Alzheimer's Disease (AD) (such as amyloid plaques and neurofibrillary tangles) and vascular diseases (related to blood vessel issues in the brain) [3].
Neuropsychiatric Aymptoms (NPSs) are a common and challenging aspect of various forms of cognitive decline, including VCI. These symptoms can have significant consequences for both the individuals with dementia and their caregivers. NPSs are associated with a more rapid cognitive decline in individuals with VCI [4]. This means that when these symptoms are present, the cognitive abilities of the person with VCI may deteriorate more quickly compared to those without such symptoms. This can make the management of the condition more challenging. Research has shown that the presence of NPSs in VCI can have a negative impact on survival [5]. Individuals who experience these symptoms may have a shorter lifespan compared to those without them. The reasons for this association are complex but could be related to factors like increased stress, physical health complications, and reduced quality of care. NPSs significantly worsen the quality of life for both individuals with VCI and their caregivers [6]. These symptoms can be distressing, disruptive, and challenging to manage. They may lead to social isolation, impaired daily functioning, and a decreased overall sense of well-being for the person with VCI. Caregivers also experience increased stress and burden when managing these symptoms. Some of the common NPSs that frequently occur in VCI include delusions, depression, aggression/agitation, disinhibition, apathy, anxiety [7].
The idea behind clustering similar NPSs together is to improve the effectiveness of studying their underlying causes (pathogenesis) and potential treatments [8]. This approach helps researchers and healthcare professionals gain a better understanding of how certain symptoms may be related and how they can be addressed collectively. Here are the clusters of NPSs that are commonly used in studies [9-11]: Affective Symptoms (AS), Hyperactivity and Behavioral Symptoms (HBS), and Psychotic Symptoms (PS).
AS includes symptoms related to mood and emotions. It typically encompasses conditions such as depression, anxiety, apathy, and sometimes includes sleep or appetite disorders. HBS focuses on symptoms related to hyperactivity and behavior. It includes symptoms like agitation, disinhibition, irritability, and aberrant motor behavior. PS involves symptoms related to psychosis, including hallucinations and delusions. Grouping them together allows for more targeted research and management strategies.
Default Mode Network (DMN) is a network of brain regions that are active when an individual is not focused on the outside world and the brain is at rest, such as during daydreaming or self-reflection [12,13]. Dysfunctions in the DMN have been observed in various neurological and psychiatric conditions. Functional Connectivity (FC) refers to the strength of communication or interaction between different brain regions. Increased or decreased FC between specific brain regions can indicate abnormalities or dysfunctions in the brain's neural networks [12,13]. While there is existing research showing a connection between DMN dysfunctions and NPSs in AD, there is limited research on the same relationship in VCI [14,15]. Treatments that target neural synapses and brain networks are suggested to improve cognitive and NPSs in individuals with neurological disorders [16,17]. Understanding the relationship between NPSs and FC alterations in VCI is important because it could provide insights into potential interventions for managing NPSs in VCI patients. Such interventions may involve modifying neural synapses and brain networks to improve cognitive and behavioral symptoms in individuals with VCI, similar to approaches being explored in AD.
In patients with VCI, there is an increased FC of the Posterior Cingulate Cortex (PCC) with various brain regions within the DMN, including the right inferior temporal gyrus, the left middle temporal gyrus, and the left superior parietal lobule [18]. This altered connectivity may be related to NPSs in VCI. Several studies have explored the relationship between NPSs and FC in patients with a history of stroke [14,15]. For example, in patients with Post-Stroke Depression (PSD), the severity of depression is positively correlated with the FC between the DMN and the salience network [14]. Another study shows decreased FC in the left Inferior Parietal Gyrus (IPG) and increased FC in the left superior frontal gyrus within the DMN in PSD patients [15]. Sub-acute ischemic stroke patients with PSD and/or anxiety symptoms have been found to have increased FC in the left IPG and the left basal nuclei within the DMN when compared to stroke controls [19]. The relationship between increased FC within DMN and NPSs suggests that NPSs in patients with VCI are associated with increased FC within the DMN. This hypothesis is based on the efficacy of antipsychotic [20,21] and antiepileptic [22,23] drugs in managing NPSs, which may be related to modulating hyperactivity within brain networks like the DMN.
Increased Physical Activity (PA) would be associated with reduced NPSs in patients with VCI. This hypothesis is supported by the idea that PA has been found to have beneficial effects on NPSs in people with dementia, especially in community-dwelling individuals [24]. Regular exercise can improve mood, reduce agitation, and enhance overall well-being [24]. When patients engage in PA, it can potentially lead to a reduction in NPSs, making care giving less stressful and burdensome for family members and caregivers [25]. PA may play a role in modulating the activity and connectivity within the DMN.
PA can increase cerebral blood flow, which is important for maintaining brain health and function. Improved blood flow can potentially enhance the functioning of brain regions involved in the DMN. Regular PA is known to reduce oxidative stress, which can be harmful to brain cells. Reduced oxidative stress may help protect brain regions associated with the DMN from damage. PA has been linked to the promotion of neurogenesis and synaptogenesis. These processes can contribute to the brain's ability to adapt and recover from damage, which may be relevant to DMN functioning [26].
To summarize, this study aims to investigate the relationship between NPSs, FC within the DMN, and PA in patients with VCI. The objectives of the present study were to: (1) perform a direct relationship analysis between the NPSs scores and the quantified measures of PA; (2) investigate the relationships among scores of NPSs, measures of PA, and strength of FC within the DMN. Through these analyses, we explored the influence of PA on FC within the DMN and on NPSs in patients with VCI.
Materials and Methods
Study Design and Subjects
The Institutional Review Committee on Human Research at Chang Gung Memorial Hospital granted approval for this study. We obtained informed consents from participants with a Clinical Dementia Rating (CDR) score of 0.5 (n=36). For participants with a CDR score of 1.0 (n=6), their designated caregivers gave informed consent. Within a four-week window, we conducted cognitive tests and Magnetic Resonance Imaging (MRI). A total of forty-two VCI patients were recruited from the Neurology Department at Chang Gung Memorial Hospital. The VCI diagnosis was determined collectively by neurologists and neuroradiologists, adhering to the criteria set by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition [27]. The causes could range from multiple or single territorial infarcts to small or strategic ones. The pathology might also encompass both neurodegenerative and cerebrovascular issues [28,29]. We excluded participants if they: (1) had significant systemic diseases, such as gastrointestinal, renal, hepatic, or respiratory issues, or (2) had other conditions or were on medication that might influence cognitive abilities.
The criteria based on MRI results are as follows: There should be no signs of cortical or watershed infarcts, hemorrhages, hydrocephalus, or specific cause White Matter Lesions (WMLs) like multiple sclerosis. Additionally, there should be no atrophy in the hippocampal or entorhinal cortex, aligning with a zero score on Schelten’s medial temporal lobe atrophy scale [30]. The MRI might show multiple supratentorial subcortical small infarcts (measuring between 3–20 mm in diameter), or any extent of WMLs, or moderate to severe WMLs (with a Fazekas score of 2 or higher [31]). It might also display multiple or single territorial infarcts or small to strategic infarcts.
Neurobehavioral Assessments
Participants underwent a general cognitive assessment using the Montreal Cognitive Assessment (MoCA). Subsequently, these MoCA scores were translated into Mini-Mental State Examination (MMSE) scores [32]. For the assessment of delayed verbal memory, we employed the Chinese Version Verbal Learning Test (CVVLT) [33]. This involved recalling a list of 9 words after a 10-minute interval (termed CVVLT-10 min) over four learning trials [34]. Memory functions were gauged using both CVVLT-10 min [34] and the modified Rey-Osterrieth complex figure recall (ROCF-recall) [35]. To measure visual-spatial capabilities, tools like the Visual Object and Space Perception (VOSP) [36] and the modified ROCF copy (ROCF-copy) [35] were utilized. The Forward Digital Span (FDS) [37] was implemented to test attention. Executive Function (EF) in participants was determined using the corrected Trail Making Test B (TMB) as well as the TMB's time to completion (measured in seconds) [38]. Lastly, the independence of each participant was gauged using the Instrumental Activities of Daily Living (IADL) scores [39].
Patients' Neuropsychiatric Symptoms (NPSs) were assessed using the NPI-12 [9,11]. The NPI-12 scores determined the severity of different symptom syndromes. The AS was represented by the Affective Composite Score (ACS), which combined scores from depression, anxiety, sleep, appetite, and apathy. The HBS was denoted by the Hyperactivity and Behavioral Composite Score (HBCS), aggregating scores from agitation/aggression, aberrant motor behavior, disinhibition, and irritability. Meanwhile, the severity of the PS was represented by the Psychotic Composite Score (PCS), derived from the combined scores of delusion and hallucination [9,11].
MRI Acquisition and Pre-processing
MRI scans for all participants were conducted using a Siemens 3T scanner, with each session lasting approximately 20 minutes. The T1-weighted imaging followed a 3D-MPRAGE protocol in a steady-state sequence featuring a 256×256 mm field of view, a 1-mm slice thickness, and a Repetition Time (TR)/Echo Time (TE) set at 2600 ms/3.15 ms. This scan spanned 5 minutes and 50 seconds.
For the Resting State Functional MRI (rs-fMRI), participants were advised to refrain from sleep deprivation, caffeine, and sedatives for eight hours prior to the scan. They were instructed to remain still, keep their eyes closed, stay awake, and avoid focusing on any specific thoughts during the procedure. Post-scan, participants were questioned about potential sleep, anxiety, or agitation incidents, with none reporting any issues. All patients had been on consistent medical treatment for a minimum of three months to minimize any impact on FC.
The rs-fMRI followed a protocol of TR=2500 ms/TE=27 ms with a voxel size of 3.4×3.4×3.4, taking 8 minutes and 27 seconds. The CONN toolbox (http://www.nitrc.org/projects/conn) [40] was utilized for artifact removal in rs-fMRI. The average signal fluctuation of blood oxygen levels between scans should not exceed 1%. The framewise displacement should stay below 0.25 mm/TR. Images underwent detrending and filtering within a 0.008 to 0.09 Hz range. The CONN toolbox also facilitated the regression of noise such as head movement, white matter signals, and cerebrospinal fluid signals from individual voxels. Our data preprocessing mirrored our past study's approach, incorporating slice time correction, realignment, segmentation, normalization to Montreal Neurological Institute (MNI) standards, spatial smoothing with a 6 mm Gaussian Kernel, and resampling to 2×2×2mm3 [41,42].
For the assessment of WMLs and hemorrhages, both Fluid-Attenuated-Inversion-Recovery (FLAIR) and T2*-weighted MRI sequences were captured. The FLAIR MRI sequence followed a protocol with TR=5000 ms/TE=393 ms and a 1 mm slice thickness, lasting 4 minutes and 37 seconds. The T2*-weighted MRI sequence took 2 minutes.
Seed-based FC
The correlation coefficients between the PCC seeds and all notable clusters depict the voxel-wise FC for every PCC seed. For the ROI-centric functional connectivity analysis, the DMN was rooted in PCC seeds on both sides, characterized by two 10 mm-radius spheres aligned with MNI coordinates (x=±2, y=-51, z=41) [43]. These PCC seeds have shown extensive connectivity with other PCC/precuneus regions and cortical zones [43]. The FC within brain structures rooted on each PCC seed underwent analysis [44].
The DMN's FC was linked to ACS, HBCS, and PCS, and this connectivity was further related to PA metrics. Employing multiple regression analysis through the CONN toolbox [40], each PA measure was associated with FC spanning each network seed and voxels across the entire brain, rooted in the average resting-state BOLD time sequence within individual correlation maps (http://www.nitrc.org/projects/conn) [40,45]. A significance bar was set with an uncorrected p-value threshold of < 0.001 at the peak level and a false discovery rate-adjusted p-value of <0.05 at the cluster level, leveraging the second-level analysis of relative inter-regional functional covariance. All-brain correlation maps underwent a conversion into z-score maps via a Fisher’s r-to-z transformation. Post determining the correlation between each NPS-CS and FC within brain networks, the FC between every seed and peak cluster was additionally extracted [40] for association with each PA metric.
The Activity Monitoring
Participants were provided with Fitbit Charge 2 devices (https://www.fitbit.com/au/charge2) and were guided on how to activate them and authorize data sharing. Fitbit Charge 2 is a GPS-equipped health band that facilitates phone-independent running and captures essential data such as steps taken, distance covered (in kilometers), and calories expended (https://www.fitbit.com/au/one). Moreover, its associated app mainly serves as the control hub for Fitbit Charge 2, supporting account setup and Bluetooth connectivity. The walking data could seamlessly integrate with Smarttrack (https://www.fitbit.com/au/smarttrack). This setup allowed the research team to view participants' live device data. When near the user’s Bluetooth-enabled smartphone, the device autonomously sent activity information to Fitbit's servers. Previous studies have verified the accuracy and consistency of the Fitbit Charge 2 in recording steps, distance [46,47], and caloric burn [48]. This activity monitor, produced by Fitbit, San Francisco, CA (https://www.fitbit.com/au/home), was utilized to gauge Physical Activity (PA). It features a 3-axis accelerometer that identifies step thresholds based on movement patterns typically associated with walking. The device supports wireless auto-syncing with smartphones and offers a battery lifespan of around 14 days.
Participants were instructed to wear the devices continuously for a minimum of 7 days. We collected PA metrics such as daily steps, distance, and calories for every participant. Preliminary checks were conducted to scrutinize data completeness, anomalies, and implausible values.
We accumulated each PA metric over the 7 days, and then determined average PA values by dividing the total by seven. Initially, daily PA data (steps, distance in kilometers, and calories burned) was collated for each participant. Subsequently, we calculated average daily PA metrics (steps/day, km/day, calories/day) for each individual. Our subsequent analyses primarily used these average daily PA values. Adherence to using the smartwatch was verified through the app's logs. Heart rates of participants were monitored at 5-minute intervals, with continuous heart rate graphs available on the app. If a participant displayed any non-adherence, evident from a recorded heart rate dropping to zero, they were asked to continue wearing the device for additional days to ensure a complete seven-day PA log.
Statistical Analyses
All results were presented as the mean ± standard deviation. For data that didn't adhere to a normal distribution, they were displayed as median with the Interquartile Range (IQR). To examine the relationships between patient-level average PA metrics, each NPS Composite Score (CS), and the strength of FC within brain networks based on each PCC seed for all VCI patients, we employed Spearman’s correlation. Statistical analyses were performed using the SPSS software (version 18 for Windows®, SPSS Inc., Chicago, IL), with a P-value<0.05 (two-tailed) deemed as statistically significant.
Results
Demographic Data
Forty-two patients diagnosed with VCI successfully finished the study (Table 1). Given that the education (in years), glycohemoglobin (HbA1c) (%), MMSE score, NPI score, each NPSs CS, along with scores from CVVLT-10 min, ROCF-recall, FDS, TMB (corrected), TMB time to completion (in seconds), VOSP, ROCF-copy, and IADL did not follow a normal distribution, these data points were subsequently presented as median (IQR) in Table 1.
Clinical and cognitive characteristics
Mean±SD
Median (IQR)
Age (years)
67.6±7.1
Education (years)
9.5±4.3
10.5(6-12)
Women/ Men
25/17
Glycohemoglobin (%)
6.4±1.3
5.9(5.7-6.7)
Total cholesterol (mg/dL)
188.1±41.2
Low-density lipoprotein (mg/dL)
109.8±35.1
Hypertension (yes/no)
16/26
MMSE
21.0±5.8
22.5(18-26)
Neuropsychiatric inventory
5.7±6.8
4(0-8)
Psychotic composite score
0.5±1.4
0(0-0)
Hyperactivity and behavioral composite score
1.1±2.7
0(0-0)
4.0±5.5
3.5(0-4)
Memory scores
CVVLT-10 min
3.0±2.9
3(0-6)
ROCF-recall
3.9±4.2
3(0-6)
Attention and executive task scores
Forward Digital Span
7.1±1.5
7.5(6-8)
TMB (corrected)
10.6±4.6
14(7.25-14)
TMB time to completion (seconds)
83.5±40.9
99(51-120)
Visuospatial task scores
VOSP
6.3±3.2
7(4.3-9)
ROCF-copy
10.9±4.8
12(9.3-14)
IADL score
15.9±5.9
16(11-22)
Measures of physical activity
Steps (steps/d)
7463.4±4724.0
Distance (km/d)
4.9±3.1
Calories (calories/d)
1616.8±272.1
Continuous variables presented as mean ± Standard Deviation (SD). Non-parametric continuous variables were further expressed as median (IQR). CVVLT-10 min, free recall after 10 minutes in Chinese version of the Verbal Learning Test; IADL: Instrumental Activities of Daily Living; IQR: Interquartile Range; MMSE: Mini-Mental State Examination; ROCF: Rey-Osterrieth Complex Figure; TMB: Trail Making Test B; VOSP: Visual Object and Space Perception.
Table 1: Demographic data for patients with vascular cognitive impairment.
The Relationship among Strength of FC within DMN, each NPSs CS, and load of PA
The DMN's FC showed a positive correlation with HBCS (P<0.05) but had no significant associations with the NPI score, ACS, or PCS (P>0.05). Due to HBCS's non-normal distribution, its relationship with the DMN's FC was assessed using Spearman’s correlation.
HBCS had a connection with the FC of the left PCC seed to the left frontal lobe (x=-18, y=-8, z=34; p=0.421; P=0.006), the right IPG (x=58, y=-36, z= 54; p=0.428; P=0.005), and the right pyramis (x=20, y=-64, z=-36; p=-0.328; P=0.036). After making adjustments for multiple comparisons, only the connections between the left PCC seed and the left frontal lobe (x=-18, y=-8, z=34; β=0.552; P<0.001) and the right IPG (x=58, y=-36, z=54; β=0.327; P=0.011) remained significant with HBCS.
HBCS also demonstrated links with the FC of the right PCC seed to several regions including the left extra-nuclear white matter (x=-22, y=-34, z=20; p=0.341; P=0.029), left corpus callosum (x=-14, y=30, z=12; p=0.440; P=0.004), SMA (x=0, y=14, z=64; p=-0.313; P=0.047), right IPG (x=58, y=-36, z=54; p=0.363; P=0.020), right supramarginal gyrus (x=40, y=-34, z=42; p=0.360; P=0.021), and right caudate (x=12, y=24, z=2; p=0.365; P=0.019). However, post multiple comparison adjustments, only its association with the left corpus callosum (x=-14, y=30, z=12; β=0.744; P<0.001) remained significant.
Upon multiple comparison adjustments, HBCS maintained its associations only with the FCs of the left PCC seed to the left frontal lobe and right IPG, and the right PCC seed to the left corpus callosum (P<0.05). Among these FCs, only the connection between the left PCC seed and the right IPG related to steps/d (p=-0.492; P=0.001) and km/d (p=-0.491; P=0.001). The FCs of the left PCC seed to the left frontal lobe and the right PCC seed to the left corpus callosum didn't show significant relations with any PA metrics (P>0.05). No FCs within the DMN had an association with calories/d (P>0.05).
The Relationship among FC within DMN, HBCS and load of PA
Many studies have linked BPSD or NPSs to the severity of the disease [49,50], and the MMSE score is commonly utilized to gauge the severity of dementia [51]. To account for the influence of disease severity, we conducted a partial correlation analysis, adjusting for the MMSE score. This adjustment aimed to isolate the impact of MMSE score on the relationship among FC, PA measures, and HBCS. Even after this adjustment, the FC between the left PCC seed and right IPG remained significantly related to HBCS (p=0.656; P<0.001; Figure 1A), steps/d (p=-0.487; P=0.002; Figure 1B), and km/d (p=-0.486; P=0.002; Figure 1B). Concerning the relationship between HBCS and PA intensity, HBCS displayed an inverse correlation with steps/d (p=-0.320; P=0.044; Figure 1C) and km/d (p=-0.313; P=0.049; Figure 1C), after accounting for the MMSE score.
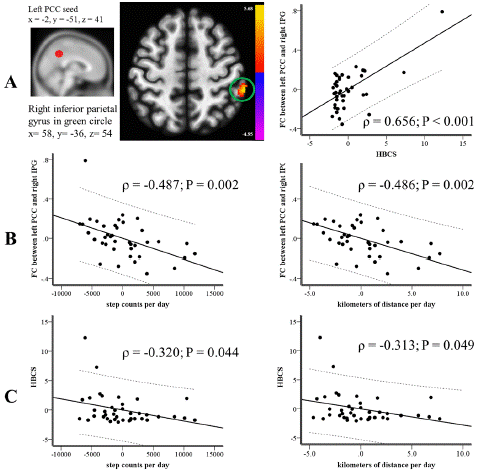
Figure 1: After adjusting for the severity of the disease, (A) The HBCS demonstrated a correlation with FC within the DMN centered on the left PCC seed, as highlighted by the peak cluster in the green circle. (B) The FC within the DMN, centered on the left PCC seed and highlighted in the green circle from part (A), was found to correlate with the average daily step counts (shown in the left panel) and average daily distances in kilometers (shown in the right panel). (C) The HBCS showed a correlation with the average daily step counts (left panel) and average distances covered in kilometers (right panel). DMN: Default Mode Network; FC: Functional Connectivity; HBCS: Hyperactivity and Behavioral Composite Score; PCC: Posterior Cingulate Cortex.
Discussion
Main Findings
The current study yielded three primary observations. Firstly, a higher HBCS directly correlated with augmented FC between the left PCC seed and the right IPG, areas generally implicated in the DMN, for VCI patients. Secondly, a boost in FC between the left PCC seed and the right IPG related to fewer steps/d and km/d, but it showed no relation to calories/d. Lastly, reduced PA in terms of steps/d and km/d corresponded to an elevated HBCS, indicating a heightened severity of HBS symptoms like agitation, disinhibition, irritability, and aberrant motor behavior in VCI patients. However, there was no correlation between calories/d and HBCS.
The HBS and FC within the DMN Anchored on the PCC seed
The initial supposition of this research was that NPSs would correlate with enhanced FC within the DMN for VCI patients. Our findings indicated a direct relationship between the heightened FC of the left PCC seed with the right IPG in the DMN and an increased severity of HBS, gauged by the HBCS, in VCI patients. An earlier investigation into the connection between DMN connectivity and NPSs in preclinical AD participants found a direct link between a higher NPI score and increased metabolism in the PCC [52]. Echoing these findings, our study also demonstrated that a higher HBCS correlated with augmented FC between the left PCC seed and the right IPG.
The heightened FC within the DMN may play a role in the pharmacological approach to managing NPSs. Common treatments for HBS like aggression and agitation include antipsychotic [20,21] and antiepileptic medications [22,23]. These function by blocking dopamine receptors with antipsychotics [53] and reducing neuronal excitability with antiepileptics [54]. The pharmacological actions of these drugs hint that the underlying cause of severe HBS may be related to enhanced neural activity or FC. Supporting this notion, our study found a connection between a higher HBCS and elevated FC within the DMN centered on the left PCC seed.
A prior research highlighted a notable link between HBS and FC within the DMN for patients suffering from AD [55]. The study [55] indicated that more severe HBS were related to diminished FC within the DMN, specifically rooted in the left medial prefrontal cortical seed. However, the HBS didn't show any association with the FC in the DMN when anchored on the PCC seed [55].
Our findings, which demonstrate a positive relationship between a higher severity of HBS and an increase in FC within the DMN, differ from the prior study [55] that found a negative correlation between HBS severity and FC within the DMN. A potential explanation for this discrepancy could be the variation in disease severity between the two studies. In the previous research [55], the average MMSE score for AD patients was 18, while in our VCI patient group, the average MMSE score was 21.
Increased FC within the DMN in the initial stages of VCI [18,19] or AD [52] has been highlighted in earlier studies. Suchamplification in FC might result from neural compensatory mechanisms, synaptic adaptability, and functional adjustments during the early phases of these conditions [52,56]. The more pronounced HBS or NPSs in the beginning stages of VCI might stem from this compensatory increase in FC within the DMN. Consequently, our findings indicate a relationship where a higher HBCS correlates with enhanced FC between the left PCC seed and the right IPG. As the disease progresses to its later stages, a decline in DMN activity is believed to have a growing influence on NPSs [55].
Increased PA, Decreased FC within DMN, and Decreased HBCS
Our study's secondary hypothesis postulated that an increase in PA would influence FC within the DMN, given that PA has been tentatively linked to promoting synaptogenesis [26]. Numerous investigations have delved into the positive impacts of PA on cognition and/or NPSs in patients suffering from mixed or AD-type dementia [24,26,57,58]. However, the majority of these studies have not explored the link between PA, cognition, and/or NPSs specifically in VCI patients. In the context of AD patients, while some studies highlight the beneficial influence of PA on NPSs [58], others have found no significant effect of PA on certain NPSs, such as depression [59].
In our study, which centered on the relationship between PA and NPSs in VCI patients, we found that a rise in PA, as measured by steps/d and km/d, correlated with a reduced severity of HBS. This aligns with findings from a prior investigation [58]. The positive impacts of PA on synaptogenesis have been linked to the mechanisms that underlie its beneficial effects on NPSs [26]. It's believed that in the early stages of vascular or neurodegenerative conditions, there's a momentary boost in FC within the DMN. This surge is thought to be a result of neural compensatory recruitment, synaptic plasticity, and functional reorganization – all strategies the brain employs to recover from neuronal harm [52,56]. Consequently, the observed correlation in our VCI patients between higher PA and reduced FC within the DMN suggests that the advantageous effects of PA on synaptogenesis [26] might be influencing the compensatory amplification of FC within the DMN caused by neural damage.
Previous studies have shown that this amplified FC of the PCC within the DMN in the initial stages of VCI [18] or AD [52] is tied to a heightened severity of HBS or NPSs [19,56]. Building on this, our findings indicate that increased PA, specifically in terms of steps/d and km/d, is linked to a decrease in both FC within the DMN and the severity of HBS in VCI patients.
PA of Steps/d and Km/d Matters more than PA of Calories/d
A comprehensive cohort study of adults indicates that a higher number of daily steps and improved step performance might correlate with a reduced risk of developing dementia [60]. Two studies, one from Taiwan [61] and another from the U.S. [62], have established a connection between a greater number of steps daily and enhanced Executive Function (EF). Another study reveals that individuals with executive dysfunction-Mild Cognitive Impairment (MCI) tend to exhibit more severe symptoms of agitation, disinhibition, and irritability than those with amnestic-MCI [63]. This implies a potential correlation between diminished EF and heightened HBS. This association, particularly concerning agitation and disinhibition, is further substantiated by a study on Alzheimer's patients [64]. Furthermore, a review article underscores the relationship between compromised EF and increased HBS severity, especially concerning symptoms like agitation and disinhibition [65]. The disruption in the fronto-subcortical circuits is believed to be a pivotal factor driving the relationship between EF and HBS [65]. Given that increased daily steps have been linked to the enhancement of fronto-subcortical circuits [66], it's plausible that more steps per day could lead to decreased HBS severity by improving these circuits. In essence, since the functioning of fronto-subcortical circuits is crucial for both EF and HBS, optimizing these circuits through increased daily steps could potentially alleviate the severity of HBS.
Future Directions
PA has been linked to the promotion of synaptogenesis [26]. Its positive effects on HBS could be attributed to its role in enhancing the fronto-subcortical circuits [66], which are crucial in determining the severity of HBS in VCI [65]. This study further posits that PA's influence on the functional connectivity within the DMN could be pivotal in understanding its relationship with HBS in VCI. Additionally, treatments like vascular risk factor modifications and the use of antipsychotics are essential for ameliorating HBS in VCI patients [67]. It's imperative for future studies to delve deeper into the interplay between PA, medication, brain network dynamics, and HBS in VCI patients.
Limitation
This study has several limitations. Firstly, individuals with moderate to severe dementia were not included. Future research should explore the impact of quantified PA metrics on HBS in VCI patients at this stage of dementia. Secondly, a longitudinal approach is required to understand how increased PA affects the evolution of FC within the DMN and HBS over time. Thirdly, the limited sample size might introduce the risk of type I error or produce false positives. To validate our initial findings, a study with a larger sample size is crucial. Nevertheless, we applied multiple corrections in our correlation analyses, which could mitigate the risk of type I error.
Conclusions
In summary, our study revealed a connection between increased daily physical activity (measured by steps and distance) and changes in functional connectivity within the DMN, as well as the severity of HBS in VCI patients. The FC within the DMN played a significant role in the observed association between reduced HBS severity and increased daily steps and distance.
This research highlights the potential role of PA in mitigating neuropsychiatric symptoms in VCI patients and suggests a link between brain network connectivity and symptom severity. Further studies may explore the mechanisms underlying these relationships and consider interventions that promote physical activity to improve the quality of life for individuals with VCI.
Author Statements
Ethics in Publishing
This study was approved by the Institutional Review Committee on Human Research of Chang Gung Memorial Hospital, and all of the participants were provided written informed consent. All procedures were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent for Publication
The Author guarantees that the Contribution to the Work has not been previously published elsewhere, or that if it has been published in whole or in part, any permission necessary to publish it in the Work has been obtained and the Author declares that any person named as co-author of the contribution is aware of the fact and has agreed to being so named.
Author Contributions
Ya-Ting Chang: Conceptualization; Formal analysis; Funding acquisition; Methodology; Project administration; Software; Roles/Writing - original draft; and Writing - review & editing.
Chun-Ting Liu: Data curation; Investigation; Methodology; Project administration.
Shih-Wei Hsu: Data curation; Investigation; Methodology; Project administration.
Chen-Chang Lee: Data curation; Investigation; Methodology; Project administration.
Pei-Ning Huang: Data curation; Project administration.
Conflict of Interest
All of the authors did not have financial or other interest in the product or distributor of the product. There was no kind of associations, such as consultancies, stock ownership, or other equity interests or patent-licensing arrangements, between the authors and the manufacturer or distributor of the product.
Acknowledgement
This work was supported by the National Research Program for Biopharmaceuticals [MOST 109-2628-B-182A-013; MOST 108-2628-B-182A-004].
References
- Dichgans M, Leys D. Vascular cognitive impairment. Circ Res. 2017; 120: 573-91.
- van der Flier WM, Skoog I, Schneider JA, Pantoni L, Mok V, Chen CLH, et al. Vascular cognitive impairment. Nat Rev Dis Primers. 2018; 4: 18003.
- Snyder HM, Corriveau RA, Craft S, Faber JE, Greenberg SM, Knopman D, et al. Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimers Dement. 2015; 11: 710-7.
- Rockwell E, Jackson E, Vilke G, Jeste DV. A study of delusions in a large cohort of Alzheimer’s disease patients. Am J Geriatr Psychiatry. 1994; 2: 157-64.
- Tun SM, Murman DL, Long HL, Colenda CC, von Eye A. Predictive validity of neuropsychiatric subgroups on nursing home placement and survival in patients with Alzheimer disease. Am J Geriatr Psychiatry. 2007; 15: 314-27.
- Torti FM, Jr., Gwyther LP, Reed SD, Friedman JY, Schulman KA. A multinational review of recent trends and reports in dementia caregiver burden. Alzheimer Dis Assoc Disord. 2004; 18: 99-109.
- Han I-W. Behavioral and psychological symptoms in vascular cognitive impairment. In: Stroke revisited: vascular cognitive impairment. 2020; 43-59.
- Gauthier S, Cummings J, Ballard C, Brodaty H, Grossberg G, Robert P, et al. Management of behavioral problems in Alzheimer’s disease. Int Psychogeriatr. 2010; 22: 346-72.
- Cheng ST, Kwok T, Lam LC. Neuropsychiatric symptom clusters of Alzheimer’s disease in Hong Kong Chinese: prevalence and confirmatory factor analysis of the Neuropsychiatric Inventory. Int Psychogeriatr. 2012; 24: 1465-73.
- van der Linde RM, Dening T, Matthews FE, Brayne C. Grouping of behavioural and psychological symptoms of dementia. Int J Geriatr Psychiatry. 2014; 29: 562-8.
- Aalten P, Verhey FR, Boziki M, Bullock R, Byrne EJ, Camus V, et al. Neuropsychiatric syndromes in dementia. Results from the European Alzheimer disease Consortium: part I. Dement Geriatr Cogn Disord. 2007; 24: 457-63.
- Zhou J, Seeley WW. Network dysfunction in Alzheimer’s disease and frontotemporal dementia: implications for psychiatry. Biol Psychiatry. 2014; 75: 565-73.
- Ballarini T, Iaccarino L, Magnani G, Ayakta N, Miller BL, Jagust WJ, et al. Neuropsychiatric subsyndromes and brain metabolic network dysfunctions in early onset Alzheimer’s disease. Hum Brain Mapp. 2016; 37: 4234-47.
- Balaev V, Orlov I, Petrushevsky A, Martynova O. Functional connectivity between salience, default mode and frontoparietal networks in post-stroke depression. J Affect Disord. 2018; 227: 554-62.
- Yao G, Zhang X, Li J, Liu S, Li X, Liu P, et al. Improving depressive symptoms of post-stroke depression using the Shugan Jieyu capsule: A resting-state functional magnetic resonance imaging study. Front Neurol. 2022; 13: 860290.
- Gottschling C, Geissler M, Springer G, Wolf R, Juckel G, Faissner A. First and second generation antipsychotics differentially affect structural and functional properties of rat hippocampal neuron synapses. Neuroscience. 2016; 337: 117-30.
- Dzyubenko E, Juckel G, Faissner A. The antipsychotic drugs olanzapine and haloperidol modify network connectivity and spontaneous activity of neural networks in vitro. Sci Rep. 2017; 7: 11609.
- Sun YW, Qin LD, Zhou Y, Xu Q, Qian LJ, Tao J, et al. Abnormal functional connectivity in patients with vascular cognitive impairment, no dementia: a resting-state functional magnetic resonance imaging study. Behav Brain Res. 2011; 223: 388-94.
- Vicentini JE, Weiler M, Almeida SRM, de Campos BM, Valler L, Li LM. Depression and anxiety symptoms are associated to disruption of default mode network in subacute ischemic stroke. Brain Imaging Behav. 2017; 11: 1571-80.
- Kongpakwattana K, Sawangjit R, Tawankanjanachot I, Bell JS, Hilmer SN, Chaiyakunapruk N. Pharmacological treatments for alleviating agitation in dementia: a systematic review and network meta-analysis. Br J Clin Pharmacol. 2018; 84: 1445-56.
- Lee PE, Gill SS, Freedman M, Bronskill SE, Hillmer MP, Rochon PA. Atypical antipsychotic drugs in the treatment of behavioural and psychological symptoms of dementia: systematic review. BMJ. 2004; 329: 75.
- Cooney C, Murphy S, Tessema H, Freyne A. Use of low-dose gabapentin for aggressive behavior in vascular and Mixed Vascular/Alzheimer dementia. J Neuropsychiatry Clin Neurosci. 2013; 25: 120-5.
- Gallagherand D, Herrmann N. Antiepileptic Drugs Treat Agitation Aggression Dem They Have Place Ther? Drugs. 2014; 74: 1747-55.
- Steichele K, Keefer A, Dietzel N, Graessel E, Prokosch HU, Kolominsky-Rabas PL. The effects of exercise programs on cognition, activities of daily living, and neuropsychiatric symptoms in community-dwelling people with dementia-a systematic review. Alzheimers Res Ther. 2022; 14: 97.
- Christofoletti G, Oliani MM, Bucken-Gobbi LT, Gobbi S, Beinotti F, Stella F. Physical activity attenuates neuropsychiatric disturbances and caregiver burden in patients with dementia. Clinics (Sao Paulo). 2011; 66: 613-8.
- Veronese N, Solmi M, Basso C, Smith L, Soysal P. Role of physical activity in ameliorating neuropsychiatric symptoms in Alzheimer disease: A narrative review. Int J Geriatr Psychiatry. 2019; 34: 1316-25.
- Tang Y, Xing Y, Zhu Z, He Y, Li F, Yang J, et al. The effects of 7-week cognitive training in patients with vascular cognitive impairment, no dementia (the Cog-VACCINE study): A randomized controlled trial. Alzheimers Dement. 2019; 15: 605-14.
- Skrobot OA, Attems J, Esiri M, Hortobágyi T, Ironside JW, Kalaria RN, et al. Vascular cognitive impairment neuropathology guidelines (VCING): the contribution of cerebrovascular pathology to cognitive impairment. Brain. 2016; 139: 2957-69.
- Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006; 37: 2220-41.
- Scheltens P, Leys D, Barkhof F, Huglo D, Weinstein HC, Vermersch P, et al. Atrophy of medial temporal lobes on MRI in ”probable” Alzheimer’s disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry. 1992; 55: 967-72.
- Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987; 149: 351-6.
- Roalf DR, Moberg PJ, Xie SX, Wolk DA, Moelter ST, Arnold SE. Comparative accuracies of two common screening instruments for classification of Alzheimer’s disease, mild cognitive impairment, and healthy aging. Alzheimers Dement. 2013; 9: 529-37.
- Chang CC, Kramer JH, Lin KN, Chang WN, Wang YL, et al. Validating the. Chinese version of the Verbal Learning Test for screening Alzheimer’s disease. J Int Neuropsychol Soc. 2010; 16: 244-51.
- Lezak MD. Neuropsychological assessment. 4th ed. New York: Oxford University Press; 2004.
- Rey A. L’examen psychologique dans les cas d’encéphalopathie traumatique. Arch Psychol. 1941; 28: 286-340.
- Warringtonand EK. M. James. The visual object and space perception battery. Bury St Edmunds, England: Thames Valley Test Company; 1991.
- Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, et al. The Alzheimer’s disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009; 23: 91-101.
- Reitan RM. The relation of the trail making test to organic brain damage. J Consult Psychol. 1955; 19: 393-4.
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969; 9: 179-86.
- Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012; 2: 125-41.
- Chang YT, Mori E, Suzuki M, Ikeda M, Huang CW, Lee JJ, et al. APOE-MS4A genetic interactions are associated with executive dysfunction and network abnormality in clinically mild Alzheimer’s disease. NeuroImage Clin. 2019; 21: 101621.
- Chang YT, Hsu SW, Huang SH, Huang CW, Chang WN, Lien CY, et al. ABCA7 polymorphisms correlate with memory impairment and default mode network in patients with APOEepsilon4-associated Alzheimer’s disease. Alzheimers Res Ther. 2019; 11: 103.
- Margulies DS, Vincent JL, Kelly C, Lohmann G, Uddin LQ, Biswal BB, et al. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Natl Acad Sci USA. 2009; 106: 20069-74.
- Kelly AM, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, et al. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb Cortex. 2009; 19: 640-57.
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007; 37: 90-101.
- Takacs J, Pollock CL, Guenther JR, Bahar M, Napier C, Hunt MA. Validation of the Fitbit One activity monitor device during treadmill walking. J Sci Med Sport. 2014; 17: 496-500.
- Ferguson T, Rowlands AV, Olds T, Maher C. The validity of consumer-level, activity monitors in healthy adults worn in free-living conditions: a cross-sectional study. Int J Behav Nutr Phys Act. 2015; 12: 42.
- Stahl ST, Insana SP. Caloric expenditure assessment among older adults: criterion validity of a novel accelerometry device. J Health Psychol. 2014; 19: 1382-7.
- Gottesmanand RT, Stern Y. Behavioral and psychiatric symptoms of dementia and rate of decline in Alzheimer’s disease. Front Pharmacol. 2019; 10: 1062.
- Tanaka H, Hashimoto M, Fukuhara R, Ishikawa T, Yatabe Y, Kaneda K, et al. Relationship between dementia severity and behavioural and psychological symptoms in early-onset Alzheimer’s disease. Psychogeriatrics. 2015; 15: 242-7.
- Vermeiren Y, Van Dam D, Aerts T, Engelborghs S, De Deyn PP. Brain region-specific monoaminergic correlates of neuropsychiatric symptoms in Alzheimer’s disease. J Alzheimers Dis. 2014; 41: 819-33.
- Ng KP, Pascoal TA, Mathotaarachchi S, Chung CO, Benedet AL, Shin M, et al. Neuropsychiatric symptoms predict hypometabolism in preclinical Alzheimer disease. Neurology. 2017; 88: 1814-21.
- Ohno Y, Kunisawa N, Shimizu S. Antipsychotic treatment of behavioral and psychological symptoms of dementia (BPSD): management of extrapyramidal side effects. Front Pharmacol. 2019; 10: 1045.
- Macdonaldand RL, Kelly KM. Antiepileptic drug mechanisms of action. Epilepsia. 1995; 36: S2-12.
- Lee JS, Kim JH, Lee SK. The relationship between neuropsychiatric symptoms and default-mode network connectivity in Alzheimer’s disease. Psychiatry Investig. 2020; 17: 662-6.
- Balthazar ML, Pereira FR, Lopes TM, da Silva EL, Coan AC, Campos BM, et al. Neuropsychiatric symptoms in Alzheimer’s disease are related to functional connectivity alterations in the salience network. Hum Brain Mapp. 2014; 35: 1237-46.
- Jia RX, Liang JH, Xu Y, Wang YQ. Effects of physical activity and exercise on the cognitive function of patients with Alzheimer disease: a meta-analysis. BMC Geriatr. 2019; 19: 181.
- Cass SP. Alzheimer’s disease and exercise: A literature review. Curr Sports Med Rep. 2017; 16: 19-22.
- Morris JK, Vidoni ED, Johnson DK, Van Sciver A, Mahnken JD, Honea RA, et al. Aerobic exercise for Alzheimer’s disease: A randomized controlled pilot trial. PLOS ONE. 2017; 12: e0170547.
- Del Pozo Cruz B, Ahmadi M, Naismith SL, Stamatakis E. Association of daily step count and intensity with incident dementia in 78 430 adults living in the UK. JAMA Neurol. 2022; 79: 1059-63.
- Chang YT, Liu CT, Hsu SW, Lee CC, Huang PC. Functional connectivity, physical activity, and neurocognitive performances in patients with vascular cognitive impairment, no dementia. Curr Alzheimer Res. 2022; 19: 56-67.
- Spartano NL, Demissie S, Himali JJ, Dukes KA, Murabito JM, Vasan RS, et al. Accelerometer-determined physical activity and cognitive function in middle-aged and older adults from two generations of the Framingham Heart Study. Alzheimers Dement (N Y). 2019; 5: 618-26.
- Rosenberg PB, Mielke MM, Appleby B, Oh E, Leoutsakos JM, Lyketsos CG. Neuropsychiatric symptoms in MCI subtypes: the importance of executive dysfunction. Int J Geriatr Psychiatry. 2011; 26: 364-72.
- Senanarong V, Poungvarin N, Jamjumras P, Sriboonroung A, Danchaivijit C, Udomphanthuruk S, et al. Neuropsychiatric symptoms, functional impairment and executive ability in Thai patients with Alzheimer’s disease. Int Psychogeriatr. 2005; 17: 81-90.
- García-Alberca JM, Lara Muñoz JPL, Berthier Torres M. Neuropsychiatric and behavioral symptomatology in Alzheimer disease. Actas Esp Psiquiatr. 2010; 38: 212-22.
- Moore D, Jung M, Hillman CH, Kangand M, Loprinzi PD. Interrelationships between exercise, functional connectivity, and cognition among healthy adults: A systematic review. Psychophysiology. 2022; 59: e14014.
- Al Jerdi S, Aleyadehand R, Imam Y. Management of Cognitive Impairment After Stroke. 2020.