
Research Article
Phys Med Rehabil Int. 2024; 11(1): 1222.
A Novel Approach to Integrative Concussion Rehabilitation: A Pragmatic Study
Lauren Ziaks¹*; Jenna Tucker²; Thomas Koc²; Meaghan Dowdell²; Giana Giorello²
¹Intermountain Rehabilitation Services, Park City Hospital, Intermountain Health, USA
²Department of Physical Therapy, Kean University, USA
*Corresponding author: Lauren Ziaks Intermountain Rehabilitation Services, Park City Hospital, Intermountain Health, 900 Round Valley Drive, Park City, Utah 84049, USA Tel: 435-658-7350; Fax: 435-658-7360 Email: Lauren.Ziaks@imail.org
Received: December 08, 2023 Accepted: January 18, 2024 Published: January 25, 2024
Abstract
Purpose: The purpose of this study is to evaluate the effectiveness of the novel Ziaks Integrative Neurological Concussion (ZINC) Protocol in evaluating and treating persistent post-concussion symptoms.
Methods: Individuals with concussion were systematically evaluated for neurological impairments and subsequently received six modules of integrative and progressive therapies. Treatment addressed motor function impairment, oculomotor and binocular vision deficits, and central vestibular deficits while incorporating postural stability, dual task and cognitive loading skills and providing instruction in cardiovascular exercise to promote autonomic stability.
Results: Of 30 patients, 15 completed all 6 protocol modules for inclusion in data analysis. Mean treatment duration was 6.6 (SD±.99) visits over 69.53 days (SD±21.92). The Post-Concussion Symptom Scale and Dizziness Handicap Inventory outcomes from pre- to post-treatment achieved clinical and statistical significance. Statistical significance was achieved for the Brain Injury Vision Symptom Survey, Brock String near point of convergence, King Devick and the novel binocular vision screening tool.
Conclusions: A structured, integrative, and progressive rehabilitation program addressing the vestibulo-oculomotor and motor function domains of concussion may be effective in the management of persistent post-concussion symptoms.
Impact Statement: This study details one potential comprehensive and sequential method to integrate the neurological domains of concussion rehabilitation while screening for impairments in the physical domains. There is a gap in knowledge regarding the most effective timing, sequencing, and implementation of intervention methods across the 4 concussion domains. This study provides the groundwork for future research to establish treatment protocols aimed to efficiently reduce symptoms associated with persistent post-concussion symptoms.
Keywords: Rehabilitation; Brain injury; Vestibular; Oculomotor; Motor function
Abbreviations: PPCS: Persistent Post-Concussion Symptoms; CPG: Clinical Practice Guidelines; PT: Physical Therapy; BPPV: Benign Paroxysmal Positional Vertigo; BBI: Brain-Body Integration; ZINC: Ziaks Integrative Neurological Concussion; PCSS: Post Concussion Symptom Scale; DHI: Dizziness Handicap Inventory; BIVSS: Brain Injury Vision Symptom Survey; MCID: Minimal Clinically Important Difference; mCTSIB: Modified Clinical Test of Sensory Interaction and Balance; KD: King Devick; HEP: Home Exercise Program; VOMS: Vestibulo-Oculomotor Screen; NPC: Near Point of Convergence; SD: Standard Deviation
Introduction
Concussions are complex injuries with a wide-range of symptoms that can involve the musculoskeletal, nervous, cardiovascular and/or psychological systems [1,2]. Most adults demonstrate full recovery within 1-2 weeks, however, 5-58% can experience symptoms for weeks or months after injury, now referred to as Persistent Post-Concussion Symptoms (PPCS) [2,3].
Historical treatment protocols focused on rest during the acute period, but recent studies demonstrate that an active rehabilitation approach is more beneficial for recovery [4-7]. The 2020 Clinical Practice Guidelines (CPG) for Physical Therapy (PT) evaluation and treatment of concussion provides evidence supporting serial evaluations for dysfunction across 4 functional domains: cervical musculoskeletal, vestibulo-oculomotor, autonomic/exertional intolerance, and motor function [2].
However, it identifies knowledge gaps for preferred evaluation strategies, recommended intervention approaches and sequencing multimodal treatment interventions, which can be complicated by the interrelationship of the vestibular, visual, and musculoskeletal systems [8].
A multifaceted evaluation used to create an individualized treatment plan can promote quicker clinical improvement, specifically in those with PPCS; however few studies have assessed effectiveness of combined interventions [9,10]. Level one evidence supports the efficacy of subthreshold aerobic exercise in reducing PPCS, with less robust research available to guide clinicians in sequencing individualized multimodal treatment plans for the remaining domains [9,11,12].
Due to the heterogeneity of concussion symptoms presenting in multiple concussion domains, adapting an individualized treatment protocol that combines the benefits of subthreshold graded aerobic exercises while addressing the other domains is warranted. Cervical musculoskeletal and vestibulo-ocular PT care models have been proposed to address this gap. Multiple studies have concluded that addressing cervical spine limitations and Benign Paroxysmal Positional Vertigo (BPPV) symptoms, integrated with visual and vestibular rehabilitation, can improve clinical and patient-reported outcomes across all systems [13,14]. In addition, Ziaks et al 2021 proposed that phased primitive reflex integration therapy targeting complex movements and Brain-Body Integration (BBI) as a precursor to vision and vestibular therapy can have improved motor function post-concussion [15]. This study expanded on the integrated vision and vestibular protocol introduced by Ziaks et al in 2019, to include a BBI protocol derived from primitive reflex integration research, theorized to target the moro reflex, asymmetrical tonic neck reflex, symmetrical tonic neck reflex, tonic labyrinthine reflex, and spinal galant reflex (Figure 1) [15,16].
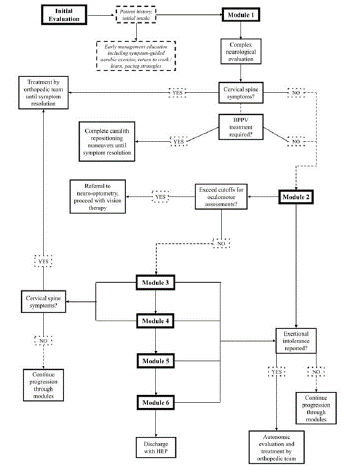
Figure 1: ZINC protocol timeline with domain integration.
The results of these studies suggest that there is opportunity for a structured multimodal assessment and treatment protocol conducted on an efficient timeline to successfully manage PPCS. This observational, pragmatic study introduces an integrative and progressive evaluation and treatment paradigm for the vestibulo-oculomotor and motor function domains of concussion, to be known as “Ziaks Integrative Neurological Concussion (ZINC) Protocol”.
This study aims to evaluate the preliminary effectiveness of the ZINC Protocol in reducing PPCS using subjective and objective outcome measures.
Materials and Methods
Role of the Funding Source
No funders played a role in the design, conduct, or reporting of this study.
Subjects
Individuals with a concussion diagnosis between October 31, 2019 and March 30, 2022 were referred from participating physicians and athletic trainers. Medical records and subjective history were screened prior to evaluation by the Principal Investigator (PI) for participation eligibility using the following inclusion criteria: (1) >10 years of age, (2) Date of injury more than 3 weeks but less than 1 year prior to screening, (3) Ability to (or the presence of a guardian who can) understand and sign a written informed consent form, obtained prior to initiation of any study procedures, (4) Willing and able to comply with visits, tests, and evaluation schedule. Individuals were excluded if they met any of the following: (1) English not as primary language, requiring translation services, (2) Participation in any other clinical trials involving investigational or marketed products within 30 days prior to entry in the study, (3) Presence of orthopedic conditions such as non-weight bearing status, and (4) Medicare as primary insurance coverage.
Outcome Measures
Intake forms included the Post Concussion Symptom Scale (PCSS), Dizziness Handicap Inventory (DHI) and Brain Injury Vision Symptom Survey (BIVSS). The PCSS is a valid and reliable tool that rates 22 self-reported concussion symptoms, including physical, cognitive, and emotional aspects, on a 0 (none) to 6 (severe) point Likert scale. The Minimal Clinically Important Difference (MCID) for the PCSS total score is 26.5 points [17]. The DHI is a reliable and valid 25-item tool scored on a 0-100 scale, assessing the impact of dizziness on physical and emotional aspects of one’s quality of life. Totaled scores are classified as follows: 16-34 points as mild handicap, 36-52 as moderate handicap, and 54+ as severe handicap, with established MCID of 19 points [18]. The BIVSS is a 28-item self-administered survey for vision symptoms related to brain injury. A preliminary study assessing BIVSS validity determined 82.2% sensitivity for predicting brain injury, however MCID has not been established [19].
Patients completed the Modified Clinical Test of Sensory Interaction and Balance (mCTSIB) using the NeuroCom Balance Master and the King Devick (KD), as well as a novel binocular vision screening tool for suppression and visual perception. The novel binocular vision screening tool is completed with the patient wearing red and green glasses with both eyes open while searching for letters A-Z with the right eye, followed by numbers 1-26 with the left eye.
Appropriate cueing is provided to allow for assessment of binocular vision, scanning efficiency, working memory, and visual endurance over time. The mCTSIB is an objective assessment of balance with variations in visual, vestibular, and somatosensory inputs. Although the Vestibular EDGE task force recommends the mCTSIB, there continues to be limited evidence regarding sensitivity, specificity, or reliability of mCTSIB in the concussion population [20]. The KD is a reliable and valid tool which measures oculomotor performance and identifies suboptimal brain function [21].
Design
Patients completed one 60-minute weekly visit for the first 4 weeks, reducing to 1 visit every other week for 2 months at a hospital-based outpatient rehabilitation clinic, as well as 20-minutes of a prescribed daily Home Exercise Program (HEP). The PI conducted evaluations and treatments, including a review of HEP compliance and progress at each session. The study was approved by the Intermountain Health Institutional Review Board and all patients/guardians signed an informed consent form.
Sessions progressed through 6 modules addressing BBI, oculomotor and binocular deficits, and central vestibular deficits, while incorporating postural stability, dual task, and cognitive loading skills each visit During initial evaluation, patients completed the PCSS, BIVSS, and DHI. Patients were screened for cervicogenic complaints and referred to a PT team member specializing in cervical spine for further evaluation and treatment for the cervical-musculoskeletal domain [13,16]. Evaluations of the motor function and vestibulo-oculomotor domains were completed and structured therapies were based on identified impairment(s). The novel Primitive Reflex Screening Tool was used to screen for atypical BBI findings [15]. Patients progressed through the modules at their own pace and were expected to achieve goals consistent with normative values prior to advancing to the next module. The PI began each module with a subjective review and assessment of HEP progress.
During Module 1, patients were provided early concussion management education including pacing strategies, sleep hygiene, blue light filters, graded aerobic exercise, return to learn/work protocols, and suggested B-complex and Docosahexaenoic acid supplements to support healing [6,22,23,24,25,26,27]. Patients were instructed to begin 20 minutes of daily cardiovascular exercise at a 3/10 rate of perceived exertion, without symptom exacerbation greater than 3/10 on the visual analog scale; they were then guided to progress per the Return to Sport Protocol until achieving prior level of function [6,28]. Individuals with suspected autonomic dysfunction were administered a Buffalo Concussion Treadmill Test by the orthopedic team consistent with the protocol detailed in Ziaks et al 2022 [29]. This strategy of dividing the “neurological” and “orthopedic/physical” facilitates streamlining of interventions. Initial BPPV screenings were completed via subjective history; Dix Hallpike and supine roll tests were used to screen symptomatic patients [2]. Those requiring intervention were guided through canalith repositioning maneuvers in the clinic and instructed to complete twice daily until symptom resolution [2]. All patients were then instructed on correlating exercises for phase one of the BBI treatment protocol based on findings from the Primitive Reflex Screening Tool (Appendix A) [14]. Patients reporting symptoms consistent with cervicogenic headache or cervicalgia were referred to the orthopedic team for assessment and treatment [2].
During Module 2, a “VOMS-plus” examination was completed, augmenting the Vestibulo-Oculomotor Screen (VOMS) with oculomotor assessments outlined in Dr. Chaikin’s Vision Screening & Vision Rehab Therapy manual [30]. This includes vergences, ocular alignment, smooth pursuits, saccades, Near Point of Convergence (NPC), gaze stabilization and visual motion sensitivity completed in yaw and pitch planes. Patients meeting established cutoffs were referred to neuro-optometry for further evaluation. Any appropriate interventions to address the results of a neuro-optometry consultation were completed in PT. Patients completed the mCTSIB on the Neurocom, the novel binocular vision screening tool and the KD. Progress on BBI exercises was evaluated to update the HEP. Patients then completed dual task and cognitive loading exercises (e.g. sitting a physioball while identifying and verbalizing odd numbers with their left hand and even numbers with their right hand).
During Module 3, saccadic exercises were introduced with “Super Saccades” objective measurements repeated each subsequent visit. Postural stability exercises in the vestibular condition, dual task and cognitive loading exercises were added [e.g. sitting on a rotating stool while completing laterality and directionality tasks simultaneously (rotating trunk and lower extremities right for the letters b/p and left for the letters d/q)]. If body-on-head movements provoked cervicogenic complaints, patients were referred to the orthopedic team. The HEP was progressed for the BBI component of the protocol (Appendix A) and low-level oculomotor control exercises were added.
During Module 4, a re-evaluation of all outcome measures was conducted. Postural demands for balance were added to cognitive loading and dual task exercises as tolerated to increase cognitive flexibility (e.g. the previously completed odd/even exercise would progress by adding marching in place on foam). Accommodation exercises using a Hart Chart and gaze stabilization exercises were added to the HEP; review of any remaining BBI protocol was completed.
During Module 5, training for convergence/divergence was added to the treatment protocol and HEP, while progressing oculomotor control exercises with postural challenges and dual tasks. Re-assessment of gaze stabilization exercises was completed for HEP progression. The remaining time focused on progressive postural stability exercise intensity in the vestibular condition, dual task and cognitive loading exercises.
Module 6 included comprehensive re-assessments of all baseline tests and measures and patient-reported outcomes. A final HEP was prescribed for patients to independently return to prior level of function over the subsequent 1-2 months, focusing on accommodation, vergences and remaining gaze stabilization impairments.
Follow-up via email was completed at 1 month post discharge to collect patient-reported outcome measures and determine if patients continued prescribed HEP until achieving goals.
Statistical Analysis
R Core Team (Vienna, Austria) was used for data analysis [31]. Subject characteristics were described using frequency counts/percentages for categorical variables and mean/Standard Deviation (SD) for continuous variables. Paired-sample t-tests analyzed mean scores for the PCSS, the BIVSS, the DHI, the Brock String convergence, the KD, and the novel binocular vision screening tool. Statistical significance was set at p<0.05.
Results
A total of 270 medical records of patients with a concussion diagnosis were referred and screened by the PI for inclusion criteria. Of the 270 patients, 30 met all inclusion criteria and 15 were included for data analysis after completing all 6 protocol modules. There were no adverse events related to treatment however, patients were lost to follow up due to: 6(17.6%, completed 2-5 modules) COVID-19 concerns, 3(8.8%, 2-3 modules) unknown, 2(5.9%, 4-5 modules) financial burden, 1(2.9%, 2 modules) psychiatric conditions, 1(2.9%, 2 modules) work demands, 1(2.9%, 4 modules) familial death, and 1(2.9%, 5 modules) orthopedic injuries unrelated to study. Four patients (26%) did not return the outcome measures at 1 month follow-up. The final sample included 11 females (73.3%) and 4 males (26.7%), with a mean age of 30.27 years (SD±17.86). There was an average of 79.13 days from initial injury to date of evaluation. Demographic characteristics are reported in Table 1.
Frequency (%)
(n=15)
Sex
Female
11(73.3%)
Male
4(26.7%)
History of Concussion
Yes
7(46.6%)
No
8(53.3%)
Mechanism of Injury
Nontraditional sport
4(26.6%)
Sports-related
4(26.6%)
Motor Vehicle accident
3(20.0%)
Fall
3(20.0%)
Struck by object
1(6.6%)
Age, years,Mean (SD)
30.27 years (±17.86)
Injury to Evaluation, Mean (SD)
79.13 days (±84.9)
Table 1: Demographic characteristics of the patients.
The average treatment duration was 6.6 (SD±.99) visits over a period of 69.53 days (SD±21.92). The average days and number of treatments between progressions of treatment integration is displayed in Table 2.
Days, Mean (SD)
Number of visits, Mean (SD)
Total duration of treatment
69.53(±21.92)
6.6(±.99)
Integrate Level 1 BBI
41.13(±24.38)
4.8(±1.01)
Integrate Level 2 BBI
60.13(±22.59)
5.93(±0.79)
Add vision to HEP (module 3)
25.93(±7.35)
4(±0.76)
Add VOR to HEP (module 4)
35.73(±14.54)
4.6(±0.74)
Add Accommodation to HEP (module 4)
33.87(±14.51)
4.47(±0.64)
Add Vergences to HEP (module 5)
52.48(±22.22)
5.53(±0.74)
Goal achieved Super Saccades
42.8(±19.46)
4.06(±1.03)
**BBI: Brain-Body Integration
**HEP: Home Exercise Program
**VOR: Vestibulo-Ocular Reflex
Table 2: Timeline to Achieve Target Measures.
Pre-treatment score
Post-treatment score
Pre to post
t-statistic
Pre to post
1 month follow-up score
(n=15)
(n=15)
Score mean difference (95% CI)
p value
(n=11)
PCSS,
55(±27.07)
15.27(±11.20)
39.73(25.83, 53.64)
6.13
<0.0001
9.18(±7.87)
Mean (SD)
BIVSS,
40.27(±23.20)
13.47(±12.72)
26.8(15.07,38.53)
4.9
<0.0001
9.36(±6.82)
Mean (SD)
DHI,
36.93(±26.14)
12.40(±15.20)
24.53(9.85,39.22)
3.58
<0.0001
5.46(±5.30)
Mean(SD)
Table 3: Subjective Outcome Measure Scores.
Results from the PCSS, DHI, BIVSS demonstrated statistically and clinical significant improvements from pre to post-treatment with mean differences of 39.73 points, 24.53 points, and 26.8 points, respectively (p<0.0001).
Eight (53.33%) patients were screened for cervicogenic complaints and referred for further evaluation/treatment of the cervical-musculoskeletal domain [2]. An additional 8 (53.33%) patients demonstrated positive dix-hallpike and/or supine roll tests. They completed a mean of 1.5 treatments of canalith repositioning maneuvers in the clinic, with instructions to complete twice daily until symptom resolution [2]. Individuals receiving treatment for cervical-musculoskeletal complaints or BPPV safely completed the ZINC protocol with no modifications required and no adverse reactions.
The novel binocular vision screening tool results demonstrated statistically significant improvements from pre- to post-treatment with a mean difference of 48.68 seconds, p=0.04 (Table 4). Brock string NPC and KD results demonstrated statistically significant improvements from pre- to post-treatment with mean differences of 6.74 inches and 14.33 seconds, respectively (p<0.0001).
Sample size
Pre-treatment (module 2)
Post-treatment (module 6)
Pre to post Score mean difference (95% CI)
t-statistic
Pre to post treatment p value
Brock String convergence, Mean (SD)
n=15
11.17 inches (±5.7)
4.43 inches (±1.69)
6.74(4.01, 9.46) inches
5.3
<0.0001
King Devick, Mean (SD)
n=15
58.56 seconds (±17.39)
44.23 seconds (±6.90)
14.33(5.94, 22.73) seconds
3.66
<0.0001
Novel binocular vision screening tool, Mean (SD)
n=14
258.17 seconds (±102.81)
209.49 seconds (±86.35)
51.95(7.58, 96.33) seconds
2.53
0.025
Table 4: Objective Outcome Measure Scores.
During the initial evaluation, patients presented with a mean of 4.87 (SD±.35) out of 5 BBI patterns on the Primitive Reflex Screening Tool (Appendix A). Upon discharge, all patients demonstrated integration of all BBI patterns. Additionally, 9 (60%) patients exhibited an emotional response (e.g. irritability or agitation) while completing BBI exercises in module 1. Emotional responses to BBI exercises resolved for all patients by module 3.
Administration of the “VOMS-plus”, using ratings based on guidelines from the Vision Screening & Vision Rehab Therapy manual, as well as the mCTSIB, were completed during module 2 and module 6. All patients demonstrated improvement in all “VOMS-plus” components, however MCID has not been established therefore descriptive statistics are reported in Table 5. Further research would determine how to best interpret these results.
Pre-Treatment (module 2)
Post-Treatment (module 6)
Saccades
Good (3, 20%)
Resolved (11, 73.33%)
Fair-Good (8, 53.33%)
Good (3, 20%)
Fair (2, 13.33%)
Fair-Poor (1, 6.67%)
Smooth Pursuits
Good (4, 26.67%)
Resolved (12, 80%)
Fair-Good (6, 40%)
Good (2, 13.33%)
Fair (4, 26.67%)
Fair-Poor (1, 6.67%)
Near Point Convergence
WNL (7, 46.67%)
WNL (13, 86.67%)
ABD (6, 40%)
ABD (1, 6.67%)
Nystagmus (2, 13.33%)
Nystagmus (1, 6.67%)
Symptoms provoked - yes (7, 46.67%)
Symptoms provoked - yes (1, 6.67%)
Vestibulo- Ocular Reflex provocative
<60 bpm (2, 13.33%)
<60 bpm (0, 0%)
61 bpm -119bpm (7, 46.67%)
61-119 bpm (1, 6.67%)
>120 bpm (6, 40%)
120-179 bpm (9, 60%)
>180 bpm WNL (5, 33.33%)
Ocular alignment
WNL (12, 80%)
WNL (14, 93.3%)
mCTSIB
WNL (8, 53.33%)
WNL (14, 93.3%)
Visual dependence (3, 20%)
Declined (1, 6.7% - same patient)
Fail all 4 conditions (3, 20%)
Declined (1, 6.7%)
**WNL: Within Normal Limits; **ABD: Abduction of the Eye; **60 beats per minute (bpm): ½ cycle per second - 1 beat per side provided
Table 5: Vision and balance evaluation findings.
Prior to treatment, 6 patients failed to maintain balance on the mCTSIB. Of these 6 individuals, 4 reported symptom provocation. All patients who completed the mCTSIB post-treatment were asymptomatic. Results are reported in Table 5.
Discussion
This study is the first to evaluate effectiveness of the ZINC Protocol, a proposed method for addressing the neurological concussion domains. Outcomes from pre to post-treatment for the PCSS and DHI achieved clinical and statistical significance, with scores exceeding established MCIDs. Statistical significance was achieved for the BIVSS, brock string NPC and KD, however MCIDs for these outcomes have not been established. These results demonstrate improvement in PPCS, supporting the efficacy of this integrative concussion rehabilitation protocol initially proposed by Ziaks et al, 2021 [15].
The ZINC Protocol is a portion of one proposed comprehensive method detailing sequenced and progressive examinations and integrated treatments. Patients had a mean difference of 39.73 points on the PCSS, in an average of 6.6 visits over 69.5 days. Similarly, Grabowski et al looked at a combination of cervicothoracic manual interventions, vestibular-oculomotor exercises, cardiovascular training and sport-specific training to treat PPCS, resulting in a 9-point PCSS score improvement over a mean of 4 visits in 84 days [32]. Another study assessed the efficacy of an active rehabilitation program for adolescents with PPCS involving subsymptom threshold aerobic training, coordination exercises, visualization and imagery techniques, and a HEP [33]. Over a mean of 3.4 visits in 35 days, they found a change of 24.7 points on the PCSS in the intervention group, as compared to 15.8 points in the control group. Other studies have concluded that individualized multimodal programs promote symptom reduction, improved self-management, and enhanced function in individuals with PPCS [34,35]. While the ZINC protocol builds on this previous research supporting a multimodal approach to evaluate and treat PCSS, larger-scale long-term studies are needed to validate these types of proposed frameworks. Optimal oculomotor function is achieved through a combination of pursuits, saccades, vergences and visual-fixation movements such as gaze-holding, optokinetic responses and the vestibulo-ocular reflex. Impairments in these components have been associated with concussion-related symptoms and contribute to difficulty maintaining balance and postural control during functional activities [36,37]. A 2020 systematic review reported that impairments in NPC post-concussion can be treated with oculomotor therapy [38]. Other studies have identified the benefits of gaze stabilization to improve the vestibulo-ocular reflex post-concussion [39]. It has been reported that standard vision therapy alone to address these types of deficits post-concussion averages 12-20 visits over 42-106.4 days [38,40,41]. After completing an average of 6.6 treatment sessions of the ZINC protocol, patients demonstrated near or full resolution of oculomotor impairments, indicated by improvement in brock string measures for NPC, reduced BIVSS scores for subjective visual symptoms, and improvement on all “VOMS-plus” components including saccades and pursuits. Additionally, KD scores improved from 58.56 seconds during module 2 to 44.23 seconds in module 6. Scores indicating abnormalities on the KD have not been established, but average KD times for healthy adults is between 44.5 to 51.24 seconds [42,43]. Although MCID is not established, post-treatment KD time was well within the reported averages for healthy adults after a relatively short treatment duration.
There is sufficient evidence demonstrating positive effects of vestibular rehabilitation to improve dizziness associated with vestibular-oculomotor deficiencies post-concussion [44-46]. The results of this study found clinically and statistically significant DHI outcomes, as the mean difference of 24.53 points exceeds the established 19-point MCID. These outcomes align with research supporting the validity of the DHI in conjunction with the VOMS, particularly in the context of vestibulo-oculomotor impairments post-concussion [47]. Eagle et al, 2022 suggests a predictive relationship between select vestibular and oculomotor components within the DHI and elevated VOMS scores, strengthening the utility of DHI in this context. A 2017 case series of 6 individuals with PPCS demonstrated statistically significant results (p=0.033) of the DHI following a comprehensive 6-month multimodal rehabilitation program [48]. These findings align with the outcomes of Alsalaheen et al 2010, where DHI improvements exceeded the MCID [49]. Following the implementation of the ZINC protocol, improved outcomes were also observed at post-treatment mCTSIB assessment. Another study demonstrated notable improvements in mCTSIB scores following an 8-session rehabilitation program for PPCS [50]. The current study results support cumulative prior findings, highlighting that individuals experiencing PPCS who participate in a multimodal approach including vestibular rehabilitation have improvement in standardized symptom reporting tools.
The ZINC protocol design utilizes the visual and vestibular systems to mirror how individuals functionally utilize multisensory processing with a complex interaction between function and structure of the brain, perception, behavior, and emotion to navigate the real world [51]. Exercises were selected or developed with multiple clinical purposes in an effort to reduce volume and duration of treatment.
This integrative and progressive method allows the clinician to determine the clinical reasoning for each exercise’s goals in individual patients, while allowing the structure of a protocol to guide treatment. For example, the “bpqd chart” starts in sitting with body-on-head rotations, allowing for a functional screen of cervicogenic dizziness, while completing a dual task and laterality / directionality skill requiring visual tracking and scanning. Due to the length of the exercise, the patient is also challenged in attention and cognitive endurance. This exercise progresses over time to include standing and potentially jump turns to increase the vestibular demand during dual task exercises. With one exercise, the clinician can address a variety of clinical goals at once.
Utilizing exercises with multiple aims and clinical relevance minimizes the number of exercises required to address these complicated clinical profiles and lack of homogeneity in the concussion population. Patients were discharged from therapy once they were able to establish understanding of their final HEP and when they had returned to a prior level of function based on community, school, and work participation.
Current literature for the neurological concussion domains focuses on the vestibular-oculomotor components, with minimal guidance for assessment and treatment of motor function [12]. Subtle motor function impairments can be difficult to detect and often persist long after other symptoms resolve, impacting daily activity participation and potentially contributing to repeat injuries [2]. It has been proposed that symptoms of suspected primitive reflex disinhibition may contribute to motor function impairments due to the negative effects on balance, coordination, impulsivity, visual tracking and convergence, motion intolerance and concentration [15]. Ziaks et al, 2021 was the first to look at individuals with PPCS who received integrated primitive reflex disinhibition, vision, and vestibular rehabilitation interventions [15].
A unique aspect of the ZINC protocol is the advancement from foundational skills through motor function with the BBI progression, building to more complex and dynamic visual and vestibular integrative exercises. In this BBI protocol, individuals are instructed to focus on slow concentrated movement to build awareness over multiple body parts simultaneously, with the intention of increasing kinesthetic and proprioceptive perception. During exercises such as the “superman with the head down” (Appendix A), the individual will automatically flex at the elbows and/or knees and with no awareness of the coupled movement pattern.
Drawing attention to the elbow flexion and having the patient focus on end range extension may be a key factor in the effectiveness of these exercises; however, further research will be required to better understand the underlying mechanisms of these exercises. Similarly, in the “Moro Bridge” exercise, increased postural sway and cognitive effort of completing the exercise will be noted by patients when completed in the target position of feet, knees, and hands at midline versus a traditional posture. The authors hypothesize that the observed improvements in form and effort are a result of increased neuromuscular control, as true strength gains would require more time to allow for muscular hypertrophy to occur. Of interesting note, the BBI exercises can provoke autonomic responses such as the emotional changes seen in 9 (60%) patients during module 1. These emotional responses were resolved by module 3, appearing to correlate with improvements in patient’s subjective reports of concentration and/or focus, and potentially improve brain processing speeds and visual perceptual skills. Symptoms of suspected primitive reflex retention do overlay with post-concussion symptoms and include impairment in mood regulation, impulsivity, visual tracking and convergence, balance and coordination, motion sickness, short term memory, and cognitive fatigability [14].
The population in this study was composed of predominantly adult female non-athletes. While these results expand on current concussion literature which has traditionally focused on youth male athletes, future research of this approach for the spectrum of subgroups would be beneficial for generalizability.
Limitations
There are several limitations for this study. There is a small sample size from a sample of convenience and therefore the results cannot be generalizable. The study was subject to selection bias because the PI was the only individual with access to patients’ records.
The inclusion criteria for time since injury was ultimately a design flaw due to the number of patients excluded. At the time of study design, it was widely accepted that 90% of injuries resolved within 3 weeks. To reduce risk of bias from spontaneous recovery, including what recent research has shown to be the positive effects of early exercise, patients were included outside of this spontaneous window (n=90). There were also concerns regarding the impact of long-term disability on recovery, which limited recruitment to less than 1 year (n=53). Due to the observational design of this pragmatic trial, active recruitment was not possible to facilitate study recruitment within the established timeline and a higher number of exclusions were identified than anticipated.
Interventions were clinic standard of care, covered by insurance. Due to absent funding, recruitment was challenging with 56 individuals screened who were unable or unwilling to participate. The COVID-19 pandemic disrupted study recruitment, subject participation and retention. One patient completed all 6 modules however, their final visit was completed over telehealth with IRB approval due to the COVID-19 pandemic, resulting in an inability to complete final assessment of the novel binocular vision screening tool.
Another limitation was difficulty obtaining follow up questionnaires. Initially questionnaires could not be completed electronically and patients were emailed PDFs of all 3 questionnaires to complete, scan, and return. Adherence to follow up questionnaires did improve upon providing an electronic version however it is ultimately unknown how great of a role this technology challenge played in the poor retention rate for follow-up measures.
Conclusion
Although the concussion domains are well outlined in the Concussion CPG, a knowledge gap remains regarding the most effective way to sequence interventions and integrate treatment across domains [2]. The ZINC Protocol is one proposed method of standardizing sequential examination and treatment while individualizing the vestibular-oculomotor and motor function domains, and screening the cervical-musculoskeletal and autonomic dysfunction/exertional intolerance domains. Although significant differences were identified across all subjective and objective measures in this study, future research is required to demonstrate effectiveness and validity of the ZINC Protocol. This pragmatic study’s results provide the groundwork for future research to close the knowledge gap in sequencing assessment and treatment across the concussion domains in an expanded patient population.
Author Statements
Declaration of Interest
Lauren Ziaks has developed continuing education related to concussion integrative management and participates in speaking engagements (paid and unpaid) on this topic.
All other authors declare there are no conflicts of interest.
Financial Disclosure Statement
No funding was provided for this research study.
Ethics Approval
This study was approved by the Intermountain Rehabilitation Services Institutional Review Board. A waiver of consent was obtained, and the patient’s rights were protected.
Acknowledgements
As well as Dr. Mark Curtis, OD, FCOVD., and Dr. Rhonda Taubin, MD., Kate Minick the authors thank Krisde Patterson, Carolyn Ure, Jen Thomas OT., and Chelsea Brown, PT, DPT., Dr. Robin Price, OD, FCOVD., and Dr. Melinda Roalstad, MS, PA-C in their assistance with the development of the ZINC Protocol and larger integrative treatment paradigm. As well as Dr. Mark Curtis, OD, FCOVD., and Kate Minick, PT, DPT, PhD., Janene Holmberg, PT, DPT., and Allison Butler, Greg Snow, Matthew Schneider, CCRP from Intermountain Health for their support and assistance with this study.
References
- Esterov D, Greenwald BD. Autonomic dysfunction after mild traumatic brain injury. Brain Sci. 2017; 7: 100.
- Quatman-Yates CC, Hunter-Giordano A, Shimamura KK, Landel R, Alsalaheen BA, Hanke TA, et al. Physical therapy evaluation and treatment after concussion/mild traumatic brain injury. J Orthop Sports Phys Ther. 2020; 50: CPG1-CPG73.
- Rickards TA, Cranston CC, McWhorter J. Persistent post-concussive symptoms: A model of predisposing, precipitating, and perpetuating factors. Appl Neuropsychol Adult. 2022; 29: 284-94.
- Carter KM, Pauhl AN, Christie AD. The role of active rehabilitation in concussion management: A systematic review and meta-analysis. Med Sci Sports Exerc. 2021; 53: 1835-45.
- Haider MN, Nowak A, Sandhur M, Leddy JJ. Sport-related concussion and exercise intolerance. Oper Tech Sports Med. 2022; 30: 150895.
- Leddy JJ, Burma JS, Toomey CM, Hayden A, Davis GA, Babl FE, et al. Rest and exercise early after sport-related concussion: a systematic review and meta-analysis. Br J Sports Med. 2023; 57: 762-70.
- Schneider KJ, Leddy JJ, Guskiewicz KM, Seifert T, McCrea M, Silverberg ND, et al. Rest and treatment/rehabilitation following sport-related concussion: A systematic review. Br J Sports Med. 2017; 51: 930-4.
- Treleaven J. Dizziness, unsteadiness, visual disturbances, and sensorimotor control in traumatic neck pain. J Orthop Sports Phys Ther. 2017; 47: 492-502.
- Reid SA, Farbenblum J, McLeod S. Do physical interventions improve outcomes following concussion: a systematic review and meta-analysis? Br J Sports Med. 2022; 56: 292-8.
- Leddy JJ, Baker JG, Merchant A, Picano J, Gaile D, Matuszak J, et al. Brain or strain? Symptoms alone do not distinguish physiologic concussion from cervical/vestibular injury. Clin J Sport. 2015; 25: 237-42.
- Lundblad M. A conceptual model for physical therapists treating athletes with protracted recovery following a concussion. Int J Sports Phys Ther. 2017; 12: 286-96.
- Rytter HM, Graff HJ, Henriksen HK, Aaen N, Hartvigsen J, Hoegh M, et al. Nonpharmacological treatment of persistent postconcussion symptoms in adults: a systematic review and meta-analysis and guideline recommendation. JAMA Netw Open. 2021; 4: e2132221.
- Wong CK, Ziaks L, Vargas S, DeMattos T, Brown C. Sequencing and integration of cervical manual therapy and vestibulo-oculomotor therapy for concussion symptoms: retrospective analysis. Int J Sports Phys Ther. 2021; 16: 12-20.
- Schneider KJ, Meeuwisse WH, Nettel-Aguirre A, Barlow K, Boyd L, Kang J, et al. Cervicovestibular rehabilitation in sport-related concussion: A randomized controlled trial. Br J Sports Med. 2014; 48: 1294-8.
- Ziaks L, Brown C, Iversen MD. Physical examination findings in patients with protracted concussion and the impact of an integrative concussion rehabilitation protocol. Internet J Allied Health Sci Pract. 2021; 19: Article 6.
- Ziaks L, Giardina R, Kloos A. Integration of vision and vestibular therapy for vestibulo-ocular post-concussion Disorder–a case study. IJAHSP. 2019; 17: 11.
- Langevin P, Frémont P, Fait P, Roy JS. Responsiveness of the post-concussion symptom scale to monitor clinical recovery after concussion or mild traumatic brain injury. Orthop J Sports Med. 2022; 10: 23259671221127049.
- Rehab measures; Updated 2014. Dizziness handicap inventory. 2017.
- Laukkanen H, Scheiman M, Hayes JR. Brain Injury Vision Symptom Survey (BIVSS) questionnaire. Optom Vis Sci. 2017; 94: 43-50.
- Murray N, Salvatore A, Powell D, Reed-Jones R. Reliability and validity evidence of multiple balance assessments in athletes with a concussion. J Athl Train. 2014; 49: 540-9.
- Hecimovich M, King D, Dempsey AR, Murphy M. The King–Devick test is a valid and reliable tool for assessing sport-related concussion in Australian football: A prospective cohort study. J Sci Med Sport. 2018; 21: 1004-7.
- Ettenhofer ML, Remigio-Baker RA, Bailie JM, Cole WR, Gregory E. Best practices for progressive return to activity after concussion: lessons learned from a prospective study of US military service members. Neurotrauma Rep. 2020; 1: 137-45.
- Fisher M, Wiseman-Hakes C, Obeid J, DeMatteo C. Does sleep quality influence recovery outcomes after post concussive injury in children and adolescents? J Head Trauma Rehabil. 2023; 38: 240-8.
- Clark J, Hasselfeld K, Bigsby K, Divine J. Colored glasses to mitigate photophobia symptoms posttraumatic brain injury. J Athl Train. 2017; 52: 725-9.
- Karmali S, Beaton MD, Babul S. Outlining the invisible: experiences and perspectives regarding concussion recovery, return-to-work, and resource gaps. Int J Environ Res Public Health. 2022; 19: 8204.
- Vonder Haar C, Peterson TC, Martens KM, Hoane MR. Vitamins and nutrients as primary treatments in experimental brain injury: clinical implications for nutraceutical therapies. Brain Res. 2016; 1640: 114-29.
- Finnegan E, Daly E, Pearce AJ, Ryan L. Nutritional interventions to support acute mTBI recovery. Front Nutr. 2022; 9: 977728.
- Bezherano I, Haider MN, Willer BS, Leddy JJ. Practical management: prescribing subsymptom threshold aerobic exercise for sport-related concussion in the outpatient setting. Clin J Sport Med. 2021; 31: 465-8.
- Ziaks L, Tucker J, Koc T, Schaefer A, Hanson K. Identifying trends of dysautonomia signs and symptoms associated with protracted concussion recovery during the Buffalo Concussion treadmill Test: A retrospective study. Brain Impairment. 2022: 1-10.
- Chaikin L. Vision screening & vision rehab therapy [manual]. Wild Iris Optometric Group. 2007.
- R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. 2022.
- Grabowski P, Wilson J, Walker A, Enz D, Wang S. Multimodal impairment-based physical therapy for the treatment of patients with post-concussion syndrome: retrospective analysis on safety and feasibility. Phys Ther Sport. 2017; 23: 22-30.
- Chan C, Iverson GL, Purtzki J, Wong K, Kwan V, Gagnon I, et al. Safety of active rehabilitation for persistent symptoms after pediatric sport- related concussion: a randomized controlled trial. Arch Phys Med Rehabil. 2018; 99: 242-9.
- Hugentobler JA, Vegh M, Janiszewski B, Quatman-Yates C. Physical therapy intervention strategies for patients with prolonged mild traumatic brain injury symptoms: a case series. Int J Sports Phys Ther. 2015; 10: 676-89.
- Germann D, Marshall C, Kazemi M. Multi-modal management of sport and non-sport related concussion by chiropractic sports specialists: a case series. J Can Chiropr Assoc. 2020; 64: 214-26.
- Pearce KL, Sufrinko A, Lau BC, Henry L, Collins MW, Kontos AP. Near point of convergence after a sport-related concussion: measurement reliability and relationship to neurocognitive impairment and symptoms. Am J Sports Med. 2015; 43: 3055-61.
- Kontos AP, Deitrick JM, Collins MW, Mucha A. Review of vestibular and oculomotor screening and concussion rehabilitation. J Athl Train. 2017; 52: 256-61.
- Santo AL, Race ML, Teel EF. Near point of convergence deficits and treatment following concussion: A systematic review. J Sport Rehabil. 2020; 29: 1179-93.
- Dunlap PM, Mucha A, Smithnosky D, Whitney SL, Furman JM, Collins MW, et al. The gaze stabilization test following concussion. J Am Acad Audiol. 2018.
- Gallaway M, Scheiman M, Mitchell GL. Vision therapy for post-concussion vision disorders. Optom Vis Sci. 2016; 93.
- Rollett P, Morandi G. Effect of vision therapy on measures of oculomotor function of patients presenting with post-concussion syndrome. Can J Optom. 2019; 81: 53-9.
- Rizzo JR, Hudson TE, Dai W, Desai N, Yousefi A, Palsana D, et al. Objectifying eye movements during rapid number naming: methodology for assessment of normative data for the King-Devick test. J Neurol Sci. 2016; 362: 232-9.
- Galetta KM, Liu M, Leong DF, Ventura RE, Galetta SL, Balcer LJ. The King-Devick test of rapid number naming for concussion detection: meta-analysis and systematic review of the literature. Concussion. 2016; 1: CNC8.
- Galeno E, Pullano E, Mourad F, Galeoto G, Frontani F. Effectiveness of vestibular rehabilitation after concussion: a systematic review of randomised controlled trial. Healthcare (Basel). 2022; 11: 90.
- Kontos AP, Eagle SR, Mucha A, Kochick V, Reichard J, Moldolvan C, et al. A randomized controlled trial of precision vestibular rehabilitation in adolescents following concussion: preliminary findings. J Pediatr. 2021; 239: 193-9.
- Zargari M, Williams K, Jo J, et al. Does earlier vestibular therapy after sport-related concussion lead to faster recovery?. J Neurosurg Pediatr. 2023; 1: 1-8.
- Eagle SR, Feder A, Manderino LM, Mucha A, Holland CL, Collins MW, et al. Concurrent validity of the Vestibular/Ocular Motor Screening (VOMS) tool with the Dizziness Handicap Inventory (DHI) among adolescents with vestibular symptoms/impairment following concussion. Phys Ther Sport. 2022; 53: 34-9.
- Adams J, Moore B. Return to meaningful activities after a multi-modal rehabilitation programme among individuals who experience persistent dizziness and debility longer than 9 months after sustaining a concussion: A case series. Physiother Can. 2017; 69: 249-59.
- Alsalaheen BA, Mucha A, Morris LO, Whitney SL, Furman JM, Camiolo-Reddy CE, et al. Vestibular rehabilitation for dizziness and balance disorders after concussion. J Neurol Phys Ther. 2010; 34: 87-93.
- Teare-Ketter A, LaForme Fiss A, Ebert J. The utility of neuromotor retraining to augment manual therapy and vestibular rehabilitation in a patient with post-concussion syndrome: A case report. Int J Sports Phys Ther. 2021; 16: 248-58.
- Arshad Q, Saman Y, Sharif M, Kaski D, Staab JP. Magnitude estimates orchestrate hierarchical construction of context-dependent representational maps for vestibular space and time: theoretical implications for functional dizziness. Front Integr Neurosci. 2022; 15: Article 806940.