
Research Article
Phys Med Rehabil Int. 2024; 11(2): 1230.
Effects of Intravenous Dexmedetomidine on Postoperative Delirium in Patients with Wilson Disease Undergoing Splenectomy: A Single-Center Double-Blind, Randomized Placebo-Controlled Trial
Shouyi Wang1,2; Xianwen Hu1*; Hong Wu2; Wei He2; Yu Liu2; Zhaoxia Chu2; Yingying Yin2; Qin Liu2; Fan Chen2; Yan Wu2; Qing Tao2
¹Department of Anesthesiology, The Second Affiliated Hospital of Anhui Medical University Hefei 230601, China
²Department of Anesthesiology, The First Affiliated Hospital of Anhui University of Traditional Chinese Medicine, Hefei 230031, China
*Corresponding author: Xianwen Hu Department of Anesthesiology, the Second Affiliated Hospital of Anhui Medical University, Hefei 230601, China. Tel: +86 15155159719 Email: huxianwen001@163.com
Received: May 07, 2024 Accepted: June 05, 2024 Published: June 12, 2024
Abstract
Background: Postoperative Delirium (POD) is a disturbed mental state that occurs after surgery. Its pathological mechanism remains unclear and may involve multiple factors, with inflammatory responses and neuroinflammation being the primary causes. This study aimed to investigate the effect of Dexmedetomidine (DEX) on the incidence of POD in patients with Wilson Disease (WD) undergoing splenectomy.
Materials and Methods: We enrolled 124 patients with WD who underwent splenectomy. Patients were divided into two groups randomly. The primary endpoint of the study was the incidence of POD at three distinct time points: one day postoperatively (T6), two days postoperatively (T7), and three days postoperatively (T8). The secondary endpoint was the serum levels of Interleukin (IL)-1Β, IL-6, and Tumor Necrosis Factor (TNF)-a at T6-T8.
Results: The incidence of POD at T6, T7, and T8 and the total incidence of POD in group D were lower than those in group C. The serum concentrations of TNF-a in group D were lower than that in group C in the POD patients at T6, T7, and T8, and the difference was statistically significant.
Conclusions: DEX can reduce the incidence of POD by suppressing TNF-a expression in patients with WD after splenectomy. Exploring different dosages or administration methods of dexmedetomidine could be a potential avenue for subsequent studies. In addition, the use of inflammatory factor inhibitors, especially IL-6, TNF-a antibodies, may be beneficial for the prevention of POD.
Keywords: Hepatolenticular Degeneration; Splenectomy; Dexmedetomidine; Delirium; Interleukin-6; TNF-alpha
Background
Wilson Disease (WD), also known as hepatolenticular degeneration, is an inherited disorder of copper metabolism caused by pathological copper accumulation in various organs, particularly the liver and brain. This accumulation leads to a wide range of symptoms [1]. Patients with WD often present with splenomegaly and pancytopenia, which are conventionally treated with splenectomy [2].
Postoperative Delirium (POD), or emergence delirium, is a disturbed mental state that occurs after surgery, with an incidence of 37%–46% depending on the type of surgical procedure performed. In some cases, it has been reported to be as high as 51% [3] and occurs in up to 50%–70% of high-risk patient groups [4], even reaching up to 73.5% [5]. POD is most prevalent in older patients, those with existing neurocognitive disorders, and those undergoing complex or emergency procedures. Preclinical and clinical research in recent years has uncovered more about the pathophysiology of postoperative delirium and may yield more potential therapeutic options. Several recent clinical trials have demonstrated that intraoperative Dexmedetomidine (DEX) infusion can prevent POD [6-8].Intraoperative DEX has been shown to reduce POD by 40%–65% [7,9], and it could be the most effective sedative agent in reducing POD [5].
The pathological mechanism of POD remains unclear, and it may be caused by multiple factors, including inflammatory responses and neuroinflammation, which appear to be the primary cause [10]. Patients with WD often experience systemic inflammation and neuroinflammation due to copper ion deposition in the liver and brain [11-13]. Moreover, surgical trauma can stimulate systemic inflammatory responses, which can further induce neuroinflammation in various ways [3]. Preliminary experiments from our center suggest that POD is more likely to occur in patients with WD after splenectomy.
DEX has been shown to reduce the incidence of POD by inhibiting peripheral inflammatory mediator expression, and animal experiments have demonstrated that DEX can also inhibit neuroinflammation [14,15]. Recently, several studies have reported that DEX can prevent POD and decrease peripheral inflammatory responses [8,10]. DEX also used during anesthesia in cirrhotic patients induced an attenuated stress response, a decrease in inflammation levels, and reduced opioid consumption, as well as improved liver function [16]. All WD splenectomy patients have severe cirrhosis, systemic inflammation and neuroinflammation, so DEX should be beneficial for such patients, but whether DEX has the effect of preventing POD in such patients is unknown. This study aims to investigate the effect of DEX on the incidence of POD in patients with WD and explore the potential mechanism of DEX in preventing POD by inhibiting inflammatory mediator expression.
Materials and Methods
Study Design
This study was a single-center double-blind, randomized placebo-controlled trial. We enrolled 124 patients with WD aged 16–58 years, with American Society of Anesthesiologists (ASA) grade I–II, Child–Pugh grades A–B, and who were scheduled for splenectomy in our hospital. The Ethics Committee of The First Affiliated Hospital of the Anhui University of Chinese Medicine approved this study, and all patients signed informed consent. The study was registered at the China Clinical Trial Registration Center (https://www.chictr.org.cn/listbycreater.aspx) with registration number of ChiCTR2100050549 on 28/8/2021, and was designed and reported using the CONSORT statement.
Hypertension is a well-known risk factor for cognitive impairment and dementia [17], the preexisting cognitive impairment and use of narcotic analgesics were significantly associated with POD [18]. The history of psychiatric illness [19], cardiovascular and cerebrovascular diseases are the potential risk factors of POD [4,20], but higher BMI mediated protective effects on POD [21]. The field of drug “allergy” has expanded to include adverse reactions associated with immunosuppressive medications, anticytokine therapies and monoclonal antibodies. In view of the above findings, in order to ensure that patients have a similar risk of POD, the following exclusion criteria are established. Exclusion criteria were patients with severe hypertension (systolic Blood Pressure [BP] =180 mmHg or diastolic BP =110 mmHg), Body Mass Index (BMI) >30 kg/m2, drug allergy, preoperative Mini-Mental State Examination (MMSE) score of =23, assessed at 1 day preoperatively (T0), preoperative psychiatric history, severe cardiovascular and cerebrovascular diseases, long-term use of opioids or other analgesic drugs, and history of addiction.
Management of General Anesthesia and Analgesia
All patients fasted for 8 h and did not receive any sedative or analgesic medications before anesthesia induction. In the operating room, the vein channel was opened for infusion, and the lactate Ringer solution (Fengyuan Pharmaceutical Company, Anhui, China) was maintained at 6–8 mL kg-1·h-1. Standard monitoring consisted of invasive arterial Blood Pressure (BP), electrocardiography, pulse oxygen saturation, end-tidal carbon dioxide (PetCO2), body temperature, and Bispectral Index (BIS), were monitored using a Mindray monitor (Mindray Corporation, Shenzhen, China). Before anesthesia induction, DEX 0.5 μg/kg was injected intravenously at 10 mins, and 0.25 μg·kg-1·h-1 was administered until 30 min before surgery ended (group D), and normal saline was used in control group (group C).
Anesthesia was inducted using midazolam 0.03 mg/kg (Renfu Pharmaceutical Company, Hubei, China), sufentanil 0.3–0.5 μg/kg Renfu Pharmaceutical Company, Hubei, China), etomidate 0.3 mg/kg (Enhua Pharmaceutical Company, Jiangsu, China), and cisatracurium 0.2 mg/kg (Hengrui Pharmaceutical Company, Jiangsu, China), with BIS = 60. Subsequently, the patient was intubated after 5 min. Mechanical ventilation parameters were the tidal volume of 8–10 mL kg-1, ventilation frequency of 12 bpm, and I:E of 1:2. The ventilation parameters were adjusted based on PetCO2, which was maintained between 35 and 45 mmHg. Five minutes after intubation, propofol 4–8 mg kg-1·h-1(Fresenius Kabi Medical LTD, Beijing, China), remifentanil 0.1–0.3 μg kg-1·min-1 (Renfu Pharmaceutical Company, Hubei, China) and cisatracurium 0.1–0.2 mg kg-1·h-1 were intravenously pumped, and BIS values were maintained between 40 and 60. The patients’ body temperature was monitored by a nasal thermometer and maintained between 36.0°C and 37.0°C. DEX infusion and sevoflurane administration were stopped 30 min before the surgery ended.
Propofol and remifentanil infusions were stopped after closing the incision, and tramadol 1 mg/kg (Xudong Haipu Pharmaceutical Co., LTD, Shanghai, China) and azasetron 0.1 mg/kg (Zhengda Tianqing Pharmaceutical Co., LTD, Nanjing, China) were immediately administered. Assisted ventilation was given when patients regained partial breathing and consciousness, and the tracheal tube was removed when patients opened their eyes and reached the standard of extubation.
After the extubation, patient-controlled intravenous analgesia (PCIA) pump was connected to the intravenous line, and the drugs were configured (sufentanil 2 μg/kg + azasetron 0.2 mg/kg, diluted to 100 mL with normal saline (Fengyuan Pharmaceutical Company, Anhui, China), background infusion rate 2 mL/h, single dose 2 ml, locking time 15 min). Subsequently, the patients were transferred to the post-anesthesia recovery room for 30 min.
Intraoperative Hemodynamic Monitoring and Management
The Mean Arterial Pressure (MAP) and Heart Rate (HR) were continuously measured and recorded before DEX or saline administration at baseline (T1), 15 min after intervention (T2), beginning of skin incision (T3), 1 h postoperatively (T4), and after skin suturing (T5). Additionally, the duration of surgery and intraoperative blood loss were recorded. Phenylephrine 40 μg was administered if hypotension occurs (a decrease of >20% of the baseline values), whereas atropine 0.3–0.5 mg was administered for bradycardia of <50 bpm. Interventions for tachycardia (HR >120 beats/min) and hypertension (systolic BP >180 mmHg or diastolic BP >100 mmHg) include adjustment of anesthesia depth and use of vasoactive drugs.
Outcome Measures
The primary endpoint was the incidence of POD at one day postoperatively (T6), two days postoperatively (T7), and three days postoperatively (T8). Confusion Assessment Method (CAM) [22] delirium scale assessment was used to discern patients with POD twice daily at T6, T7, and T8 (morning between 9:00 and 11:00 a.m., and afternoon between 3:00 and 5:00 p.m.). A psychiatrist or psychologist who was blinded to group allocation screened patients with positive CAM tests for delirium based on the Diagnostic and Statistical Manual of Mental Disorders 5 (see the supplementary material).
Bradycardia, tachycardia, hypotension, hypertension, hypoxemia, nausea, vomiting, shivering, headache, and postoperative pain were recorded at T6, T7, and T8. At 7:30 a.m. at T0, T6, T7, and T8, 5 mL of venous blood was collected from the side without infusion and centrifuged at 4000 rpm for 10 min. The serum was separated and stored at -80°C. Before the assay, all samples were thawed to room temperature and mixed by gentle swirling or inversion. Serum IL-1Β, IL-6, and TNF-a levels were measured using ELISA kits (Nanjing Jiancheng Biological Project Company, Nanjing, China).
Randomization and Blinding
Once consent was obtained, the patients were randomized by an assistant, not involved in data collection, using a computer-generated randomization program. The assist a randomly assigned the participants into two groups and kept the assigned tasks in sealed opaque envelopes. On the morning of the surgery, the assistant opened a sealed envelope, and prepared the dexmedetomidine or saline in identical syringes according to the group allocation. The anesthesiologist who administered the injections, the investigators who assessed outcomes, as well as the patients were all blinded as to which group the patient had been allocated.
Sample Size and Statistical Analyses
The sample size was determined by the incidence of POD, the primary endpoint. In high-risk patients, the incidence of POD can be as high as 50%–70% [4,23], even up to 73.5% [5], and DEX can reduce the occurrence of POD by 40%–65% [4,7,9]. Patients with WD undergoing splenectomy may be at high risk of developing POD due to preoperative systemic inflammation, neuroinflammation, and inflammation caused by surgical trauma. Combined with the preliminary experimental results of our center, this study conservatively estimated the POD incidence as 50%, and DEX could reduce the POD incidence by 50%. To achieve a power of 0.8 with a significance level of 0.05, each group should have 55 patients. Considering the lost-to-follow-up rate of approximately 10%, the final sample size was 61 and 63 patients in groups D and C, respectively.
SPSS version 21.0 (SPSS Inc., Chicago, IL, USA) software was used for statistical analysis with two-tailed tests wherever appropriate, and P < 0.05 was considered statistically significant. Continuous variables were presented as mean ± SD ( ± s) for normally distributed data and analyzed using the independent t-test. Data with non-normal distribution were presented as median (interquartile range) and analyzed using the Mann–Whitney U test. Two-way repeated-measures analysis of variance with Bonferroni correction for both within- and between-group comparisons was used to analyze IL-1Β, IL-6, and TNF-a levels at T0, T6, T7, and T8, as well as intraoperative MAP and HR at T1–T5. The IL-1Β, IL-6, and TNF-a levels at T6, T7, and T8 in the POD patients were analyzed by the independent samples t-test. Categorical variables were presented as frequencies and percentages and analyzed using the chi-square test or Fisher exact test.
Results
Figure 1 shows the consolidated standards of reporting trials flow diagram of this study. We assessed 140 patients with WD for study eligibility; however, seven patients refused to participate, and three patients did not meet the inclusion criteria. Finally, 130 patients were randomly divided into two groups, with 65 patients in each group. Subsequently, four and two patients in groups D and C dropped out of the study, respectively. Moreover, two patients who sustained hypoxia required breathing support in each group, and two patients in group D had wound disruption, which required reoperation, and had to be transferred to the intensive care unit postoperatively. Therefore, 124 patients completed the study: 61 and 63 in groups D and C, respectively.
Patients’ Characteristics and Operative Data
No difference was found in age, gender, height, weight, ASA classification, and Child–Pugh classification between the two groups (Table 1). Additionally, the duration of surgery, time to estuation, blood loss, fluid balance, propofol, remifentanil, and sufentanil were not different between the two groups. The consumption of atropine, esmolol, phenylephrine, and urapidil was not statistically different between the two groups during the perioperative period (Table 2). Moreover, the MAP and HR were not statistically different between the two groups at T1–T5.
Group D
Group C
P-value
(N = 61)
(N = 63)
Age (years)
29.98 ± 9.04
30.22 ± 8.81
0.882
Gender (M/F)
27/34
30/33
0.708
Height (cm)
163.72 ± 8.13
165.16 ± 8.91
0.350
Weight (kg)
65.59 ± 9.99
63.44 ± 9.60
0.225
ASA status (I/II)
33/28
28/35
0.282
Child–Pugh (A/B)
40/21
38/25
0.545
Data are presented as mean ± standard deviation or numbers.
M: Male, F: Female, ASA: American society of anesthesiologists.
Table 1: Comparison of different baseline characteristics between two groups.
Group D
Group C
P-value
(N = 61)
(N = 63)
Duration of surgery (min)
97.62 ± 17.12
103.02 ± 18.37
0.094
Time to extubation (min)
10.11 ± 4.34
8.96 ± 3.81
0.121
Blood loss (mL)
190.25 ± 34.21
179.67 ± 30.30
0.071
Fluid balance (mL)
1678.03 ± 80.68
1701.75 ± 88.95
0.123
Propofol (mg)
355.21 ± 8.88
351.83 ± 11.75
0.073
Remifentanil (mg)
1.64 ± 0.15
1.65 ± 0.17
0.665
Sufentanil (μg)
34.77 ± 8.81
32.65 ± 8.30
0.170
Atropine
7 (11.5)
5 (7.9)
0.505
3 (4.9)
7 (11.1)
0.324
Phenylephrine
7 (11.5)
8 (12.7)
0.835
Urapidil
2 (3.3)
5 (7.9)
0.463
Data are presented as mean ± standard deviation or median (IQR).
Fluid balance: input (fluid) - output (blood loss + urine + abdominal tube drainage).
Table 2: Comparison of different surgical data between two groups.
Primary Endpoint
The incidence of POD at T6, T7, and T8 and total POD incidence in group C were higher than that in group D, and the difference was statistically significant (Table 3). The serum concentrations of TNF-a in group D were lower than that in group C in the POD patients at T6, T7, and T8, and the difference was statistically significant (Figure 2).
Group D
Group C
P-value
(N = 61)
(N = 63)
T6
10 (16.4)
26 (41.3)
0.002
T7
12 (19.7)
25 (39.7)
0.015
T8
8 (13.1)
20 (31.7)
0.013
Total
14 (23.0)
33 (52.4)
0.001
Data are presented as the number of subjects (percentage).
T6 = 1 day postoperatively, T7 = 2 days postoperatively, T8 = 3 days postoperatively.
Table 3: The incidence of postoperative delirium between the two groups at different time points.
Secondary Endpoints and Postoperative Characteristics
IL-1β serum concentrations were similar at T0, T6, T7, and T8 in both groups (Figure 3A).Compared with T0, serum IL-6 concentrations increased significantly at T6, T7, and T8 in both groups. Compared with T6, serum IL-6 concentrations decreased significantly at T7 and T8. Changes in serum IL-6 concentrations were similar in both groups, but the difference was not significant(F(time) = 814.500, P < 0.001; F(group) = 0.445, P = 0.506; F(time*group) = 1.812, P = 0.179) (Figure 3B). Compared with T0, the serum TNF-a concentrations of the two groups were significantly decreased at T6, T7, and T8, with group D showing significantly lower serum TNF-a concentrations compared with group C(F(time) = 1120.987, P < 0.001; F(group) = 247.951; P < 0.001, F(time/group) = 269.743; P < 0.001) (Figure 3C).
Table 4 shows the main adverse events and postoperative pain in two groups at 72 h postoperatively. The incidence of bradycardia in group D was higher than that in group C (11.5% vs. 7.9%, P = 0.505), and the incidence of tachycardia in group D was lower than that in group C (4.9% vs. 11.1%, P = 0.349). The incidence of hypertension, hypotension, nausea, shivering, and pruritus in group D was lower than that in group C (P > 0.05). No hypoxemia and vomiting were observed in both groups. The incidence of postoperative headache in group C was significantly higher than that in group D (23.8% vs. 6.6%, P = 0.008). Postoperative pain was not statistically different between the two groups.
Table 4: Postoperative adverse events and postoperative pain between the two groups.
Discussion
This study gathered data from 124 patients with WD who underwent splenectomy from August 2021 to December 2022 at a medical university-affiliated hospital, including 61 and 63 patients in groups D and C, respectively (Figure 1).
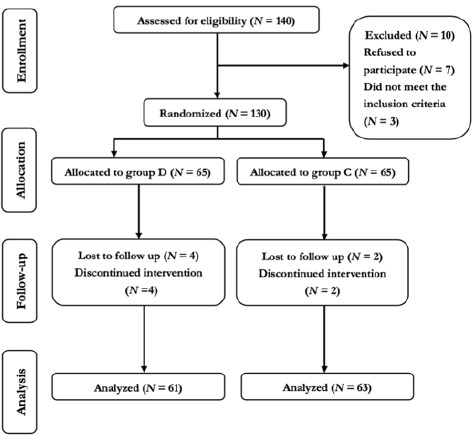
Figure 1: Consolidated standards of reporting trails flow diagram.
A previous study reported that a loading dose of DEX at 0.6 μg/kg administered 10 minutes before anesthesia induction, followed by a continuous infusion at 0.5 μg·kg-1·h-1, reduced the risk of POD by half in elderly patients undergoing major non-cardiac surgery [6]. The use of DEX at 0.5 μg/kg 10 minutes before anesthesia induction, followed by a continuous infusion at 0.2 μg·kg-1·h-1, also decreased adverse responses and the occurrence of POD [24]. Another study showed that a loading dose of DEX at 0.4 μg/kg 15 minutes before anesthesia induction, followed by a maintenance DEX infusion of 0.1 μg·kg-1·h-1, reduced the incidence of POD in older adults undergoing esophagectomy [8]. Based on these findings, we hypothesized that a loading dose of DEX at 0.5 μg/kg 10 minutes before anesthesia induction, followed by a continuous infusion of 0.25 μg·kg-1·h-1, could reduce the incidence of POD in patients with WD after splenectomy. Our study demonstrated that the incidence of POD at T6, T7, and T8, as well as the total incidence of POD in group D, was lower than that in group C (16.4% vs. 41.3%, P = 0.002; 19.7% vs. 39.7%, P = 0.015; 13.1% vs. 31.7%, P = 0.013; 23% vs. 52.4%, P = 0.001, respectively) (Table 1). The serum concentrations of TNF-a in group D were lower than that in group C in the POD patients at T6, T7, and T8 (Figure 2). These results indicated that DEX can significantly reduce the incidence of POD by inhibiting TNF-a expression in patients with WD after splenectomy. The high POD rate in this study may be associated with patients' pathophysiological states, such as long-term high-baseline peripheral inflammation and neuroinflammation [11], or acute peripheral inflammation caused by surgical trauma. Alternatively, it may be the result of their combined effects.
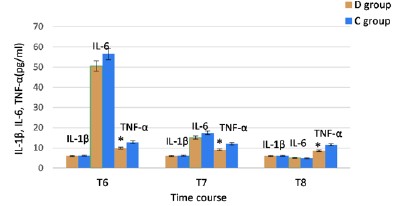
Figure 2: Comparison of the serum concentrations of Il-1β, IL-6 and TNF-a in the POD patients at T6, T7 and T8. T6, 1 day postoperatively; T7, 2 days postoperatively; T8, 3 days postoperatively; IL interleukin; TNF, tumor necrosis factor.
*P<0.05 vs Group C. Source: Microsoft® Excel ® 2019MSO, version 2307 Build 16.0.16626.20086.
Patients with WD frequently experience both systemic inflammation and neuroinflammation. Surgical trauma can further exacerbate systemic inflammation. Peripheral proinflammatory cytokines might also interact with the central nervous system, even when their levels in the circulating blood are low [25]. Additionally, the inflammatory response triggered by surgery can contribute to neuroinflammation and the development of POD [26]. POD may represent a unique manifestation of perioperative neurocognitive deficits [27], with neuroinflammation being a key characteristic of POD [28]. Currently, the impact of inflammatory mediators on POD and the potential use of these mediators as therapeutic targets for preventing POD are gaining attention.
Several studies have shown that peripheral inflammatory mediators [such as IL-1β, TNF-a, and interleukin-6 (IL-6)] can affect the brain through the vagus nerve pathway, directly through the blood-brain barrier or periventricular area, via production of inflammatory mediators by microglial cells, and via neural in- flammatory reactions; all ultimately leading to neurodegenerative changes and cognitive function impairment [29].
IL-1β is closely associated with both neuroinflammation and systemic inflammation, and it may predict cognitive dysfunction [30]. It plays a crucial role in neuroinflammatory diseases [31] and serves as a molecular link between the immune system, the vagus nerve, and the central nervous system [32]. However, in this study, serum levels remained stable without significant changes between the two groups at T0, T6, T7, and T8 (Figure 3A). The serum concentrations of IL-1β were similar in both groups in the POD patients at T6, T7, and T8 (Figure 2). This suggests that surgical trauma and DEX had no significant impact on serum IL-1β expression. Moreover, the relationship between serum IL-1β and POD was not significant. But previous studies have shown that DEX can prevent POD by inhibiting IL-1β expression in the brain [15,33], and serum IL-1β expression is closely related to the surgical approach [34]. These contradictions may be due to the chronic inflammatory state of WD patients, which makes them not very sensitive to IL-1β changes caused by surgical trauma or DEX, or due to the small sample size of our study. Therefore, the effect of IL-1β on POD and whether DEX affect the occurrence of POD in WD patients through IL-1β are worthy of further exploration.
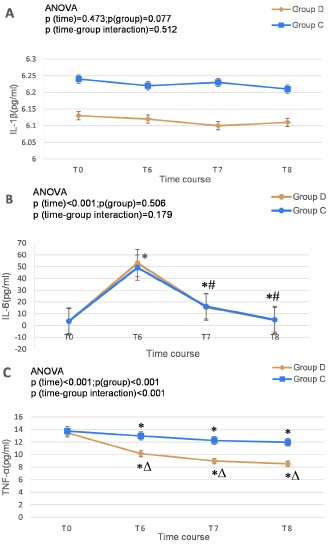
Figure 3: Comparison of serum concentrations of IL-1β (A), IL-6 (B), TNF-a (C) between the two groups. T0, 1 day preoperatively; T6, 1 day postoperatively; T7, 2 days postoperatively; T8, 3 days postoperatively; IL, interleukin; TNF, tumor necrosis factor. Source: Microsoft® Excel® 2019MSO, version 2307 Build 16.0.16626.20086.
*P < 0.05 vs T0; #P < 0.05 vs T6; ΔP < 0.05 vs Group C.
Our results showed that IL-6 increased significantly at T6, and then decreased significantly at T7 and T8 in both groups, and there was no significant difference between the two groups (Figure 3B), indicating that the change of IL-6 had nothing to do with DEX and might be a normal phenomenon caused by surgical trauma. This study demonstrated that the incidence of POD was significantly reduced in group D compared to group C (Table 3), however, serum IL-6 levels were not different in the POD patients between the two groups (Figure 2). These indicated that DEX decreased the incidence of POD, but it was unrelated to IL-6 expression. Bin Mei et al. also reported that intraoperative DEX improved postoperative neurocognitive function, but this effect was unrelated to IL-6 [35,36]. In a multivariate logistic regression analysis, Brattinga et al. found no significant relationship between IL-6 and POD [26]. Although a preoperative high serum IL-6 level was significantly associated with POD onset [37], DEX reduced the incidence of POD without affecting serum IL-6 levels. A study suggested that patient and surgical variables might have a more significant impact on postoperative inflammatory responses than the anesthetic technique [38]. Higher levels of IL-6 will increase neuronal damage [29], the onset and high incidence of POD in patients with WD could be due to long-term high baseline serum IL-6 and/or increased serum IL-6 levels induced by surgical trauma. In summary, DEX can reduce the occurrence of POD, but it does not affect serum IL-6 expression.
TNF-a is a critical mediator of chronic neuroinflammation-induced neuronal dysfunction and cognitive impairment [39], and it is associated with a high cognitive decline [40]. Chronic inflammatory status appears to be responsible for glial cell activation, triggering a more persistent inflammatory state that leads to neuronal damage and neurodegeneration [41]. Acute peripheral inflammation caused by surgical trauma disrupts the Blood-Brain Barrier (BBB) through the release of TNF-a, impairing BBB integrity, modifying the resistance of the tight
junctions of brain vessels, and resulting in cognitive impairment [29,42,43]. Therefore, targeting TNF-a could prevent and/or resolve postoperative neuroinflammation and cognitive decline [44]. Our results showed that patients with WD had high baseline TNF-a levels, putting them at high risk for POD after splenectomy, which our study also confirmed. But postoperative serum TNF-a concentrations decreased in both groups (Figure 3C). Copper ion deposition in the spleen can lead to chronic splenic inflammation, increasing TNF-a expression but after splenectomy, serum TNF-a levels may decrease. There have been reports of no increase or even decrease in TNF-a concentrations after surgery. Kvarnström et al. discovered that in major abdominal surgery, serum TNF-a levels did not increase until 24 hours postoperatively [45]. In an animal trial, abdominal surgery reduced rat spleen cells' ability to synthesize and secrete active TNF-a through multilevel regulation [46]. Combined total intravenous and inhalation anesthesia might lead to decreased TNF-a levels. Our study also found the similar phenomenon and the results showed that compared with T0, TNF-a levels in the two groups was significantly decreased, and the serum TNF-a concentration in group D was lower than that in group C at T6-T8 (Figure 3C). Moreover, the TNF-a levels in group D was lower than that in group C significantly in the POD patients at T6-T8 (Figure 2), it indicated that although TNF-a may decrease significantly after surgery for various reasons, DEX could decrease the incidence of POD by further inhibiting TNF-a expression. Therefore, we can conclude that reducing serum TNF-a concentration can significantly reduce the incidence of POD, and DEX prevents POD by inhibiting TNF-a expression.
We hypothesize that the long-term high-baseline proinflammatory cytokine levels in patients with WD might be the foundation for the high incidence of POD. The increased serum IL-6 concentrations caused by surgical trauma could potentially be a trigger factor for POD onset. DEX not only inhibited TNF-a expression but also reduced POD incidence, it does not seem to be related to IL-1β and IL-6. In future studies, researchers should explore more effective methods or specific inflammatory mediator antibodies, beyond DEX, to inhibit perioperative inflammatory mediators and thus reduce the occurrence of POD. For instance, further research is necessary to investigate the use of IL-6 antibodies to decrease POD onset and TNF-a antibodies to reduce the incidence of POD in WD patients especially.
Regarding safety, a loading dose of DEX 1 μg/kg over 10 minutes, followed by a continuous infusion at a rate of 0.2–0.8 μg·kg -1·h-1 has been shown to be safe for patients older than 70 years [47]. As such, the dosage and administration of DEX in this study should also be considered safe.
Our findings demonstrate that patients' intraoperative hemodynamics and body temperature remained stable, and there were no significant differences in anesthetic and vasoactive drug usage during the perioperative period. Moreover, postoperative adverse reactions, such as bradycardia, tachycardia, hypertension, hypotension, and hypoxia, did not differ significantly between the two groups, which may be attributable to the use of low-dose DEX. Headaches are common in patients with WD [48], and animal studies have shown that rapid withdrawal after long-term DEX usage can also cause headaches [49]. Although none of the included patients experienced headaches preoperatively, the incidence of headaches after splenectomy was relatively high. Furthermore, the incidence of headaches in group C was significantly higher than in group D, suggesting that surgery and anesthesia may have induced headaches, and DEX provided some protection. Previous research has shown that DEX can alleviate headaches [50], which could be related to its anti-neuropathic pain and neuroprotective functions [51].
This study has several limitations. Firstly, its single-center nature is crucial in acknowledging potential biases and the implications for the generalizability of the findings. Secondly, serum proinflammatory factors may not accurately reflect neuroinflammation, and obtaining direct evidence of neuroinflammation is challenging. Even if peripheral levels of proinflammatory factors indicate the inflammatory state of the central nervous system, future studies should measure markers specific to neuroinflammation. Thirdly, there are numerous types and subtypes of proinflammatory factors, and this study only examines three, which may not comprehensively represent the relationship between inflammatory mediators and POD. Lastly, the interactions between proinflammatory factors and their impact on neuroinflammation have not been clearly defined.
In conclusion, a loading dose of DEX at 0.5 μg/kg administered 10 minutes before anesthesia induction, followed by a continuous infusion at a rate of 0.25 μg·kg-1·h-1, can decrease the occurrence of Postoperative Delirium (POD) in patients with Wilson's Disease (WD) undergoing splenectomy. The onset and increased incidence of POD in WD patients may be attributed to long-term elevated baseline serum IL-6 levels and/or a surge in serum IL-6 levels induced by surgical trauma. DEX helps prevent POD by suppressing TNF-a expression.
Conclusions
DEX can reduce the incidence of POD by suppressing TNF-a expression in patients with WD after splenectomy. Exploring different dosages or administration methods of dexmedetomidine could be a potential avenue for subsequent studies. In addition, the use of inflammatory factor inhibitors, especially IL-6, TNF-a antibodies, may be beneficial for the prevention of POD.
Author Statements
Declaration of Figures Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Acknowledgements
We thank all the individuals who participated in the present study.
Data Availability
Authors report that the datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
Authors have no competing interest.
Funding
This study was supported by the Joint Foundation of The Second Affiliated Hospital of Anhui Medical University and Hefei Research Institute of Chinese Academy of Sciences (No. LHJJ202004), Anhui Provincial Health Commission (No. AHWJ2021a017), and Education Department of Anhui Province (No. KJ2021ZD0030).
Ethics Approval
Written informed written consent was signed from all participants (or their parent or legal guardian in the case of children under 16). The study was performed in agreement with the Declaration of Helsinki. Ethical approval for this study was obtained from the Ethics Committee of The First Affiliated Hospital of the Anhui University of Chinese Medicine. The ethical committee approval number is 2021-AH-41.
References
- Czlonkowska A, Litwin T, Dusek P, Ferenci P, Lutsenko S, Medici V, et al. Wilson disease. Nat Rev Dis Primers. 2018; 4: 21.
- Li LY, Yang WM, Chen HZ, Wu YH, Fang X, Zhang J, et al. Successful Splenectomy for Hypersplenism in Wilson’s Disease: A Single Center Experience from China. PLoS One. 2015; 10: e0124569.
- Alam A, Hana Z, Jin Z, Suen KC, Ma D. Surgery, neuroinflammation and cognitive impairment. Ebio Medicine. 2018; 37: 547-56.
- Jin Z, Hu J, Ma D. Postoperative delirium: perioperative assessment, risk reduction, and management. Br J Anaesth. 2020; 125: 492-504.
- Cui Y, Li G, Cao R, Luan L, Kla KM. The effect of perioperative anesthetics for prevention of postoperative delirium on general anesthesia: A network meta-analysis. J Clin Anesth. 2020; 59: 89-98.
- Li CJ, Wang BJ, Mu DL, Hu J, Guo C, Li XY, et al. Randomized clinical trial of intraoperative dexmedetomidine to prevent delirium in the elderly undergoing major non-cardiac surgery. Br J Surg. 2020; 107: e123-e32.
- Su X, Meng ZT, Wu XH, Cui F, Li HL, Wang DX, et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet. 2016; 388: 1893-902.
- Hu J, Zhu M, Gao Z, Zhao S, Feng X, Chen J, et al. Dexmedetomidine for prevention of postoperative delirium in older adults undergoing oesophagectomy with total intravenous anaesthesia: A double-blind, randomised clinical trial. Eur J Anaesthesiol. 2021; 38: S9-S17.
- Zeng H, Li Z, He J, Fu W. Dexmedetomidine for the prevention of postoperative delirium in elderly patients undergoing noncardiac surgery: A meta-analysis of randomized controlled trials. PLoS One. 2019; 14: e0218088.
- Zhang W, Wang T, Wang G, Yang M, Zhou Y, Yuan Y. Effects of Dexmedetomidine on Postoperative Delirium and Expression of IL-1β, IL-6, and TNF-a in Elderly Patients After Hip Fracture Operation. Front Pharmacol. 2020; 11: 678.
- Wu P, Dong J, Cheng N, Yang R, Han Y, Han Y. Inflammatory cytokines expression in Wilson’s disease. Neurol Sci. 2019; 40: 1059-66.
- Goyal MK, Sinha S, Patil SA, Jayalekshmy V, Taly AB. Do cytokines have any role in Wilson’s disease? Clin Exp Immunol. 2008; 154: 74-9.
- Dong J, Wang X, Xu C, Gao M, Wang S, Zhang J, et al. Inhibiting NLRP3 inflammasome activation prevents copper-induced neuropathology in a murine model of Wilson’s disease. Cell Death Dis. 2021; 12: 87.
- Zheng B, Zhang S, Ying Y, Guo X, Li H, Xu L, et al. Administration of Dexmedetomidine inhibited NLRP3 inflammasome and microglial cell activities in hippocampus of traumatic brain injury rats. Biosci Rep. 2018; 38: BSR20180892.
- Zhang L, Xiao F, Zhang J, Wang X, Ying J, Wei G, et al. Dexmedetomidine Mitigated NLRP3-Mediated Neuroinflammation the Ubiquitin-Autophagy Pathway to Improve Perioperative Neurocognitive Disorder in Mice. Front Pharmacol. 2021; 12: 646265.
- Ingustu D-G, Pavel B, Paltineanu S-I, Mihai D-I, Cotorogea-Simion M, Martac C, et al. The Management of Postoperative Cognitive Dysfunction in Cirrhotic Patients: An Overview of the Literature. Medicina (Kaunas). 2023; 59: 465.
- Neerland BE, Krogseth M, Juliebø V, Hylen Ranhoff A, Engedal K, Frihagen F, et al. Perioperative hemodynamics and risk for delirium and new onset dementia in hip fracture patients; A prospective follow-up study. PLoS One. 2017; 12: e0180641.
- Litaker D, Locala J, Franco K, Bronson DL, Tannous Z. Preoperative risk factors for postoperative delirium. Gen Hosp Psychiatry. 2001; 23: 84-9.
- Bin Abd Razak HR, Yung WYA. Postoperative Delirium in Patients Undergoing Total Joint Arthroplasty: A Systematic Review. J Arthroplasty. 2015; 30: 1414-7.
- Wang X, Yu D, Du Y, Geng J. Risk factors of delirium after gastrointestinal surgery: A meta-analysis. J Clin Nurs. 2023; 32: 3266-76.
- Deng X, Qin P, Lin Y, Tao H, Liu F, Lin X, et al. The relationship between body mass index and postoperative delirium. Brain Behav. 2022; 12: e2534.
- Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990; 113: 941-8.
- Saczynski JS, Inouye SK, Kosar C, Tommet D, Marcantonio ER, Fong T, et al. Cognitive and Brain Reserve and the Risk of Postoperative Delirium in Older Patients. Lancet Psychiatry. 2014; 1: 437-43.
- Huang H, Xu X, Xiao Y, Jia J. The Influence of Different Dexmedetomidine Doses on Cognitive Function at Early Period of Patients Undergoing Laparoscopic Extensive Total Hysterectomy. J Healthc Eng. 2021; 2021: 3531199.
- van Munster BC, Korevaar JC, Zwinderman AH, Levi M, Wiersinga WJ, De Rooij SE. Time-course of cytokines during delirium in elderly patients with hip fractures. J Am Geriatr Soc. 2008; 56: 1704-9.
- Brattinga B, Plas M, Spikman JM, Rutgers A, de Haan JJ, Absalom AR, et al. The association between the inflammatory response following surgery and post-operative delirium in older oncological patients: a prospective cohort study. Age Ageing. 2022; 51: afab237.
- Daiello LA, Racine AM, Yun Gou R, Marcantonio ER, Xie Z, Kunze LJ, et al. Postoperative Delirium and Postoperative Cognitive Dysfunction: Overlap and Divergence. Anesthesiology. 2019; 131: 477-91.
- Subramaniyan S, Terrando N. Neuroinflammation and Perioperative Neurocognitive Disorders. Anesth Analg. 2019; 128: 781-8.
- Yu S, Leng Y, Wang Y, Zhao G. A Review of the Biological Mechanisms of Dexmedetomidine for Postoperative Neurocognitive Disorders. Med Sci Monit. 2022; 28: e937862.
- Skelly DT, Griffin ÉW, Murray CL, Harney S, O’Boyle C, Hennessy E, et al. Acute transient cognitive dysfunction and acute brain injury induced by systemic inflammation occur by dissociable IL-1-dependent mechanisms. Mol Psychiatry. 2019; 24: 1533-48.
- Mendiola AS, Cardona AE. The IL-1β phenomena in neuroinflammatory diseases. J Neural Transm (Vienna). 2018; 125: 781-95.
- Goehler LE, Gaykema RP, Nguyen KT, Lee JE, Tilders FJ, Maier SF, et al. Interleukin-1beta in immune cells of the abdominal vagus nerve: a link between the immune and nervous systems? J Neurosci. 1999; 19: 2799-806.
- Sun Y-B, Zhao H, Mu D-L, Zhang W, Cui J, Wu L, et al. Dexmedetomidine inhibits astrocyte pyroptosis and subsequently protects the brain in in vitro and in vivo models of sepsis. Cell Death Dis. 2019; 10: 167.
- Helmy SA, Wahby MA, El-Nawaway M. The effect of anaesthesia and surgery on plasma cytokine production. Anaesthesia. 1999; 54: 733-8.
- Mei B, Xu G, Han W, Lu X, Liu R, Cheng X, et al. The Benefit of Dexmedetomidine on Postoperative Cognitive Function Is Unrelated to the Modulation on Peripheral Inflammation: A Single-center, Prospective, Randomized Study. Clin J Pain. 2020; 36: 88-95.
- De Cosmo G, Sessa F, Fiorini F, Congedo E. Effect of remifentanil and fentanyl on postoperative cognitive function and cytokines level in elderly patients undergoing major abdominal surgery. J Clin Anesth. 2016; 35: 40-6.
- Capri M, Yani SL, Chattat R, Fortuna D, Bucci L, Lanzarini C, et al. Pre-Operative, High-IL-6 Blood Level is a Risk Factor of Post-Operative Delirium Onset in Old Patients. Front Endocrinol (Lausanne). 2014; 5: 173.
- O’Bryan LJ, Atkins KJ, Lipszyc A, Scott DA, Silbert BS, Evered LA. Inflammatory Biomarker Levels After Propofol or Sevoflurane Anesthesia: A Meta-analysis. Anesth Analg. 2022; 134: 69-81.
- Belarbi K, Jopson T, Tweedie D, Arellano C, Luo W, Greig NH, et al. TNF-a protein synthesis inhibitor restores neuronal function and reverses cognitive deficits induced by chronic neuroinflammation. J Neuroinflammation. 2012; 9: 23.
- Holmes C, Cunningham C, Zotova E, Woolford J, Dean C, Kerr S, et al. Systemic inflammation and disease progression in Alzheimer disease. Neurology. 2009; 73: 768-74.
- Calsolaro V, Edison P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimers Dement. 2016; 12: 719-32.
- Lyman M, Lloyd DG, Ji X, Vizcaychipi MP, Ma D. Neuroinflammation: the role and consequences. Neurosci Res. 2014; 79: 1-12.
- Yang S, Gu C, Mandeville ET, Dong Y, Esposito E, Zhang Y, et al. Anesthesia and Surgery Impair Blood-Brain Barrier and Cognitive Function in Mice. Front Immunol. 2017; 8: 902.
- Terrando N, Eriksson LI, Ryu JK, Yang T, Monaco C, Feldmann M, et al. Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol. 2011; 70: 986-95.
- Kvarnström AL, Sarbinowski RT, Bengtson JP, Jacobsson LM, Bengtsson AL. Complement activation and interleukin response in major abdominal surgery. Scand J Immunol. 2012; 75: 510-6.
- Lahat N, Rahat MA, Brod V, Cohen S, Weber G, Kinarty A, et al. Abdominal surgery reduces the ability of rat spleen cells to synthesize and secrete active tumour necrosis factor-alpha (TNF-alpha) by a multilevel regulation. Clin Exp Immunol. 1999; 115: 19-25.
- Silva-Jr JM, Katayama HT, Nogueira FAM, Moura TB, Alves TL, de Oliveira BW. Comparison of dexmedetomidine and benzodiazepine for intraoperative sedation in elderly patients: a randomized clinical trial. Reg Anesth Pain Med. 2019; 44: 319-24.
- Ala A, Walker AP, Ashkan K, Dooley JS, Schilsky ML. Wilson’s disease. Lancet. 2007; 369: 397-408.
- Kaye AD, Chernobylsky DJ, Thakur P, Siddaiah H, Kaye RJ, Eng LK, et al. Dexmedetomidine in Enhanced Recovery After Surgery (ERAS) Protocols for Postoperative Pain. Curr Pain Headache Rep. 2020; 24: 21.
- Li X, Tan F, Jian C-J, Guo N, Zhong Z-Y, Hei Z-Q, et al. Effects of small-dose dexmedetomidine on hyperdynamic responses to electroconvulsive therapy. J Chin Med Assoc. 2017; 80: 476-81.
- Zhao Y, He J, Yu N, Jia C, Wang S. Mechanisms of Dexmedetomidine in Neuropathic Pain. Front Neurosci. 2020; 14: 330.