
Research Article
Phys Med Rehabil Int. 2024; 11(3): 1232.
The Relationship between Anxiety and Cognition in Stroke Patients: A Cross-Sectional Study
Zixiu Zheng1,2; Runluo Song3; Yang Song3; Yanqing Wang1; Yanjun Zhuang2; Cong Yu4*; Lixia Zhao1*
1Third Affiliated Hospital of Inner Mongolia Medical University, China
2Henan University of Science and Technology, China
3Department of Neurology, The First Affiliated Hospital of Henan University of Science and Technology, China
4Department of Nursing, Shenzhen Second People’s Hospital, China
*Corresponding author: Cong Yu, Department of Geriatrics, Third Affiliated Hospital of Inner Mongolia Medical University, China; Lixia Zhao, Third Affiliated Hospital of Inner Mongolia Medical University, China Email: 346344254@qq.com; khfyzzx@126.com
Received: July 08, 2024 Accepted: July 29, 2024 Published: August 05, 2024
Abstract
Background: There is limited evidence on the relationship between anxiety and cognition in stroke patients, and no precise relationship between the two has been indicated.
Objective: We aimed to explore the precise relationship between anxiety and cognition in Chinese stroke patients.
Methods: This study was a cross-sectional study, 384 hospitalized stroke patients were assessed with questionnaires and scales, including the Demographic Characteristics Questionnaire, the Hamilton Anxiety Inventory (HAMA), and the Montreal Cognitive Assessment (MoCA).
Results: Anxiety was present in 55.47% of the 384 patients. Univariate analysis showed that age, gender, marital status, smoking, and alcohol consumption were associated with cognition, and multiple linear regression results showed that HAMA was not independently associated with MoCA after adjusting for potential confounders (β=-0.16, 95% CI: -0.29 to- 0.03), which would be inconsistent with HAMA (subgroup) as a categorical variable (P trend of 0.004) A non-linear relationship was detected between HAMA and MoCA with an inflection point of 9. The effect sizes and confidence intervals to the left and right of the inflection point were -0.54 (-0.78 to -0.30) and 0.02 (-0.14 to -0.17), respectively.
Conclusion: The relationship between anxiety and cognition is nonlinear. When the HAMA score is less than 9, anxiety and cognition are negatively correlated, and when it is greater than or equal to 9, the cognitive score will no longer decrease and is saturated.
Keywords: Anxiety; Cognition; Stroke; Nonlinearity; Cross-sectional study
Introduction
Anxiety is one of the most common psychological problems in stroke patients and is a subjective experience for patients, such as nervous and worried thoughts, as well as physiological changes including sweating, dizziness, increased blood pressure and heart rate [1]. Several neurophysiological studies have shown that anxiety is highly correlated with cognitive performance [2-4]. A 1-year longitudinal study has also shown that anxiety has a detrimental effect on functional prognosis in a stroke population [5]. In addition, higher levels of anxiety increase the risk of stroke recurrence [6]. Cognitive impairment is a major cause of poststroke morbidity and mortality worldwide [7], and approximately half of patients have some degree of Poststroke Cognitive Impairment (PSCI) [8]. Cognitive dysfunction may involve impairment in cognitive domains such as memory, attention, executive function, or visual construction [9], and even in patients with mild stroke, cognitive impairment occurs in 30-40% of patients after three months [10]. In China, ZHU et al. [11] prospectively investigated 104 patients 3-6 months after stroke and confirmed the occurrence of Cognitive Impairment (PSCI) in 63.46% of patients, with low cognitive function possibly leading to vascular dementia and possibly to Alzheimer's disease. Currently, there are more studies proving the correlation between depression and cognition [12-14], but the relationship between anxiety and cognition is complex and less studied, so it is necessary to study the correlation between anxiety and cognition. Regarding the relationship between anxiety and cognition, Gigi et al. [15] showed that anxiety is a risk factor for cognitive decline through a study of 50 patients. Nyberg et al. [16] showed an association between cognitive function and anxiety severity, while Gimson et al. [17] found a positive correlation between clinical anxiety and future dementia through a systematic evaluation, and Ma L et al. [18] concluded that anxiety is a possible risk factor for cognitive decline and progression to dementia through a systematic evaluation. In summary, previous studies have demonstrated that there is a strong relationship between anxiety and cognition and that early assessment of anxiety is important for the prognosis of patients. However, previous studies have not taken into account the nonlinear relationship during data analysis, as well as the lack of precise quantification of the relationship between anxiety levels and cognition, and the differences in study population and ethnicity. Therefore, we aimed to explore the precise relationship between anxiety and cognition in Chinese stroke patients.
Methods
Study Population
The present study was a cross-sectional study. We collected data on stroke patients admitted to the Department of Neurology, First Affiliated Hospital of Henan University of Science and Technology, China, from February 2022 to February 2023. The collection of information on these patients was nonselective as well as continuous. A total of 384 patients were included in this study when we are presenting (Figure 1 for the final sample size). Inclusion criteria: (1) age =18 years; (2) meeting the diagnostic criteria for cerebral hemorrhage and cerebral infarction in the Diagnostic Points for Various Types of Cerebrovascular Diseases adopted at the Fourth National Cerebrovascular Symposium in 1996 [19]; (3) being conscious and able to cooperate in completing the questionnaire; and (4) informed consent. Exclusion criteria: (1) patients with previous psychiatric diseases and Alzheimer's disease; (2) those in critical condition or combined with other serious physical diseases.
Figure 1: Description of the participant screening process.
Materials
The questionnaire consisted of 3 parts:
(1) a demographic characteristics questionnaire, designed by the researcher, which included gender, age, marital status, smoking, alcohol consumption, and whether hypertension or diabetes was combined.
(2) Hamilton Anxiety Inventory (HAMA), developed by Hamilton [20] in 1959, includes 14 items. Each item is scored from 0 to 4 on a 5-point scale. The higher the total score, the more severe the anxiety, <7 means no anxiety symptoms, 7-13 is mild to moderate anxiety, =14 has severe anxiety. The Cronbach's alpha coefficient for this scale was 0.93 [21].
(3) The Montreal Cognitive Assessment (MoCA), designed by Nasreddine et al. [22], includes 8 cognitive domains such as attention, 11 items, and a total score of 30, with higher scores associated with better cognitive functioning. The scale has a Cronbach's alpha coefficient of 0.782 and a retest reliability of 0.972. The HAMA and MoCA have been widely used in clinical practice and research since their introduction, and they have high sensitivity and reliability [23,24].
Statistical Analysis
Continuous variables are described as mean ± standard deviation (M+SD), and categorical variables are described as number of cases (n) and percentage (%). We used χ 2 test to determine differences in categorical variables between groups and Student's t test to determine differences in continuous variables between groups. Multiple linear regression analysis was used to determine the relationship between variables and perceptions. Unadjusted and adjusted models for multiple linear regression analysis are presented, and inflection point values for variables were calculated by threshold and saturation effect analysis. All analyses were conducted using the statistical packages R (http://www.r-project.org, The R Foundation) and Empower Stats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA). P values less than 0.05 (two-sided) were considered statistically significant.
Results
A Description of The Participant Screening Process
The study initially included 405 patients with stroke. Eighteen patients with incomplete baseline information and three patients with combined Alzheimer's Disease (AD) were excluded, leaving 384 for the final data analysis, as detailed in the flow chart (Figure 1).
Description of Baseline Characteristics of Participants
The baseline characteristics of the patients are listed in Table 1. We grouped them according to HAMA scores: 0-6 in the no anxiety group, 7-13 in the mild and moderate anxiety group, and =14 in the severe anxiety group. After grouping, we observed the trend of the distribution of each variable among the different groups. The mean age of stroke patients was 60.7 ± 14.7 years, the prevalence of anxiety was 55.47% (213/384), the distribution of MoCA scores was statistically different between the different HAMA subgroups (all P values < 0.05), and we also observed a statistically significant distribution of age and gender between the different subgroups (all P values < 0.05). However, we did not observe statistically significant differences in marital status, smoking, alcohol consumption, combined hypertension, diabetes, or both hypertension and diabetes (all P values > 0.05).
HAMA Team
T1
<7T2
=7 to<14T3
=14P-value
No. of participants
171
130
83
MoCA
20.37 ± 6.21
16.89 ± 6.50
16.26 ± 6.55
<0.001
Age (years)
61.98 ± 12.59
62.99 ± 12.89
0.010
Sex
0.003
Male
60 (35.09%)
49 (37.69%)
47 (56.63%)
Female
111 (64.91%)
81 (62.31%)
36 (43.37%)
Marital
0.927
Not married
8 (6.90%)
5 (6.10%)
4 (7.84%)
Married
108 (93.10%)
77 (93.90%)
47 (92.16%)
Smoke
0.800
Never smoke
75 (64.10%)
58 (70.73%)
37 (69.81%)
Quit smoke
11 (9.40%)
5 (6.10%)
5 (9.43%)
Smoke
31 (26.50%)
19 (23.17%)
11 (20.75%)
Alcohol
0.632
Never drink alcohol
78 (66.67%)
58 (70.73%)
41 (77.36%)
Quit drinking
10 (8.55%)
5 (6.10%)
4 (7.55%)
Drinking
29 (24.79%)
19 (23.17%)
8 (15.09%)
Comorbidities
0.146
None
52 (45.22%)
33 (40.24%)
21 (40.38%)
Hypertension
41 (35.65%)
38 (46.34%)
19 (36.54%)
Diabetes
21 (18.26%)
9 (10.98%)
8 (15.38%)
Suffering from high blood pressure and diabetes
1 (0.87%)
2 (2.44%)
4 (7.69%)
Abbreviations: HAMA: Hamilton Anxiety Scale; MoCA: Montreal Cognitive Assessment
Table 1: Characteristics of participants (n=384).
A Description of The Results for The Linear Relationship Between HAMA and Moca
Results of univariate analysis of HAMA and MoCA: The results of the univariate analysis are shown in Table 2. The results of the analysis showed that HAMA scores were negatively correlated with MoCA scores, and age, gender, marital status, smoking, and alcohol consumption were related to cognition. However, we also found that the type and number of comorbid chronic diseases were not related to cognition.
Statistics
β/OR (95%CI)
P-value
HAMA Team
<7
171 (44.53%)
reference
=7 to<14
130 (33.85%)
-3.48 (-5.10, -1.85)
<0.0001
=14
83 (21.61%)
-4.11 (-5.98, -2.25)
<0.0001
Age
60.66 ± 14.65
-0.19 (-0.24, -0.14)
<0.0001
Sex
Male
183 (39.35%)
reference
Female
282 (60.65%)
3.36 (1.88, 4.84)
<0.0001
Marital
Not married
20 (6.78%)
reference
Married
275 (93.22%)
-6.59 (-10.21, -2.98)
0.0004
Smoke
Never smoked
203 (68.12%)
reference
Quit smoking
27 (9.06%)
1.74 (-1.34, 4.81)
0.2693
Smoking
68 (22.82%)
2.17 (0.12, 4.23)
0.0395
Alcohol
Never drink alcohol
212 (71.14%)
reference
Quit drinking
25 (8.39%)
0.49 (-2.70, 3.67)
0.7644
Drinking
61 (20.47%)
2.65 (0.54, 4.76)
0.0147
Comorbidities
None
125 (42.66%)
reference
Hypertension
114 (38.91%)
1.00 (-0.95, 2.96)
0.3156
Diabetes
45 (15.36%)
0.18 (-2.48, 2.85)
0.8939
Suffering from high blood pressure and diabetes
9 (3.07%)
4.11 (-2.18, 10.41)
0.2011
Abbreviations: CI: Confidence Interval; OR: Odds Ratio.
Table 2: Univariate analysis for HAMA.
Results of multiple linear regression analysis of HAMA and MoCA: We observed the association between HAMA and MoCA through different covariate adjustment strategies and present the results in Table 2. In the unadjusted model, every 1-point increase in HAMA was associated with a 0.28-point decrease in MoCA (β=-0.28, 95% CI: -0.38 to -0.17). After adjusting for age, sex, MoCA decreased by 0.18 points for every 1-point increase in HAMA (β=-0.18, 95% CI: -0.28 to -0.07). When adjusting for age, sex, marital, smoking, alcohol, and comorbidities, we found that for every 1-point increase in HAMA, MoCA decreased by 0.16 points (β=-0.16, 95% CI: -0.29 to- 0.03). For the purpose of sensitivity analysis, we transformed HAMA scores into categorical variables by grouping and calculated P values for trend tests. The results showed that the results when HAMA was used as a continuous variable were not consistent with the results when HAMA was used as a categorical variable (see Table 3).
Exposure
Crude Model
Adjust I
Adjust II
(β, 95%CI, P)
(β, 95%CI, P)
(β, 95%CI, P)
HAMA
-0.28 (-0.38, -0.17) <0.0001
-0.18 (-0.28, -0.09) 0.0003
-0.16 (-0.29, -0.03) 0.0160
HAMA Team
<7
reference
reference
reference
=7 to<14
-3.48 (-5.10, -1.85) <0.0001
-2.86 (-4.34, -1.38) 0.0002
-3.48 (-5.30, -1.66) 0.0002
=14
-4.11 (-5.98, -2.25) <0.0001
-2.86 (-4.58, -1.14) 0.0013
-2.54 (-4.62, -0.45) 0.0180
P for trend
<0.0001
0.0002
0.0043
Abbreviations: CI: Confidence Interval.
Crude model: did not adjust for other covariants.
Adjust I model adjust for: age; sex
Adjust II model adjust for: age; sex; marital; smoke; alcohol; comorbidities
Table 3: Relationship between HAMA and MoCA.
Non-Linear Correlation Results Between HAMA and Moca
We observed the nonlinear association between HAMA and MoCA by smoothed curve fitting and a Generalized summation Model (GAM) with a log-likelihood ratio test with P less than 0.05. This result suggests that a two-segment linear regression model should be used to fit the association between HAMA and MoCA. Our results showed (Figure 2) that the association between HAMA and MoCA showed a saturation effect after adjusting for age, gender. Using the two-piecewise linear model and the recursive algorithm, we calculated that the inflection point of HAMA was at the HAMA score of 9. On the left side of the inflection point, the cognitive score decreased by 0.54 points for every 1-point increase in anxiety (β=-0.54, -0.78to -0.30, P<0.01), i.e., the higher the HAMA score, the lower the MoCA score, and the lower the cognitive function. The lower the score of MoCA, the worse the cognitive function. To the right of the inflection point, no association was found between HAMA and MoCA (β=0.02, -0.14 to 0.17, P>0.05), i.e., at a HAMA score greater than or equal to 9, as the HAMA score increased, the MOCA score no longer changed significantly and was saturated, see Table 4.
Inflection points of HAMA
Effect size (β)
95% CI
P value
-0.54
-0.78 to -0.30
<0.001
=9
0.02
-0.14 to -0.17
0.817
P for log likelihood ratio 0.001
Effect: MoCA, Cause: HAMA
Adjusted: age, sex.
Table 4: Threshold Effect Analysis of HAMA and MoCA using Piece-wise Linear Regression.
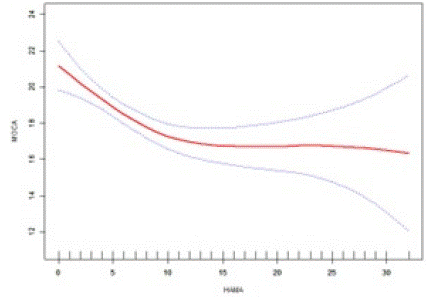
Figure 2: Association between HAMA and MoCA.
A threshold, nonlinear association between HAMA and MoCA was found in a generalized additive model (GAM). A solid red line represents the smooth curve fit between variables. Blue bands represent the 95% confidence interval of the fit. All adjusted for age and sex.
Discussion
In the present study, our results are in line with the findings of Gigi [15], Nyberg [16] and Gallagher [25], who found anxiety to be a risk factor for cognitive decline in an experimental study of 50 community adults aged 59-82 years through a class of questionnaires. Gallagher surveyed 161 patients with cognitive impairment and concluded that anxiety was correlated with mild cognitive impairment and Alzheimer's disease, and Nyberg showed an association between cognition and anxiety severity in a cross-sectional study that included 189 Swedish patients aged 18-65 years. This is the same result we found in a larger sample, but we targeted stroke patients in the Chinese population. The results were adjusted for a few more covariates, and we precisely calculated an inflection point value of 9 points for anxiety, and to the left of the inflection point, each 1-point increase in anxiety was associated with a 0.54-point decrease in cognitive function score (β=-0.54, -0.78to -0.30, P<0.01), the higher the HAMA score, the lower the MoCA score, i.e., the more anxious the patient, the worse the cognition. To the right of the inflection point, no association was found between HAMA and MoCA (β=0.02, -0.14 to 0.17, P>0.05), and at a HAMA score greater than or equal to 9, as the HAMA score increased, the MoCA score no longer changed significantly and was saturated, i.e., as anxiety increased, the cognitive level no longer decreased and was in a steady state.
Our results are inconsistent with those of Williams et al. [26] and Bierman EJ [27], who showed no association between cognition and anxiety in following patients six months after stroke. In a cross-sectional study, Bierman EJ investigated 3,107 elderly people in the Netherlands and showed that severe anxiety symptoms were negatively associated with cognitive function. We speculate that the main reasons for the different results are: (1) inconsistency with the Williams et al. study, probably because the present study was conducted in hospitalized patients with stroke, and the suddenness of the stroke event hit the patients harder, while with discharge and later recovery, the patients' anxiety decreased, so there was no significant association with cognition; and, furthermore, studies have demonstrated that stroke patients have a sharp decrease in cognitive function at the time of the event (2) and Bierman EJ [28], and it has also been demonstrated that approximately one third of stroke survivors are found to have a severe degree of cognitive impairment in the first few months after the event [29]. (2) Unlike the study by Bierman EJ et al., the reason may be that the mean age of the population in this study was 60.7 ± 14.7 years, an age group that is assuming important family and social responsibilities and where stroke events are more likely to cause anxiety and cognitive impairment in patients. (3) Our regression description studies were inconsistent in that they did not regress anxiety, whereas we performed regression analyses and adjusted for age, sex, marital, smoke, alcohol, and comorbidity variables; (4) The linear regression results of this study also showed a negative association between anxiety and cognition, but this study, for sensitivity reasons, targeted non-linear association with the Generalized Additive Model (GAM).
The clinical value of this study is to speculate the precise relationship between anxiety and cognition, i.e., the precise pointat which anxiety scores suggest early warning, timely intervention to reduce patient anxiety, identify and stop or mitigate the onset or progression of cognitive dysfunction earlier, and improve the quality of life of stroke patients.
The main strengths of this study are: (1) the sample size of this study is large (N=384 cases); (2) this study analyzes anxiety variance in 3 groups according to different anxiety levels, and the results are more stable; (3) this study uses sensitivity analysis and an algorithm that elucidates nonlinearity to better reflect the true relationship between anxiety and cognitive function.
Limitations
Our study has some drawbacks, as follows: Patients with Alzheimer's Disease (AD) was excluded from this study. Therefore, future use of the findings of this study in the AD population requires caution; this study was a cross-sectional study and, therefore, was inevitably confounded by confounders, but we rigorously adjusted for confounders and assessed the accuracy of the results by sensitivity analysis. Limited to the nature of observational studies, we could only observe associations and not assess causality; we could only adjust for measurable confounders but not for unmeasurable confounders; therefore, future clinical studies with higher levels of evidence in larger populations to validate our findings are warranted.
Conclusion
The relationship between anxiety and cognition is nonlinear; we speculate that when the HAMA score is less than 9, anxiety and cognition are negatively correlated, and when it is greater than or equal to 9, the cognitive score will no longer decrease and is saturated.
Author Statements
Acknowledgements
The authors would like to thank Miss. Xinlin Chen and Mr. Chi Chen of the Empower Institute for their help in processing the data.
Authors Contributions
ZZ contributed to the drafting of the manuscript. YS and YZ contributed to the data collection. YW and RS contributed to the proofreading of the manuscript. CY and LZ contributed to the conception and critical revision of the manuscript, analysis and interpretation of the data and approved the final version of the submitted manuscript.
Funding
This work was supported by the [Department of Neurology, The First Affiliated Hospital of Henan University of Science and Technology, China #1] under Grant [LHGJ20220676]; [Third Affiliated Hospital of Inner Mongolia Medical University, China#2] under Grant [wsjkkj098].
Availability of Data and Materials
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This trial was registered (registration number: 2022038004-FS01) and approved by the Clinical Research Ethics Committee of the Second People's Hospital of Shenzhen, China. The registration number of the Chinese Clinical Trials Registration Center is ChiCTR2200060103.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Calhoon GG, Tye KM. Resolving the neural circuits of anxiety. Nature Neuroscience. 2015; 18: 1394–1404.
- Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: attentional control theory. Emotion (Washington, D.C.). 2007; 7: 336–353.
- Osinsky R, Gebhardt H, Alexander N, Henning J. Trait anxiety and the dynamics of attentional control. Biological Psychology. 2012; 89: 252–259.
- Tseng YL, Lu CF, Wu SM, Shimada S, Huang T, Lu GY. A functional near-infrared spectroscopy study of state anxiety and auditory working memory load. Frontiers in Human Neuroscience. 2018; 12: 313.
- Lee EH, Kim JW, Kang HJ, Kim SW, Kim JT, Park MS, et al. Association between anxiety and functional outcomes in patients with stroke: a 1-year longitudinal study. Psychiatry Investigation. 2019; 16: 919–925.
- Lambiase MJ, Kubzansky LD, Thurston RC. Prospective study of anxiety and incident stroke. Stroke; a journal of cerebral circulation. 2014; 45: 438–443.
- Rost NS, Brodtmann A, Pase MP, van Veluw SJ, Biffi A, Duering M, et al. Post-stroke cognitive impairment and dementia. Circulation Research. 2022; 130: 1252–1271.
- Pantoni L, Salvadori E. Location of infarcts and post-stroke cognitive impairment. The Lancet. Neurology. 2021; 20: 413–414.
- Gottesman RF, Hillis AE. Predictors and assessment of cognitive dysfunction resulting from ischaemic stroke. Lancet neurology. 2010; 9: 895–905.
- Einstad MS, Saltvedt I, Lydersen S, Ursin MH, Munthe-Kaas R, Ihle-Hansen H, et al. Associations between post-stroke motor and cognitive function: a cross-sectional study. BMC Geriatrics. 2021; 21: 103.
- Zhu Y, Zhao S, Fan Z, Li Z, He F, Lin C, et al. Evaluation of the mini-mental state examination and the montreal cognitive assessment for predicting post-stroke cognitive impairment during the acute phase in chinese minor stroke patients. Frontiers in Aging Neuroscience. 2020; 12: 236.
- Kanellopoulos D, Wilkins V, Avari J, Oberlin L, Arader L, Chaplin M, et al. Dimensions of post-stroke depression and neuropsychological deficits in older adults. The American journal of geriatric psychiatry: official journal of the American Association for Geriatric Psychiatry. 2020; 28: 764–771.
- Douven E, Aalten P, Staals J, Schievinke SHJ, van Oostenbrugge RJ, Verhey FRJ, et al. Co-occurrence of depressive symptoms and executive dysfunction after stroke: associations with brain pathology and prognosis. Journal of Neurology, Neurosurgery, and Psychiatry. 2018; 89: 859–865.
- Yang W, Xiao L, Yuan Z, Huang H, Xiang Y, Liu Z, et al. Anxiety and depression in patients with physical diseases and associated factors: a large-scale field survey in general hospitals in china. Frontiers in Psychiatry. 2021; 12: 689787.
- Gigi A, Papirovitz M. Association of anxiety awareness with risk factors of cognitive decline in mci[J]. Brain Sciences. 2021; 11: 135.
- Nyberg J, Henriksson M, Wall A, Vestberg T, Westerlund M, Walser M, et al. Anxiety severity and cognitive function in primary care patients with anxiety disorder: a cross-sectional study. BMC Psychiatry. 2021; 21: 617.
- Gimson A, Schlosser M, Huntley JD, Marchant NL. Support for midlife anxiety diagnosis as an independent risk factor for dementia: a systematic review. BMJ Open. 2018; 8: e019399.
- Ma L. Depression, anxiety, and apathy in mild cognitive impairment: current perspectives. Frontiers in Aging Neuroscience. 2020; 12: 9.
- Xiang Hua,Wang Chunxiu Mei Liping et al. Advances in stroke epidemiology. Chinese Journal of Epidemiology. 2011: 847-853.
- Hamilton M. The assessment of anxiety states by rating. The British Journal of Medical Psychology, 1959; 32: 50–55.
- Moazen-Zadeh E, Bayanati S, Ziafat K, Rezaei F, Mesgarpour B, Akhonet al. Vortioxetine as adjunctive therapy to risperidone for treatment of patients with chronic schizophrenia: a randomised, double-blind, placebo-controlled clinical trial. Journal of Psychopharmacology (Oxford, England). 2020; 34: 506–513.
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society. 2005; 53: 695–699.
- Hughes D, Judge C, Murphy R, Loughlin E, Costello M, Whiteley W, et al. Association of blood pressure lowering with incident dementia or cognitive impairment: a systematic review and meta-analysis. JAMA. 2020; 323: 1934-1944.
- Brenes GA, Danhauer SC, Lyles MF, Hoagn PE, Miller ME. Telephone-delivered cognitive behavioral therapy and telephone-delivered nondirective supportive therapy for rural older adults with generalized anxiety disorder. JAMA psychiatry. 2015; 72: 1012–1020.
- Gallagher D, Coen R, Kilroy D, Belinski K, Bruce I, Coakley D, et al. Anxiety and behavioural disturbance as markers of prodromal alzheimer’s disease in patients with mild cognitive impairment. International Journal of Geriatric Psychiatry. 2011; 26: 166–172.
- Williams OA, Demeyere N. Association of depression and anxiety with cognitive impairment 6 months after stroke. Neurology. 2021; 96: e1966–e1974.
- Bierman EJM, Comijs HC, Jonker C, Beekman ATF. Effects of anxiety versus depression on cognition in later life. The American Journal of Geriatric Psychiatry: Official Journal of the American Association for Geriatric Psychiatry. 2005; 13: 686–693.
- Levine DA, Galecki AT, Langa KM, Unverzagt FW, Kabeto MU, Giordani B, et al. Trajectory of cognitive decline after incident stroke. JAMA. 2015; 314: 41–51.
- Gorelick PB, Nyenhuis D. Stroke and cognitive decline. JAMA. 2015; 314: 29–30.