
Research Article
Phys Med Rehabil Int. 2024; 11(3): 1234.
Causal Inference for Scoliosis and Strabismus: A 2-Sample Mendelian Randomization Study
Changsui Yu1,2; Zifeng Xu5*; Xiaofeng Zhang3; Zhongbao Yu4; Kejian Lu2; Fengyuan Zhan4; Xinyue Zhang2; Liguo Zhu1
1The Second Department of Spine, Wangjing hospital of China Academy of Chinese Medical Sciences, China
2Department of Traditional Chinese Osteopathy & Traumatology, Beijing Electric Power Hospital, China
3The Second Affiliated Hospital of Heilongjiang university of Chinese Medicine, China
4Chinese Medicine Department, Liaoning Yu Zhongbao Clinic of Traditional Chinese Medicine, China
5Health News, China
*Corresponding author: Zifeng Xu, Health News. No. 6, Dongzhimen Wai Street, Beijing, 100027, China. Email: xuzifeng200406@163.com
Received: July 13, 2024 Accepted: July 31, 2024 Published: August 08, 2024
Abstract
Bachground: Some studies have shown an association between spinal curvature and strabismus, but the genetic association has not been clarified. Therefore, the present study is proposed to be a Mendelian randomization study aiming to investigate the genetic causal association between spinal curvature and strabismus.
Purpose: Genetic causal associations between strabismus, Convergent Concomitant Strabismus (Ccs), Divergent Concomitant Strabismus (Dcs), Other Specified and Unspecified Strabismus (Osus), Other Strabismus (Os) and spinal curvature were investigated by a bidirectional Mendelian randomization study to provide a basis for the prevention and treatment of spinal curvature.
Methods: Significant and independent Single Nucleotide Polymorphisms (SNPs) in genome-wide association studies were selected as Instrumental Variables (IVs) for Mendelian Randomization (MR) analysis. Inverse Variance Weighted (IVW), MR-Egger regression, weighted median (WME), Simple Mode (SM), and Weighted Mode (WM) were used to analyze causal association; Heterogeneity and multiplicity tests were also performed and analyzed using the leave-one-out method to assess the stability of the results.
Results: MR and reverse MR was utilized to assess the impact of scoliosis on strabismus, revealing that the 95% confidence intervals of all instrumental variables OR values spanned 1 and the P values were all above 0.05. These results indicate a lack of evidence supporting a causal relationship between scoliosis and strabismus.
Conclusion: There is currently no conclusive evidence of a genetic causal relationship between scoliosis and strabismus, including their subtypes. Further laboratory studies are needed to confirm these findings, and future research with larger sample sizes is necessary to provide more robust support.
Keywords: Spinal curvature; strabismus; subgroup; Mendelian randomization; Causal association analysis
Introduction
Scoliosis is a three-dimensional deformity of the spine characterized by abnormal curvature and vertebral rotation. Diagnosis typically involves a Cobb Angle exceeding 10° on the coronal plane of the spine orthograph [1]. While the condition can have various causes, over 80% of cases are classified as idiopathic scoliosis [2]. Factors such as congenital or acquired vertebral structural diseases, brain stem asymmetry, sensory and balance issues, as well as platelet and collagen dysfunction can contribute to the development of scoliosis [3]. The resulting spinal curvature and deformation can lead to movement and proprioception issues, along with restricted thorax movement that can impact lung expansion and ventilation, affecting the physical and mental health of adolescents [4]. Severe cases of scoliosis may result in organ damage such as spinal cord compression, respiratory failure, and cardiovascular disease [5]. The pathogenesis of scoliosis may involve a combination of genetic, hormonal, endocrinological, and muscular factors [6], with a higher prevalence in females typically around the age of 10 [7]. The prevalence of scoliosis in children and adolescents ranges from 0.47% to 5.20% [8]. In recent years, the incidence of scoliosis has been increasing, making it a significant health concern alongside myopia and obesity. Scoliosis can be difficult to detect early on, and the progression tends to accelerate with greater angles, emphasizing the importance of timely intervention to avoid missing the optimal window for correction. Currently, scoliosis treatment options are categorized into non-surgical and surgical approaches. Surgical treatment is known for its complexity, high risk, and resource-intensive nature. Non-surgical treatments are typically recommended for patients with mild scoliosis and include exercise therapy, breathing exercises, suspension therapy, chiropractic massage, electrical stimulation, traction therapy, and bracing. These methods are crucial for both preventing and managing scoliosis in most patients [9].
Strabismus, the misalignment of the eyes, encompasses various types such as esotropia and exotropia [10]. According to the 2017 clinical guidelines for esotropia and exotropia from the American Academy of Ophthalmology and the 'Expert Consensus on the Classification of Strabismus' by the Strabismus and Pediatric Ophthalmology Group of the Chinese Medical Association, esotropia includes infantile esotropia, acquired esotropia, and other types, while exotropia includes infantile exotropia, intermittent exotropia, convergence deficiency, and other types [10]. Classification based on eyeball and eye movement changes and strabismus angle includes Convergent Concomitant Strabismus (Ccs), Divergent Concomitant Strabismus (Dcs), Other Specified and Unspecified Strabismus (Osus), and Other Strabismus (Os) [11,12]. Treatment is recommended for all types of esotropia, with early detection and intervention being crucial for improving long-term visual, motor, and perceptual outcomes, as highlighted in the 2017 Preferred Practice Pattern. The guidelines also emphasize the impact of strabismus on children's quality of life and the negative effects of exotropia on children and parents' quality of life [13].
Recent clinical observational studies have indicated a potential association between visual and spinal curvature. The prevalence of scoliosis among both congenitally and acquired blind individuals has been reported to range from 42.9% to 59% [14]. A recent meta-analysis of literature involving a large sample of nearly 20,000 individuals demonstrated a 2.91 times increased risk of scoliosis in the visually impaired population. Subgroup analyses revealed that the risk of scoliosis was over 7 times higher in individuals with complete blindness, with three studies in the hyporefractive subgroup showing an elevated risk of scoliosis [15]. Two additional studies involving subjects with strabismus reported a 3.09-fold increased risk of scoliosis [15]. However, the causal relationship between strabismus and scoliosis remains uncertain, highlighting the need for further research to elucidate a potential link between these conditions.
Traditional observational epidemiological studies have faced challenges in identifying disease causes and making causal inferences, including issues like reverse causal association, potential confounders, microexposure factors, and multiple tests. Randomized Control Trials (RCTs) are often difficult to implement due to ethical constraints and experimental limitations when trying to establish a direct correlation between exposure factor X and disease outcome Y. Mendelian Randomization (MR) design, inspired by Instrumental Variable (IV) concepts from econometrics, uses gene variation as an instrumental variable for studying exposure factors [16]. This approach aims to estimate potential causal relationships between exposures and outcomes, offering a solution to the aforementioned challenges [17]. Genetic variation, randomly assigned during meiosis, is free from confounding and reverse causality, mimicking the effects of randomized controlled trials18 and providing more accurate insights into causality [19]. In this study, strabismus and its subtypes were considered as exposure factors, scoliosis as outcome variables, and a two-sample MR Analysis was conducted to assess the causal relationship between strabismus and scoliosis, followed by reverse MR Validation analysis.
Materials and Methods
Research Design
This study utilized strabismus as the exposure factor, and Single Nucleotide Polymorphisms (SNPs) significantly associated with it as Instrumental Variables (IVs).
Scoliosis was selected as the outcome variable, and genetic causal association analysis was conducted using the Two Sample MR package in R. The reliability of the results was verified through Cochran Q heterogeneity test, pleiotropy test, and sensitivity analysis. The study methodology adhered to three instrumental variable assumptions [20]: (1) significant association between instrumental variables and strabismus; (2) instrumental variables are unrelated to any potential confounding factors; (3) instrumental variables are not significantly associated with scoliosis (Figure 1). Q heterogeneity test, multiplicity test, and sensitivity analysis.
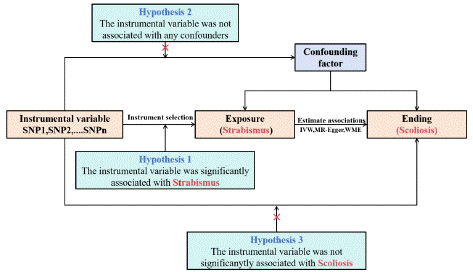
Figure 1: Design of the two-sample Mendelian randomization study.
Data Sources
In this study, genome-wide association study data for scoliosis and strabismus and strabismus related subtyping were obtained from the website "https://gwas.mrcieu.ac.uk/".The included population was the European population.
Instrumental Variables
First, SNPs closely related to both strabismus and scoliosis with genome-wide significance (P<5×10-5). Subsequently, SNPs strongly associated with strabismus were integrated with scoliosis data as instrumental variables, harmonizing the data with the effect allele frequency, while removing palindromic SNPs with intermediate allele frequencies and SNPs associated with confounding factors. Finally, the strength of the instrumental variable was evaluated by calculating the F value, with F>10 indicating no weak instrumental variables. Ultimately, SNPs that are mutually independent and significantly associated with strabismus were obtained as the final instrumental variables [21]. The process of selecting strabismus-associated subtypes instrumental variables is consistent with the above.
MR Analysis
Statistical software R 4.3.1 and a significance level of a=0.05 were utilized in this research. Five MR Analyses were conducted to assess the causal relationship between strabismus and scoliosis. These analyses included Inverse Variance Weighted (IVW), MR-Egger regression, Weighted Median (WME), Simple Mode (SM), and Weighted Mode (WM) [22]. IVW, the primary analysis model, utilized the inverse variance of each instrumental variable as weights and estimated the overall effect using the ratio method and weighted regression. MR-Egger regression method differs from IVW by considering an intercept term and using the inverse of the outcome variance as weights. WME required at least 50% effective instrumental variables, and after sorting SNPs by weight, the median was used to estimate causal effects with good consistency. SM and WM are modal-based estimation models that group SNPs with similar causal effects and provide estimates for most clustered SNPs. WM, in particular, assigns weights to each SNP based on the inverse variance of its effect.
Sensitivity Analysis
Cochran Q test, MR-Egger regression, and leave-one-out analysis were employed to assess the robustness of the findings [23]. Cochran Q was utilized to evaluate differences among SNPs, with a significance level of p<0.05 indicating heterogeneity. A MR-Egger regression intercept term with p<0.05 suggests potential horizontal pleiotropy among the instrumental variables. The leave-one-out method involved systematically removing SNPs one by one to assess the collective impact of the remaining SNPs on the results, thus examining the influence of individual SNPs.
Results
Population Characteristics and SNP Information for Instrumental Variables
There were six strabismus-related datasets including the UK Biobank (UKB) FinnGen (Finn) databases Convergent Concomitant Strabismus (Ccs), Divergent Concomitant Strabismus (Dcs), Other specified and unspecified strabismus (Osus), and Subtype classification of Other strabismus (Os). The UKB dataset focused on patients undergoing surgical correction of strabismus and had the largest sample size, consisting of 6117 patients, 456,816 controls, and 9.8 million SNPs. Additionally, a scoliosis dataset from the Finnish database comprised 1,168 individuals with the disease and 164,682 controls, with a total of 16.38 million SNPs. All datasets represented European populations and included both male and female individuals, with reference genomes of HG19/GRCh37 (Table 1).
ID
Trait
Case(n)
Control
(n)SNPs
(n)Population
Build
Year
UKB-b-15527
Strabismus
6117
456816
9851867
European
HG19/GRCh37
2018
Finn-b-M13-SCOLIOSIS
Scoliosis
1168
164682
16380270
European
HG19/GRCh37
2021
Finn-b-H7-CONVERSTRAB
Ccs
967
210931
16380461
European
HG19/GRCh37
2021
Finn-b-H7-STRABISMUS
Strabismus
4620
214172
16380466
European
HG19/GRCh37
2021
Finn-b-H7-DIVERGSTRAB
Dcs
1348
210931
16380456
European
HG19/GRCh37
2021
Finn-b-H7-STRABISMUS
Osus
140
210931
16380456
European
HG19/GRCh37
2021
Finn-b-H7-STRABOTH
Os
3829
210931
16380463
European
HG19/GRCh37
2021
Note: Ccs: Convergent Concomitant Strabismus; Dcs: Divergent Concomitant Strabismus; Osus: Other Specified and Unspecified Strabismus; Os: Otherstrabismus.
Table 1: Basic information about the data set.
Forward MR Analysis Results
Genetic causal relationship analysis between Strabismus and Scoliosis: This study utilized two datasets on strabismus (UKB-b-15527, finn-b-H7_STRABISMUS) as exposure variables and scoliosis (finn-b-M13_SCOLIOSIS) as the outcome to perform MRanalyses on two separate samples. Following the screening for SNP sites with genome-wide significance and removal of palindromic SNPs with intermediate allele frequencies, 44 and 15 SNPs were identified as instrumental variables, respectively. Various methods were employed to estimate the causal relationship between strabismus and scoliosis, with the results depicted in Figure 2 and Figure 3. The data analysis of ukb_Strabismus and finn_Scoliosis revealed odds ratio (OR) values for the MR-Egger, WME, IVW, SM, and WM methods, with corresponding p-values of 0.101, 0.825, 0.791, 0.966, and 0.830, respectively. The IVW method suggested no causal link between strabismus and scoliosis, as all p-values 0.05. Similar results were observed in the analysis of finn_Strabismus and finn_Scoliosis.

Figure 2: MR results of Strabismus and Scoliosis.
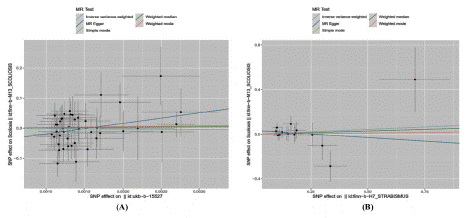
Figure 3: Mendelian randomized scatterplot of strabismus and scoliosis.
((A)Mendelian randomized scatterplot of finn_Scoliosis and ukb_Strabismus; (B) Mendelian randomized scatterplot of finn_Scoliosis and finn_Strabismus.)
Heterogeneity tests were conducted on MR-Egger and IVW results. In the dataset analysis model of ukb_Strabismus, finn_Strabismus, and scoliosis, all heterogeneity test results were p>0.05 (Table S1 and Figure S1), indicating the absence of heterogeneity. The intercept of the MR-Egger regression was utilized to assess pleiotropy in the study. The results revealed that the Egger-intercept values were all close to 0, with the regression intercept term p>0.05, suggesting no horizontal pleiotropy and no interference effects in the MR results (Table S2). A 'Leave-one-out' sensitivity analysis demonstrated that excluding a specific SNP did not significantly alter the IVW analysis results of the remaining SNPs, indicating no SNPs had a substantial impact on the estimated causal association (Figure S2).
Genetic causal relationship between strabismus subtypes and scoliosis: The genetic causal relationship between Ccs, Dcs, Os, Osus, and scoliosis in strabismus subtypes was further analyzed. MR results for Ccs and scoliosis are presented in Figure 4 and Figure 5A. The P-values for the five MR inspection methods (MR-Egger, WME, IVW, SM, and WM) are 0.184, 0.422, 0.208, 0.651, and 0.617, respectively. Based on the IVW and MR-Egger models, it is concluded that there is no causal relationship between Ccs and scoliosis. Similarly, MR results for Dcs and scoliosis are shown in Figure 4 and Figure 5B. The P-values for the five test methods are 0.599, 0.109, 0.104, 0.083, and 0.698, respectively. Again, based on the IVW and MR-Egger models, it is concluded that there is no causal relationship between Dcs and scoliosis.
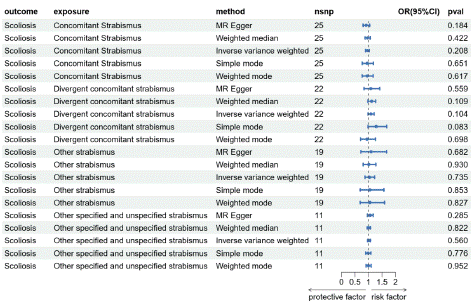
Figure 4: Mendelian randomized Results of strabismus Subtyping and Scoliosis.
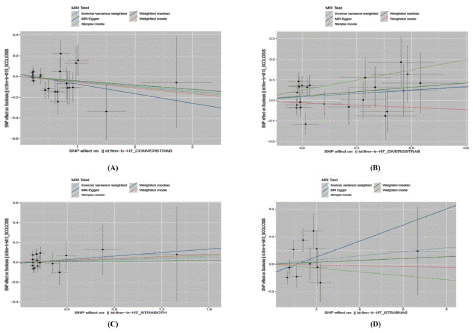
Figure 5: Mendelian randomized scatterplot of strabismus subtyping and scoliosis.
((A)Mendelian randomized scatterplot of Ccs and scoliosis; (B) Mendelian randomized scatterplot of Dcs and scoliosis; (C)Mendelian randomized scatterplot of Os and scoliosis; (D)Mendelian randomized scatterplot of Osus and scoliosis.)
The MR results for Os and scoliosis are illustrated in Figure 4 and Figure 5C. The P-values for the five test methods were 0.682, 0.930, 0.735, 0.853, and 0.827, respectively. Based on the IVW and MR-Egger models, it can be inferred that there is no causal relationship between Os and scoliosis. The MR results for Osus and scoliosis are presented in Figre 4 and Figure 5D. The P-values t methods were 0.285, 0.822, 0.560, 0.776, and 0.952, respectively. Similarly, the IVW and MR-Egger models suggest no causal relationship between Osus and scoliosis.
Model evaluation of causality between strabismus subtyping and scoliosis: Heterogeneity tests were conducted on MR-Egger and IVW, with all results indicating no heterogeneity between models (Table S3 and Figure S3). The intercept of MR-Egger regression was utilized to assess pleiotropy in the study. The Egger-intercept values were all near 0, with regression intercept termp>0.05, suggesting no horizontal pleiotropy and no interference in the MR results (Table S4). The 'Leave-one-out' sensitivity analysis demonstrated that excluding a specific SNP did not significantly alter the IVW analysis results of the remaining SNPs, indicating no single SNP had a substantial impact on the estimated causal association (Figure S4).
Results of reverse MR Analysis
Based on the reverse MR analysis results (Figure 6-7), the IVW OR for ukb_Strabismus is 0.99 with a 95% CI of 0.998-1.000 and a P-value of 0.265. For finn_Scoliosis, the IVW OR is 1.010 with a 95% CI of 0.967-1.056 and a P-value of 0.630. It is currently inconclusive to state a clear association between scoliosis and strabismus. No significant causal relationship was found between the subtypes Ccs and Dcs of scoliosis and strabismus (Figure 8-9). Furthermore, no SNPs related to scoliosis were identified in Os and Osus. Cochran's IVW and MR-Egger's Q tests indicate no significant heterogeneity in the model (Tables S4, S7 and Figures S5, S7). MR-Egger regression intercept analysis (Tables S5, S6) and MR-PRESSO analysis also did not reveal significant horizontal pleiotropy (Figures S6, S8).
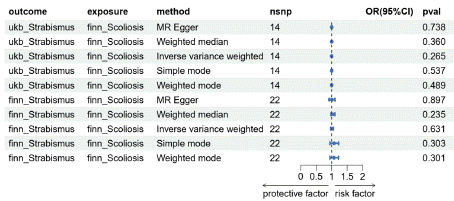
Figure 6: MR Results of strabismus and scoliosis.
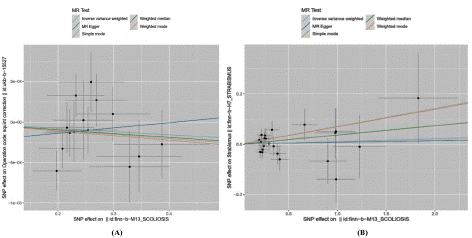
Figure 7: Mendelian randomized scatterplot of strabismus and scoliosis.
((A) Scatterplot of ukb_Strabismus for finn_Scoliosis; (B) Scatter plot of finn_Strabismus for finn_Scoliosis.)
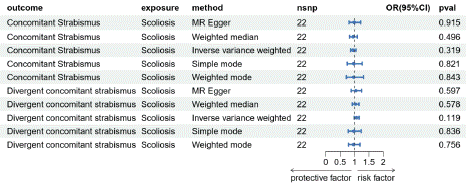
Figure 8: Mendelian randomized Results of strabismus subtyping and scoliosis.
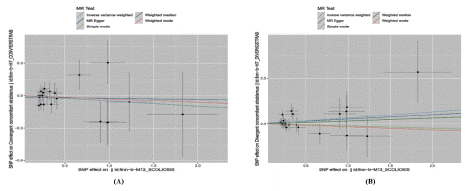
Figure 9: Mendelian randomized scatterplot of strabismus subtyping and scoliosis.
((A) Scatterplot between Ccs and finn_Scoliosis (B) scatterplot between Dcs and finn_Scoliosis.)
Discussion
This study explores the causal effect of strabismus and its subtypes on scoliosis using MR results. It suggests a genetic direction for further investigation, finding no evidence to support a causal effect of specific type of strabismus on scoliosis. These findings offer a fresh perspective on the mechanisms of visual abnormalities in scoliosis.
Scoliosis is a complex disease with genetic factors playing a significant role in its development. Despite extensive research, the exact genetic mechanisms are still not fully understood and show a lot of variation. Familial idiopathic scoliosis has been linked to various chromosomal regions, suggesting a polygenic inheritance pattern that requires further investigation to identify specific genetic factors [24]. For instance, genetic factors related to connective tissue structure, bone formation, melatonin signaling, puberty, and growth have all been associated with idiopathic scoliosis, indicating a complex genetic component influenced by ethnic and genetic diversity. Studies have demonstrated that idiopathic scoliosis may have a genetic basis with different inheritance patterns such as autosomal dominant, X-linked, polygenic, or multifactorial inheritance, leading to a complex genetic pattern due to locus and allelic heterogeneity [25]. Dysregulation of Wnt signaling, as observed in Ptk7 mutant zebrafish, is linked to congenital and idiopathic scoliosis [26]. Polymorphisms in the chromodomain helicase DNA binding protein 7 Gene (CHD7) gene are associated with idiopathic scoliosis [27,28]. Gene-environment interactions, like the interplay between Notch signaling pathway gene haploinsufficiency and prenatal hypoxia, contribute to congenital scoliosis development, underscoring the intricate role of genetic and environmental factors [29]. A compound inheritance pattern involving rare null mutations and hypoalleles of the T-box transcription factor 6 (TBX6) gene was identified in a subset of congenital scoliosis cases, accounting for 11% of cases [30]. Overall, research indicates a significant genetic component in scoliosis, with multiple genes and pathways playing a role in its pathogenesis. Various inheritance patterns and gene-environment interactions further contribute to its complexity.
Many family members have a shared strabismus, indicating that strabismus may have a genetic component. Studies have found that nucleotide polymorphisms in certain genes are highly correlated with strabismus subtypes [31]. Studies have shown that gene mutations necessary for the normal development and connection of brainstem ocular motor neurons, such as PHOX2A, SALL4, KIF21A, ROBO3, and HOXA1, are associated with congenital strabismus syndrome. Meanwhile, strabismus in families is associated with chromosome 7p22.1. Genetic susceptibility exists at susceptibility loci, indicating genetic heterogeneity between strabismus and families [32]. The heritability of strabismus has a great impact on esotropia, and the genetic effect is limited to esotropia and is not related to ametropia, suggesting that genetic factors may not play an important role in exotropia [33]. Genetic variants within the NPLOC4-TSPAN10-PDE6G gene cluster on chromosome 17q25 are associated with an increased risk of strabismus, with a population attributable risk of approximately 8.4% [34]. In summary, this suggests that certain types of strabismus have a strong genetic component. Genetic heterogeneity is evident, with different genes and loci associated with different forms of the disease. These findings highlight the complexity of strabismus genetics and point to specific biological pathways and brain regions that may be involved in its pathogenesis.
Previous research has indicated that the CHD7 gene is associated with susceptibility to adolescent idiopathic scoliosis and shares commonalities with the rare CHARGE syndrome. Mutations, such as missense and splicing mutations, in the coding exon of the CHD7 gene have been linked to CHARGE syndrome, with approximately 60% of patients exhibiting symptoms like eye diseases and heart defects. However, the mortality rate among infants with CHARGE syndrome is notably high, and there is a scarcity of research data on this condition. Studies on ROBO3 gene polymorphisms have revealed a connection to the development of horizontal gaze palsy. Notably, a specific ROBO3 gene variation (rs74787566) has been significantly associated with adolescent idiopathic scoliosis [35]. The correlation between certain ROBO3 gene polymorphisms and strabismus disorder aligns with previous findings that individuals with strabismus have a higher prevalence of idiopathic scoliosis, particularly those with exotropia [36,37]. Visual impairments associated with idiopathic scoliosis can range from severe visual impairment to myopia and heterosopia (refractive errors in both eyes). Animal studies have shown that early-onset strabismus can disrupt the astigmatism process, leading to heteroopia. Early hyperopic strabismus is a significant risk factor for amblyopia, while early esotropia can result in both strabismus and amblyopia [38]. Research has suggested that thinning of the choroidal layer of the eye may underlie the development of anisotropy, with amblyopia, refractive error, and strabismus potentially coexisting. Therefore needs to be confirmed by further studies [39].
There is currently insufficient evidence to support a genetic risk for both strabismus and scoliosis. Previous research has indicated that various visual impairments, including blindness, refractive errors, and strabismus, may increase the likelihood of developing scoliosis [15]. However, further studies are required to comprehensively understand the genetic link between these conditions. Our study utilized data from large cohorts in the UK Biobank and Finnish databases to explore the genetic relationship between strabismus and scoliosis in terms of single nucleotide polymorphisms. Our findings suggest that there is no direct causal connection between strabismus and scoliosis. This conclusion is supported by the limitations of existing studies, which often rely on cross-sectional methods and lack consistency in evaluating scoliosis cases. Additionally, our study is limited by its focus on European populations, which may not be generalizable to other groups. Furthermore, subgroup analyses based on age, health status, and gender were not feasible due to data constraints [40]. This study offers several advantages. While randomized controlled trials are considered a robust clinical research method, they can be costly, challenging to track over time, and complex to execute in practice. In this study, the two-sample Mendelian randomization research design was utilized to minimize the impact of confounding variables and reverse causality, effectively emulating a randomized controlled trial. Additionally, the study carefully selected single nucleotide polymorphisms highly correlated with the exposure of interest as instrumental variables, and employed sensitivity analysis techniques to validate the research findings.
Conclusion
Ultimately, the study concludes that there is no current evidence supporting a causal relationship between strabismus and its associated subtypes with scoliosis. The findings of this study offer a fresh perspective for further investigating the underlying mechanisms of scoliosis in individuals with visual impairments.
Author Statements
Funding
This study was supported by Supported by the National Natural Science Foundation of China (No.81774343); National Traditional Chinese Medicine examination 2023 annual research project youth special project (No.TE2023001); Research topic of education and teaching reform in Capital Medical University (No. 2023JYY484) and Beijing Electric Power Hospital Doctor fund project (No. Y2023003).
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Availability of Data and Material
All data used to support the findings of this study are included within the article.
Authors’ Contributions
DrLiguo Zhu conceptualized conceived and designed the review. DrChangsui Yu and DrXiaofeng Zhang wrote the first draft of the manuscript and critically revised the final manuscript. Dr Zhongbao Yu, Shuren Wang and Kejian Lu wrote sections and guided part of the manuscript. Dr Fengyuan Zhan, Daoxiong Gong and Kun Zhang conceived and drafted the figures. All of the authors contributed to the article and approved the submitted version.
References
- Lai B, Jiang H, Gao Y, Zhou X. Causal effects of gut microbiota on scoliosis: a bidirectional two-sample mendelian randomization study. Heliyon. 2023; 9: e21654.
- Negrini S, Donzelli S, Aulisa AG, Czaprowski D, Schreiber S, de Mauroy JC, et al. 2016 SOSORT guidelines: orthopaedic and rehabilitation treatment of idiopathic scoliosis during growth. Scoliosis Spinal Disord. 2018; 13: 3.
- Vasiliadis ES, Grivas TB, Kaspiris A. Historical overview of spinal deformities in ancient greece. Scoliosis. 2009; 4: 6.
- Xu S, Su Y, Wang Z, Liu C, Jin L, Liu H. The prevalence and characteristics of primary and middle-school students in China: a Meta-analysis based on 72 epidemiological research. Chin J Spine Spinal Cord. 2021; 31: 901-910.
- Weinstein SL, Dolan LA, Spratt KF, Peterson KK, Spoonamore MJ, Ponseti IV. Health and function of patients with untreated idiopathic scoliosis: a 50-year natural history study. Jama-J Am Med Assoc. 2003; 289: 559-567.
- Peng Y, Wang SR, Qiu GX, Zhang JG, Zhuang QY. Research progress on the etiology and pathogenesis of adolescent idiopathic scoliosis. Chin Med J. 2020; 133: 483-493.
- Zhu Z, Tang NL, Xu L, Qin X, Mao S, Song Y, et al. Genome-wide association study identifies new susceptibility loci for adolescent idiopathic scoliosis in chinese girls. Nat Commun. 2015; 6: 8355.
- Konieczny MR, Senyurt H, Krauspe R. Epidemiology of adolescent idiopathic scoliosis. J Child Orthop. 2013; 7: 3-9.
- Wang A, Ji Z, Chen H, Yang Q, Yang Q. Research progress on influencing factors and clinical treatment of adolescent scoliosis. IMHGN. 2023; 29: 3176-3180.
- Zhao C, Yao J. Standardizing the diagnosis and treatment of strabismus: interpreting the 2017 clinical guidelines for esotropia and exotropia of the American Academy of Ophthalmology. Chin J Ophthalmol. 2020; 56: 176-182.
- Hao J, Wang M, Liu J, Yusufu M, Cao K, Fu J. Alteration of neurotrophic factors and innervation in extraocular muscles of individuals with concomitant esotropia. Invest Ophthalmol Vis Sci. 2024; 65: 1.
- Hashemi H, Pakzad R, Heydarian S, Yekta A, Aghamirsalim M, Shokrollahzadeh F, et al. Global and regional prevalence of strabismus: a comprehensive systematic review and meta-analysis. Strabismus. 2019; 27: 54-65.
- Jones-Jordan L, Wang X, Scherer RW, Mutti DO. Spectacle correction versus no spectacles for prevention of strabismus in hyperopic children. Cochrane Database Syst Rev. 2014; 8: CD7738.
- Aulisa L, Bertolini C, Piantelli S, Piazzini DB. Axial deviations of the spine in blind children. Ital J Orthop Traumatol. 1986; 12: 85-92.
- Gallego-Siles JR, Siles-Fuentes MJ, Ibanez-Vera AJ, Cortes-Perez I, Obrero-Gaitan E, Lomas-Vega R. Idiopathic scoliosis in subjects with eye diseases: a systematic review with meta-analysis. Ann NY Acad Sci. 2024; 1533: 81-88.
- Birney E. Mendelian randomization. Cold Spring Harb Perspect Med. 2022; 12: a041302.
- Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey SG. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008; 27: 1133-1163.
- Ni JJ, Li XS, Zhang H, Xu Q, Wei XT, Feng GJ, et al. Mendelian randomization study of causal link from gut microbiota to colorectal cancer. Bmc Cancer. 2022; 22: 1371.
- Wang C, Wu W, Yang H, Ye Z, Zhao Y, Liu J, et al. Mendelian randomization analyses for pcos: evidence, opportunities, and challenges. Trends Genet. 2022; 38: 468-482.
- Carrasquilla GD, Garcia-Urena M, Fall T, Sorensen T, Kilpelainen TO. Mendelian randomization suggests a bidirectional, causal relationship between physical inactivity and adiposity. Elife. 2022; 11: e70386.
- Weith M, Beyer A. The next step in mendelian randomization. Elife. 2023; 12: e86416.
- Yuan S, Mason AM, Titova OE, Vithayathil M, Kar S, Chen J, et al. Morning chronotype and digestive tract cancers: mendelian randomization study. Int J Cancer. 2023; 152: 697-704.
- Sanderson E. Multivariable mendelian randomization and mediation. Cold Spring Harb. Perspect Med. 2021; 11: a038984.
- Miller NH. Genetics of familial idiopathic scoliosis. Clin Orthop Rel Res. 2007; 462: 6-10.
- Heary RF, Madhavan K. Genetics of scoliosis. Neurosurgery. 2008; 63: 222-227.
- Hayes M, Gao X, Yu LX, Paria N, Henkelman RM, Wise CA, et al. Ptk7 mutant zebrafish models of congenital and idiopathic scoliosis implicate dysregulated wnt signalling in disease. Nat Commun. 2014; 5: 4777.
- Borysiak K, Janusz P, Andrusiewicz M, Chmielewska M, Kozinoga M, Kotwicki T, et al. Chd7 gene polymorphisms in female patients with idiopathic scoliosis. Bmc Musculoskelet Disord. 2020; 21: 18.
- Wang W, Chen T, Liu Y, Wang S, Yang N, Luo M. Predictive value of single-nucleotide polymorphisms in curve progression of adolescent idiopathic scoliosis. Eur Spine J. 2022; 31: 2311-2325.
- Sparrow DB, Chapman G, Smith AJ, Mattar MZ, Major JA, O’Reilly VC, et al. A mechanism for gene-environment interaction in the etiology of congenital scoliosis. Cell. 2012; 149: 295-306.
- Wu N, Ming X, Xiao J, Wu Z, Chen X, Shinawi M, et al. Tbx6 null variants and a common hypomorphic allele in congenital scoliosis. N Engl J Med. 2015; 372: 341-350.
- Engle EC. The genetic basis of complex strabismus. Pediatr Res. 2006; 59: 343-348.
- Parikh V, Shugart YY, Doheny KF, Zhang J, Li L, Williams J, et al. A strabismus susceptibility locus on chromosome 7p. Proc Natl Acad Sci USA. 2003; 100: 12283-12288.
- Sanfilippo PG, Hammond CJ, Staffieri SE, Kearns LS, Melissa LS, Barbour JM, et al. Heritability of strabismus: genetic influence is specific to eso-deviation and independent of refractive error. Twin Res Hum Genet. 2012; 15: 624-630.
- Plotnikov D, Shah RL, Rodrigues JN, Cumberland PM, Rahi JS, Hysi PG, et al. A commonly occurring genetic variant within the nploc4-tspan10-pde6g gene cluster is associated with the risk of strabismus. Hum Genet. 2019; 138: 723-737.
- Zhang Z, Zhang Z, Shu L, Meng Y, Ma J, Gao R, et al. A genetic variant of the robo3 gene is associated with adolescent idiopathic scoliosis in the chinese population. Spine. 2023; 48: E20-E24.
- Pan XX, Huang CA, Lin JL, Zhang ZJ, Shi YF, Chen BD, et al. Prevalence of the thoracic scoliosis in children and adolescents candidates for strabismus surgery: results from a 1935-patient cross-sectional study in china. Eur Spine J. 2020; 29: 786-793.
- Zhu B, Wang X, Fu L, Yan, J. Pattern strabismus in a tertiary hospital in southern china: a retrospective review. Med Lith. 2022; 58: 1018.
- Smith ER, Hung LF, Arumugam B, Wensveen JM, Chino YM, Harwerth RS. Observations on the relationship between anisometropia, amblyopia and strabismus. Vision Res. 2017; 134: 26-42.
- Karaca EE, Cubuk MO, Akcam HT, Uzun F, Yuksel E. Choroidal thickness in turkish children with anisometric amblyopia. Semin Ophthalmol. 2017; 32: 291-296.
- Damask A, Paulding C, Baras A, Carey D, Abecasis GR. Mendelian randomization study of acly and cardiovascular disease. N Engl J Med. 2020; 383: e50.