
Research Article
Phys Med Rehabil Int. 2024; 11(4): 1239.
Sex-Specific Impacts of High-Versus Moderate-Intensity Training on AT1R mRNA Expression and Cardiovascular Stress
Sara Pouriamehr; Valiollah Dabidi Roshan*
Department of Physical Education and Sport Sciences, University of Mazandaran, Iran
*Corresponding author: Valiollah Dabidi Roshan Professor, Department of Physical Education and Sport Sciences, University of Mazandaran, Mazandaran, 4741613534, Iran. Tel: +989113151509; Fax: +981135254547 Email: v.dabidi@umz.ac.ir
Received: July 19, 2024 Accepted: September 09, 2024 Published: September 17, 2024
Abstract
Objectives: This study aimed to investigate the responses of AT1R mRNA expression, cortisol, and cardiovascular stress to high- versus moderate-intensity training (HIT vs. MIT) among women and men.
Methods: 144 participants were allocated to the exercise training groups for both sexes. Right after the HIT and MIT running were performed on a treadmill, AT1R mRNA expression and physiologic stress were measured by real-time PCR and a 12-lead ECG system.
Results: Regarding the exercise intensity, although AT1R mRNA expression and O2 pulse values were abruptly higher among men compared to women (p < 0.001), the SpO2 amounts did not change during the recovery period (p > 0.05). Moreover, more time was required for MvO2 to be recovered in men who performed HIT.
Conclusion: Despite the fact that various exercise intensities result in temporary physiological stress on the cardiovascular system, especially in men, these challenges are required to improve cardiovascular fitness and it is temporary.
Keywords: Myocardial Stress; Renin-angiotensin System; Exercise Intensity; Cardiovascular Hemodynamic
Introduction
The Renin-Angiotensin System (RAS) maintains cardiovascular homeostasis by regulating arterial Blood Pressure (BP). Ample evidence proves that RAS is one of the key modulators in cardiomyopathy development [1]. The RAS amplifies hypertrophy, elevates oxidative stress, and applies pro-inflammatory effects in the heart. A secretory glycoprotein, called angiopoietins I (ANG), exists in the blood. Then, the Angiotensin-Converting Enzyme (ACE) cleaves ANG I to ANG II. This biologically active peptide acts on the Angiotensin Type 1 Receptor (AT1R) [2]. Afterward, activated AT1R promotes and elevates BP through direct (i.e., vasoconstriction, increased water and sodium uptake in the kidney), inflammatory Indies such as; C-Reactive Protein (CRP), and oxidative stress, which means ANG II causes apoptosis, necrosis, fibrosis, and myocardial remodeling that consequently promotes cardiac failure. Furthermore, hypertension, resulting from the high level of AT1R, has a key role in leading to stroke by either hemorrhagic or ischemia [3]. While most of the evidence measuring the effects of exercise on AT1R applied on animal samples, it reported that physical (High Intensity [HIT] versus Moderate Intensity [MIT]) training acutely boosts the amount of counter-regulatory RAS axis, especially the MIT. Additionally, genetic studies have shown that physical exercise modulates RAS. Meanwhile, exercise influences the cardiovascular system by reducing BP as a result of inhibiting the interaction between ACE, Angio II, and AT1R [4]. Furthermore, the low tissue levels of ACE-2 are insufficient to provide enough Ang-(1–7) generation to modulate aldosterone and cortisol secretion [5]. Even though the further increase of AT1R expression leads to pathological remodeling in adult cardio-myocytes [6]. Physiological stressors in response to exposure to stimulators, such as intensive exercise, cause an increase in cortisol secretion [7,8] and Myocardial Volume Oxygen (MVo2) [9] among male populations, which are still unclear that how this response would be in females. MVo2, known as the Rate Pressure Product (RPP) and multiplied HR to SBP, is a cardiovascular parameter that indicates the myocardial oxygen demand indirectly. Some believe that MVo2 is a comprehensive way to measure the quality of hemodynamic response and cardiac workload to exercise [10]. In adulthood, the growth of cardiac mass slows dramatically, but some factors including exercise and hemodynamic overload stimulate further development. Any disability to inhibit growth may cause pathological remodeling via the reaction of expression of genes which is normally restricted to the expanding heart. Also, hypertension precedes the disorder of ventricular hypertrophy leading to heart failure [11]. Additionally, it has been described that the further increase of AT1R expression leads to pathological remodeling in adult cardio-myocytes [6]. Studies have illustrated that the classic RAS pathway activation declares following physical exercises [12]. For instance, it is noted that moderate-intensity endurance exercise after an 8-week endurance training protocol diminished the AT1R expression among animal samples [13,14]. Furthermore, Ang-II is the more potent stimulator of aldosterone secretion and cortisol [15]. However, according to our knowledge, it is still unknown how AT1R mRNA expression would respond to moderate-intensity and/or high-intensity exercise among healthy populations regarding sex differences.
To date, little studies have addressed the sex-specific roles of different types of exercise on cardiovascular systems. Therefore, it is essential to answer the key questions; (1) Are the cardiovascular protective and stress effects of acute exercise related to sex and training intensity specific? 2) If so, responses of gender-dependent cardiovascular indicators following which intensity of interval training (HIT versus MIT) are more pronounced? and 3) whether the exercise intensity would affect the recovery duration of the physiological indexes (i.e., MvO2, and SpO2). Therefore, we aimed to monitor the response of AT1R mRNA expression, cortisol, oxygen pulse (O2 Pulse), MvO2, and blood oxygen saturation (SpO2) following one session of moderate and high-intensity trials among healthy women and men.
Material and Methods
Ethical Approval
The present study was approved by the local institutional ethics committee (Ethical code: IR.UMZ.REC.1399.011), and was conducted based on the 1964 Helsinki declaration [16]; informed consent was obtained from all participants (72 men, 72 women).
Inclusion & Exclusion Criteria
To prevent the influence of disturbing intervention, individuals having similar physiological conditions participated. We settled some required conditions to remain in the research process. For example, the age range was between 20–40 years old. Also, other criteria for entering the research process were: no smoking, no antioxidant supplements for at least three weeks before the study, no signs of chronic cardiopulmonary or inflammatory diseases or any other medical contraindications such as physical disability and limited mobility, having a Hemoglobin (HGB) level =11g/Dl and no symptom of hemoglobinopathies, such as thalassemia, which could interfere in the oxygen-carrying capacity of red blood cells. Additionally, those females who were in their follicular phases participated. On the other hand, to avoid ‘silent hypoxemia’ [17], we assessed the level of oxygen saturation via means of a finger oximeter pulse (Brisk, Model PO16, China). In this study, we excused those having oxygen saturation less than 95%.
The Subjects’ Classification and Anthropometry Measurement
In this study, 144 healthy sedentary individuals (72 males, 72 females) volunteered and participated. They were randomly categorized into two running trial groups (high intensity, n = 72; moderate intensity, n = 72) for both sexes. Therefore, the groups were organized as 1. HIT-men; 2. HIT-women; 3. MIT-men; 4. MIT-women.
Before data collection, males and females were familiarized with the testing procedures, protocols, and equipment. Additionally, the participants could ask about any part of the research progress whenever it was not clear and they also had the right to either withdraw or leave the experiment at any time with no consequences.
In addition, the anthropometric characteristics of participants were also assessed before the test [18]. Their weights and heights were measured by a stadiometer. The stadiometer had an accuracy of 0.1 cm and 0.1 kg for height and weight; respectively. Also, a body composition analyzer device (Medigate Inc., BoCA x1, Korea) was used to calculate the Body Mass Index (BMI). The participants’ demographic characteristics are summarized in Table 1.
Group
Age (years)
Height (meter)
Weight (kg)
BMI (kg.m-2)
Fat mass (%)
Vo2MAX (ml/kg/min)
MIT
Male
(n=36)
31.22 ± 5.8
1.8 ± 0.05
90.3 ± 16.17
28.8 ± 4.84
25.4 ± 7.3
39.74 ± 2.6
Female
(n=36)
28.67 ± 6.3
1.6 ± 0.05
61.6 ± 6.4
23.3 ± 2.7
29.8 ± 5.4
30.35 ± 7.4
Total
(n=72)
29.94 ± 6.2
1.7 ± 0.09
75.95 ± 19
26.03 ± 4.8
27.6 ± 6.75
35.04 ± 7.3
HIT
Male
(n=36)
28.78 ± 7.5
1.8 ± 0.1
79 ± 13.5
24.7 ± 3.2
19.5 ± 5.01
43.5 ± 3.6
Female
(n=36)
30.22 ± 4.2
1.65 ± 0.06
60.5 ± 5.7
22.46 ± 3.3
27.63 ± 7.43
32.9 ± 4.2
Total
(n=72)
29.5 ± 6.05
1.71 ± 0.1
69.7 ± 13.8
23.6 ± 3.42
23.6 ± 7.5
38.2 ± 6.6
Abbreviations: MIT: Moderate intensity trial; HIT: High intensity trial; BMI: Body mass index; VO2max: Maximal oxygen consumption.
Table 1: Demographic characteristics of the participants (means ± standard deviations).
High and Moderate Intensity Training
During the data collection period, the laboratory temperature was 24-26 °C and the relative humidity was 50-60%. The experimental protocols were explained to all participants. Men and women performed one session of the familiarization period training protocol, including walking or jogging at speed of 1 mile per hour with no slopes for 5 to 10 minutes, and then performed the main exercise training protocol. The details of the High Intensity Trial (HIT) versus Moderate Intensity Trial (MIT) protocols employed in the present study have previously been described elsewhere [19]. In summary, the participants of MIT groups performed a submaximal intensity consisting of walking or jogging at a speed of 1.34 m/s with a 5% grade at a moderate workload of 50–65% of the predicted maximum HR for 20 minutes on a treadmill (h/p/cosmos Sports and Medical GmbH, Mercury model, Nussdorf-Traunstein Germany). In HIT groups, individuals performed the Bruce protocol consisting of a 3-minute stage exercise with a subsequent gradual increase in both speed and grade until exhaustion. participants were encouraged to exercise to their maximum tolerance; when 80–90% of HRmax was reached.
ECG and Physiologic Stress Indices Measurements
During the HIT and MIT protocols, participants were fitted with a 12-lead ECG system (Ottobrunn. Germany, Custo med GmbH. 2000, XP, Vista Leibnizstr. 7. 85521), and Heart Rate (HR, beat per minute (bpm)) and systolic blood pressure (mmHg) was continuously recorded. Moreover, peripheral blood oxygen saturation was continuously measured using a wearable finger pulse oximeter (Brisk, Model PO16, China). The aforesaid protocols were terminated upon some conditions; based on the 2002 update of the ACC/AHA guidelines: 1) moderate/intense angina; 2) any signs of perfusion (cyanosis or pallor); 3) increase of > 10 mmHg in diastolic blood pressure compared to the baseline; 4) increased nervous system symptoms (ataxia, dizziness, or near-syncope); 5) once participant himself requested to stop the test; 6) sustained ventricular tachycardia; 7) ST-segment elevation greater than 1.0 mm (0.1 mV) in two or more contiguous precordial leads or more adjacent limb leads, and 8) whenever exercise specialist deemed continuing test unsafe for the participant.
The VO2max then was calculated using the equation described elsewhere. Moreover, Ratings of Perceived Exertion (RPE) (Borg 6–20 scale) were assessed during the aforesaid protocols. The MvO2 was calculated by multiplying HR (bpm) and systolic blood pressure (mmHg) [20]. It also should be mentioned that we report the MvO2 and SpO2 values immediately after exercise trial, and at the first (RECO1), second (RECO2), third (RECO3), and fifth (RECO5) minutes of recovery. The Oxygen Pulse (O2 Pulse; mL.kg-1.min-1.bpm-1) was measured regarding the formula of Wasserman et al., which is the division of VO2max to maximal heart rate [21].
Blood Sampling, AT1R mRNA Expression, and Cortisol Analysis
The blood samples were collected before (rest) and immediately post-exercise (post) for both HIT and MIT exercise. All blood collection procedures were performed according to WHO guidelines on drawing blood [22]. A venous blood sample (3 ml) was collected from antecubital veins in the sitting position and inserted in a tube containing anticoagulant and then used to analyze AT1R profiles, using an automated hematology analyzer (Hitachi 7180 automatic analyzer, Japan).
Cortisol was measured in offspring blood samples using electrochemical luminescence immunoassay and reported in International System of Units (nmol/L) (COBAS E411, Roche). Cortisol stock was prepared by dissolving 1 mg/mL in methanol (technical grade) and diluted further in either 0.5 M NaCl/PBS, 50 kDa filtered plasma with 0.5 M NaCl, or full plasma, with concentrations ranging from 30 μM down to 123 nM.
RNA Extraction and Real-Time PCR
Total RNA was extracted from human blood samples by a blood RNA isolation kit. Following isolation, total RNA was quantified via a NanoDrop spectrophotometer (Nanomabna Iranian, Iran). One microgram of total RNA per sample was reverse transcribed with oligo dT primer, RNA inhibitor, Reverse Transcriptase (M-MLV RT), and deoxyribonucleotides (YTA, Iran). Detection of mRNA amounts (AT1R) was measured by real-time PCR (Rotor gene 6000; Corbett, Research, Australia). In this study, the level of Β-actin as a housekeeping gene was also determined to normalize the relative expression of each specific gene. Specific forward and reverse primers were designed using Primer Premier 5 software and used in QRT-PCR. The reaction mixture set up in a volume of 20 μl included 8 μl Real Q Plus 2× Master Mix Green (Ampliqon, Denmark), 2 μl reverse transcription product, 0.5 μl of each specific forward and reverse primers (10pmol), and 9 μl DNase/RNase free water. Amplification reactions were run in duplicate. Amplification conditions were carried out as follows: enzyme activation at 95 °C for 15 min; 40 cycles at 95 °C for 20 secs, 57 °C for 30 secs, and 72 °C for 30 sec. The fold changes in mRNA expression were calculated using the 2-ΔΔCT method (Table 2).
Gene name
Sequence
Accession Number
Amplicon Size
(Base Pair)AGTR1
F-5'-GCATTGATCGATACCTGGCTATT-3'
R-5'-AGCAGCCAAATGATGATGCA-3'NM_000685.5
97
Table 2: The characteristic of the sequence of forwarding and reverse primers used in RT-PCR.
Statistical Analysis
All statistical analyses were performed with SPSS software (version 28.0 for Windows, IBM, Armonk, NY, USA) while GraphPad Prism®, version 8.0.2 (GraphPad Software, Inc., La Jolla, CA, USA) was used for creating figures. Initially, the Kolmogorov-Smirnov test was used to measure the normality distribution of data. One-way ANOVA analysis was used to assess distribution characteristics. To perform the between-group comparisons, Bonferroni’s test was conducted. Also, a Wilcoxon matched-pairs test was used to conduct within-group comparisons (rest vs. post status and/or different time frames of recovery). Data are expressed as mean standard deviation. The significant value was set at P < 0.05.
Results
Cortisol, AT1R mRNA Expression, and Pulse O2 at Post-Exercise Status
Regarding sex differences, the cortisol amounts were significantly lower in the MIT-men group compared to HIT-men and HIT-women groups (p = 0.004 and p = 0.003; Figure 1A). Where as, as for AT1R mRNA expression, the values were abruptly higher in men who performed either moderate-intensity trial (MIT-men) or high-intensity trial (HIT-men) in comparison with women performing either moderate- or high-intensity trials (MIT-women, HIT-women) (p < 0.001, p = 0.005; Figure 1B). In addition, the Pulse O2 values were sharply higher in the HIT-men group compared to all other three groups (i.e., MIT-men, MIT-women, and HIT-women) (p < 0.001), while its amounts were significantly higher in the MIT-men group in comparison to women who performed either MIT or HIT (p < 0.001; Figure 1C).
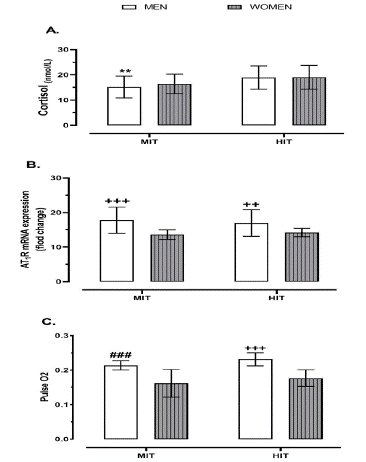
Figure 1: Impact of exercise intensity trial on AT1R mRNA expression and physiological stress indices (cortisol, Oxygen pulse) at post-exercise status regarding different sexes (i.e., men and women). Abbreviations; AT1R, angiotensin type 1 receptors expression; HIT, high intensity trial; MIT, moderate intensity trial. *** p < 0.001, ** p < 0.01, * p < 0.05 differences between the exact group and HIT groups (HIT-male and HIT-female). +++ p < 0.001, ++ p < 0.01, + p < 0.05 differences between the exact group and two women groups (MIT-women and HIT-women). ### p < 0.001, ## p < 0.01, # p < 0.05 differences between the exact group and the other three groups (MIT-female, HIT-male, and HIT-female).
MVO2 and SpO2 at Post-Exercise Status
Regarding sex differences, although slight differences were found in its values between MIT-male and HIT-female at second minute of recovery (p = 0.044; Figure 2C) and also between MIT-male and MIT-female at third minute of recovery (p = 0.034; Figure 2E), no differences were noted in SpO2 values among four groups (i.e., MIT-men, MIT-female, HIT-male, and HIT-female) at different recovery time frames, especially immediately, first, and fifth minutes of recovery (p > 0.05; Figure 2A, B & F).
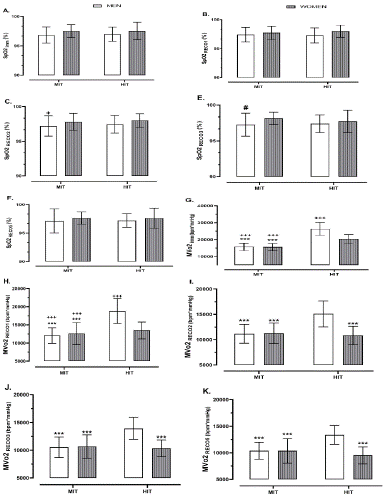
Figure 2: Impact of exercise intensity trial on MVO2 and SpO2 at different time frames (i.e., Imm, RECO1, RECO2, RECO3, and RECO5) of post-exercise status regarding different sexes (i.e., men and women). Abbreviations; MVo2, myocardial volume oxygen; SpO2, oxygen saturation; HIT, high intensity trial; MIT, moderate intensity trial. Imm, immediately after exercise; RECO1, first minute of post-exercise; RECO2, second minute of post-exercise; RECO3, third minute of post-exercise; RECO5, fifth minute of post-exercise. *** p < 0.001, ** p < 0.01, * p < 0.05 differences between the exact group and HIT-male. +++ p < 0.001, ++ p < 0.01, + p < 0.05 differences between the exact group and HIT-female. ### p < 0.001, ## p < 0.01, # p < 0.05 differences between the exact group and MIT-female.
As for MvO2, there were abrupt higher values in men and women who performed high intensity trial compared to those performing moderate intensity trial immediately and at first minute of recovery (p < 0.001; Figure 2G & H). Also, regarding sex differences, the MvO2 amounts were significantly lower in women compared to men performing HIT (HIT-women vs. HIT-men) immediately and at first minute of recovery (p < 0.001; Figure 2G & H). These sharp differences remained the same between HIT-men group in comparison with all other three groups (i.e., MIT-men, MIT-female, and HIT-women) at second, third, and fifth minutes of recovery (p < 0.001; Figure 2I, J & K), while no differences were noted in its values between HIT-women compared to women and men performing MIT (i.e., MIT-women and MIT-men groups) (p > 0.05; Figure 2I, J & K).
Despite all of these, according to response of SpO2 at different time frames of recovery status (i.e., imm, RECO1, RECO2, RECO3, and RECO5), there were no differences at different time frames of recovery (p > 0.05) except for MIT-women, which means a significant decreased was noted in this group at fifth minute compared to the third minute of recovery (p = 0.004; Figure 3A). The MvO2 amounts were significantly diminished till the second minute of recovery in both sexes who participated in MIT protocol (i.e., MIT-women and MIT-men) (p < 0.001), while no differences were noted in its values in males and females at third minute compared to fifth minute of recovery (p = 0.307 and p = 0.226; Figure 3B). As for those performing high intensity trial, the MvO2 values remained significantly higher in men until the second minute of recovery in comparison with third minute of recovery (p < 0.001), while MvO2 values were abruptly higher in women until the first minute compared to second minute of recovery (p < 0.001; Figure 3B).
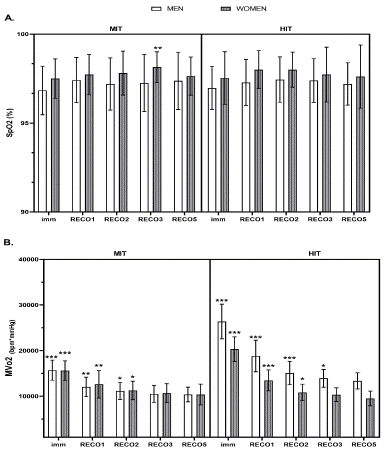
Figure 3: The response of MVO2 and SpO2 to exercise intensity trials (MIT vs. HIT) at different time frames of recovery status. Abbreviations; MVo2, myocardial volume oxygen; SpO2, oxygen saturation; HIT, high intensity trial; MIT, moderate intensity trial. Imm, immediately after exercise; RECO1, first minute of post-exercise; RECO2, second minute of post-exercise; RECO3, third minute of post-exercise; RECO5, fifth minute of post-exercise. *** p < 0.001, ** p < 0.01, * p < 0.05 differences between the exact time frame and the next time frame.
Discussion
The most important finding of the present study, regarding exercise intensity trial and sex differences, was that the High-Intensity Running Trial (HIT) significantly increased cortisol in both sexes. However, according to exercise intensity, the AT1R mRNA expression was abruptly higher in men compared to women groups, especially men performing MIT. More importantly, the present study found that O2 Pulse was higher in men compared to women regardless of exercise intensities. On the other hand, although neither sex differences nor exercise intensities affected the SpO2 at various recovery time frames, they caused higher MvO2 values among people performing HIT until the first minute of recovery. Moreover, this significant difference remained the same for men who performed HIT compared to MIT-men and women performing MIT and/or HIT until the fifth recovery minute.
The evidence shows that some conditions, such as stress-induced hypertension, increase the expression of ACE and AT1R [23]. Although moderate physical exercise could attenuate hypertension and moderate AT1R expression in animal studies [24], our findings showed that performing exercise trials increased the AT1R expression among men. For instance, AT1R was 23.6% and 20.3% higher in men performing moderate-intensity trial compared to women who performed MIT and HIT, respectively. In addition, men performing HIT had 21.4% and 18% higher AT1R values in comparison with women who performed MIT and HIT, respectively. Possibly, it would be due to the protective role of sex differences in metabolic effects of hormonal mediators such as the renin-angiotensin system against obesity-related metabolic and cardiovascular complications in females [25]. Although scientists are still wondering about how physical exercise affects the ACE2/Angio1-7/MAS pathway, it has been noted that regular physical activity increases the expression of aortic MAS receptors in rodents, which improves the vasodilator effect of Angio1-7 [26].
Another issue of the present research was to elucidate how the AT1R, as one of the main mediator factors in classical RAS signaling, as well as MVO2, cortisol, oxygen pulse, and oxygen saturation differs between males and females. To our knowledge, no experiment to date has attempted to address this important question. On the other hand, it is reported that the generation of Ang-(1–7) modulates cortisol secretion [5], which expresses the reason for 25.8% lower cortisol levels in men performing MIT compared to HIT-women at post-exercise status. Additionally, the cortisol level was 25.8% lower in men performing MIT compared to those performing HIT, which is aligned with the previous study that reported provoking circulating cortisol level increases by high-intensity exercise [8]. Etiologically, cortisol implicates in a wide range of mental and physical health outcomes [27,28], including both the onset and progression of mental and physical health disorders [27,29].
Known sex differences in the RAS components may favor a greater pressure response to ANG II in men than women. Activation of AT1R mediates most of the well-known biological functions of ANG II, including vasoconstriction, sodium reabsorption, mesangial cell proliferation, vascular hypertrophy, inflammation, and an increase in oxidative stress [26]. There are numerous studies that men have greater AT1R expression in the kidneys than women, at both the RNA and protein levels [26]. In addition to decreased receptor density in the kidneys of women than in men, it has been recently shown that specific AT1R binding is 40% lower in glomeruli from female Sprague-Dawley rats than in males, and this is dependent on the presence of 17Β-estradiol [30]. Therefore, a decrease in AT1R activation in women likely may contribute to a smaller presser response in response to ANG II. Moreover, sex differences in how men and women respond to various stress are likely related to the observation that among females, there appears to be discordance between activation of the RAS and blood pressure, so that in males, the values of hypertension and renal injury closely parallels increases in RAS activation, while the same relationship is not detected in females [26]. It seems differences in hormonal stimulation may play a role in the mechanism of sex-related differences in myocardial metabolism [31]. In response to Lower Body Negative Pressure (LBNP) experiments, which are known to activate the RAS, circulating renin and ANG II levels have been shown to the maximum during the luteal phase of the menstrual cycle when plasma 17Β-estradiol levels are highest [32]. Despite increased RAS activation during the luteal phase, mean arterial blood pressure fell in response to incremental increases in LBNP, and vasoconstrictor responses to RAS activation were blunted. Therefore, the systemic effects of acute ANG II were comparable in males and females; however, there were sex differences in renal functional responses to acute ANG II and blood pressure responses to chronic ANG II treatment. In the present study, those females who were in their follicular phases participated.
As such, greater post-exercise gene expression of AT1R might be related to greater changes in blood gases and resultant sympathy-excitation, which our research illustrates higher oxygen pulse in men compared to women. For example, O2 pulse was 24.3% and 17.8% higher in men performing moderate intensity trial compared to women who performed MIT and HIT, respectively. In addition, men performing HIT had 30.2%, 24.14%, and 5.2% higher O2 pulse values in comparison with MIT-women, HIT-women, and MIT-men, respectively. Whereas, at recovery time frames, no alternations were found in SpO2 values among women and men performing either MIT or HIT, especially immediately after exercise, at the first and fifth minutes of recovery. Also, on general, no differences were noted in SpO2 during recovery time frames for each group. However, it had a light reduction about 0.84% and 0.92% in men performing moderate-intensity trial compared to HIT-women (at the second minute of recovery) and MIT-women (at the third minute of recovery), respectively.
The mechanism of the high-intensity running trial on the autonomic cardiovascular system and increasing the modulating role of the vagal nervous system on the cardiovascular system has been reported by many researchers. Researchers reported regular physical activity can cause sinus bradycardia, i.e., a decrease in the resting sympathetic tone and an increase in parasympathetic activity thus decreasing resting HR and Systolic Blood Pressure (SBP) [33]. Also, improvement in baroreflex sensitivity and reduction in arterial stiffness induced by regular exercise may be considered as adaptation enhances cardiac vagal tone [34]. Furthermore, researchers have also suggested that HIT may increase shear stress, therefore, having a critical role in releasing nitric oxide in the arteries and leading to vasodilatations [35].
MVo2, as a marker of myocardial oxygen consumption, is related to HR, SBP, and internal workload exerted on the myocardial during stress testing. Furthermore, the alteration of post-exercise Mvo2 is known as the strongest risk factor for cardiac mortality in heart disease patients with type II diabetes [10]. Although in the present study, the acute effects of high-intensity and moderate-intensity training protocols in men and women were investigated, a study stated that physiological and cardiovascular responses, including BP, HR, heart rate recovery, and MVo2, were improved after 8 weeks of exercise in the neck and head cancer patients [36]. Consistent with these findings, regarding exercise intensity trials, our study showed the MvO2 values were higher about 40.4% and 34% in HIT-men compared to MIT-men and about 23.2% and 6.4% in HIT-women in comparison with MIT-women immediately after exercise, and at the first minute of recovery, respectively. According to sex differences, the MvO2 values were higher at about 23% and 28.33 in HIT-men compared to HIT-women immediately after exercise, and at the first minute of recovery, respectively. Regarding exercise intensity trial and sex-different interventions, the MvO2 values were higher about 41% and 34% in HIT-men compared to MIT-women and about 22.6% and 10.6% in HIT-women in comparison with MIT-men immediately after exercise, and at the first minute of recovery, respectively. Interestingly, MvO2 recovery happened swiftly among HIT-women which caused no significant differences between MIT groups from the second to the fifth minute of recovery time frames, while the MvO2 values remained abruptly higher in HIT-men compared to all other three groups (i.e., MIT-men, MIT-women, and HIT-women) until the fifth minute of recovery.
As for MvO2 recovery during post-exercise time frames, its values decreased about 23.3%, 7.94%, and 5.7% from immediately after exercise to the first minute, from the first to the second minute of recovery, and from the second to the third minute of recovery among men performing MIT, respectively. As for the MIT-women group, the reduction of MvO2 values was about 19.14%, 12%, and 5.31% from immediately after exercise to the first minute, from the first to the second minute of recovery, and from the second to the third minute of recovery. However, no significant alternation was found after that in both groups, which illustrates that the most MvO2 recovery happened until the second minute of post-exercise and its values remained the same after the third minute of recovery time frame.
On the other hand, MvO2 values significantly diminished about 28.7%, 24.7%, and 7.71% from immediately after exercise to the first minute, from the first to the second minute of recovery, and from the second to the third minute of recovery among men performing HIT, respectively. As for the HIT-women group, the sharp reduction of MvO2 values was about 33.7% and 24.42% from immediately after exercise to the first minute and from the first to the second minute of recovery, respectively, although this drop was slightly about 4.6% from the second to the third minute of recovery. This finding expresses that the MvO2 recovery was swift among women compared to men, especially till the first minute following a high-intensity exercise. However, at the third minute compared to the fifth minute of recovery, no significant alternation was found in MvO2 values among women performing HIT, while an insignificant reduction (about %) was found among male MvO2 amounts who performed HIT. These results show that the most MvO2 recovery happened until the second minute of post-exercise for women while it continued to the fifth minute among men who did HIT protocols. It should be mentioned that our study had some limitations. First, all subjects were healthy, therefore findings from this study cannot be generalized to clinical conditions, including individuals suffering from cardio-metabolic disease, Chronic Obstructive Pulmonary Disease (COPD), Heart Failure (HF), etc. Second, due to the special conditions of the COVID-19 pandemic, only males and females between the ages of 20–45 years were included in the present study and whether such findings can be achieved in children and older populations need to be investigated. Third, due to the effect of smoking on arterial blood oxygen saturation [17], as well as MvO2 [37], only healthy non-smoker individuals were included, and therefore findings from current research cannot be generalized to smoker people. Therefore, we encourage the future studies to take these limitations into consideration while accommodate various training intensity protocols to measure the responses of AT1R mRNA expression, cortisol, and the physiological stress indices among male and female populations.
Conclusions
Our study contributes some novel information about the responses of AT1R mRNA expression, cortisol, and the physiological stress indices (i.e., O2 pulse, MvO2, and SpO2) to High-Intensity Training (HIT) versus Moderate-Intensity Training (MIT) among healthy women and men. Firstly, we found that performing exercise trials increased the AT1R expression among men. Consequently, regarding the exercise intensities (HIT vs. MIT) and sex differences, the cortisol level was lower in men, especially those who performed MIT. Secondly, greater post-exercise gene expression of AT1R might be related to higher oxygen pulse in men compared to women. Generally, the MvO2 recovered mostly up to the second minute of post-exercise, while the male MvO2 values recovered up to the third minute.
Author Statements
Conflict of Interest
The authors declare no conflict of interest to disclose.
Acknowledgments
We would like to appreciate all participants who consented to participate in the present study. The authors reported there is no funding associated with the work featured in this article. This research was conducted with the financial support of the responsible author.
Author Contribution
Study design: VDR; data management: VDR, data acquisition: SP; draft of the article: VDR, SP; revision of the article: VDR, SP. The authors declare that all data were generated in-house and that no paper mill was used.
Competing Interests and Funding
The authors reported there is no funding associated with the work featured in this article. This research was conducted with the financial support of the responsible author.
References
- Tan Y, Li X, Prabhu SD, Brittian KR, Chen Q, Yin X, et al. Angiotensin II plays a critical role in alcohol-induced cardiac nitrative damage, cell death, remodeling, and cardiomyopathy in a protein kinase C/nicotinamide adenine dinucleotide phosphate oxidase–dependent manner. Journal of the American College of Cardiology. 2012; 59: 1477-1486.
- Werner C, Baumhakel M, Teo KK, Schmieder R, Mann J, Unger T, et al. RAS blockade with ARB and ACE inhibitors: current perspective on rationale and patient selection. Clinical Research in Cardiology. 2008; 97: 418-431.
- McFall A, Nicklin SA, Work LM. The counter regulatory axis of the renin angiotensin system in the brain and ischaemic stroke: Insight from preclinical stroke studies and therapeutic potential. Cellular signalling. 2020; 76: 109809.
- Domingo R, Sturrock E, Collins M. ACE activity and endurance performance during the South African Ironman triathlons. International journal of sports medicine. 2012; 34: 402-408.
- Caroccia B, Vanderriele PE, Seccia TM, Piazza M, Lenzini L, Prisco S, et al. Aldosterone and cortisol synthesis regulation by angiotensin-(1-7) and angiotensin-converting enzyme 2 in the human adrenal cortex. Journal of Hypertension. 2021; 39: 1577-1585.
- Ainscough JF, Drinkhill MJ, Sedo A, Turner NA, Brooke DA, Balmforth AJ, et al. Angiotensin II type-1 receptor activation in the adult heart causes blood pressure-independent hypertrophy and cardiac dysfunction. Cardiovascular research. 2009; 81: 592-600.
- Gottschall JS, Davis JJ, Hastings B, Porter HJ. Exercise time and intensity: how much is too much? International Journal of Sports Physiology and Performance. 2020; 15: 808-815.
- Hill E, Zack E, Battaglini C, Viru M, Viru A, Hackney AC. Exercise and circulating cortisol levels: the intensity threshold effect. Journal of endocrinological investigation. 2008; 31: 587-591.
- Kiviniemi AM, Kentta TV, Lepojarvi S, Perkiomaki JS, Piira OP, Ukkola O, et al. Recovery of rate-pressure product and cardiac mortality in coronary artery disease patients with type 2 diabetes. Diabetes Research and Clinical Practice. 2019; 150: 150-157.
- McKinsey TA, Olson EN. Toward transcriptional therapies for the failing heart: chemical screens to modulate genes. The Journal of clinical investigation. 2005; 115: 538-546.
- Magalhães DM, Nunes-Silva A, Rocha GC, Vaz LN, de Faria MHS, Vieira ELM, et al. Two protocols of aerobic exercise modulate the counter-regulatory axis of the renin-angiotensin system. Heliyon. 2020; 6: e03208.
- Agarwal D, Welsch MA, Keller JN, Francis J. Chronic exercise modulates RAS components and improves balance between pro-and anti-inflammatory cytokines in the brain of SHR. Basic research in cardiology. 2011; 106: 1069-1085.
- Li X, Wang K. Effects of moderate-intensity endurance exercise on angiotensin II and angiotensin II type I receptors in the rat heart. Molecular medicine reports. 2017; 16: 2439-2444.
- Oki K, Kopf PG, Campbell WB, Lam ML, Yamazaki T, Gomez-Sanchez CE, et al. Angiotensin II and III metabolism and effects on steroid production in the HAC15 human adrenocortical cell line. Endocrinology. 2013; 154: 214-221.
- Baker R, Schmidt U, Frewer A. The Declaration of Helsinki and the foundations of global bioethics. Ethical research: The declaration of Helsinki, and the past, present, and future of human experimentation. 2020: 47-58.
- Couzin-Frankel J. The mystery of the pandemic’s ‘happy hypoxia’. American Association for the Advancement of Science. 2020.
- Bergman RN, Stefanovski D, Buchanan TA, Summer AE, Reynolds JC, Sebring NG, et al. A better index of body adiposity. Obesity. 2011; 19: 1083-1089.
- Khodarahmi B, et al. Effect of respiratory protection equipments wear on heart rate in different workload. International Journal of Environmental Health Engineering. 2013; 2: 26.
- Ansari M, Javadi H, Pourbehi M, Mogharrabi M, Rayzan M, Semnani S, et al. The association of rate pressure product (RPP) and myocardial perfusion imaging (MPI) findings: a preliminary study. Perfusion. 2012; 27: 207-213.
- Wasserman K. Principles of exercise testing and interpretation. Measurements during integrative cardiopulmonary exercise test. 1999.
- Organization WH. Coronavirus disease 2019 (COVID-19) strategic preparedness and response plan: Accelerating readiness in the Eastern Mediterranean Region: World Health Organization. Regional Office for the Eastern Mediterranean. 2020.
- Durik M, van Veghel R, Kuipers A, Rink R, Jimoh Akanbi MH, Moll G, et al. The effect of the thioether-bridged, stabilized Angiotensin-(1–7) analogue cyclic ang-(1–7) on cardiac remodeling and endothelial function in rats with myocardial infarction. International journal of hypertension. 2012; 2012: 536426.
- Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. New England Journal of Medicine. 2001; 345: 1667-1675.
- White MC, Fleeman R, Arnold AC. Sex differences in the metabolic effects of the renin-angiotensin system. Biology of Sex Differences. 2019; 10: 1-18.
- Sullivan JC. Sex and the renin-angiotensin system: inequality between the sexes in response to RAS stimulation and inhibition. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2008; 294: R1220-R1226.
- Adam EK, Quinn ME, Tavernier R, McQuillan MT, Dahlke KA, Gilbert KE. Diurnal cortisol slopes and mental and physical health outcomes: A systematic review and meta-analysis. Psychoneuroendocrinology. 2017; 83: 25-41.
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders: overview of physical and behavioral homeostasis. Jama. 1992; 267: 1244-1252.
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008; 33: 693-710.
- Rogers JL, Mitchell AR, Maric C, Sandberg K, Myers A, Mulroney SE. Effect of sex hormones on renal estrogen and angiotensin type 1 receptors in female and male rats. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2007; 292: R794-R799.
- Peterson LR, Soto PF, Herrero P, Schechtman KB, Dence C, Gropler RJ. Sex differences in myocardial oxygen and glucose metabolism. Journal of nuclear cardiology. 2007; 14: 573-581.
- Chidambaram M, Duncan JA, Lai VS, Cattran DC, Floras JS, Scholey JW, et al. Variation in the renin angiotensin system throughout the normal menstrual cycle. Journal of the American Society of Nephrology. 2002; 13: 446-452.
- Ramos JS, Dalleck LC, Borrani F, Beetham KS, Mielke GI, Dias KA, et al. High-intensity interval training and cardiac autonomic control in individuals with metabolic syndrome: a randomised trial. International journal of cardiology. 2017; 245: 245-252.
- Heydari M, Boutcher YN, Boutcher SH. High-intensity intermittent exercise and cardiovascular and autonomic function. Clinical autonomic research. 2013; 23: 57-65.
- Soltani M, Baluchi MJ, Boullosa DA, Daraei A, Govindasamy K, Dehbaghi KM, et al. Endurance training intensity has greater effects than volume on heart rate variability and arterial stiffness adaptations in sedentary adult men: A Randomized Controlled Trial. 2021.
- Yen CJ, Hung CH, Kao CL, Tsai WM, Chan SH, Cheng HC, et al. Multimodal exercise ameliorates exercise responses and body composition in head and neck cancer patients receiving chemotherapy. Supportive Care in Cancer. 2019; 27: 4687-4695.
- Rezk-Hanna M, Benowitz NL. Cardiovascular effects of hookah smoking: potential implications for cardiovascular risk. Nicotine and Tobacco Research. 2019; 21: 1151-1161.