
Case Report
Phys Med Rehabil Int. 2024; 11(5): 1243.
Cervical Spinal Cord Stimulation Provides Analgesic Relief for Post Stroke Facial Pain
Anand Pooleri, MD¹; Michael Sabia, MD¹*; Kingsuk Ganguly, MD¹; Harrison Jordan, DO, PGY²; Jasjit Sehdev, MD¹
¹Cooper University Hospital, 1 Cooper Plaza, Camden, NJ 08103, USA
²Department of physical medicine and rehab East Carolina University, USA
*Corresponding author: Mike Saba, Cooper University Hospital, 1 Cooper Plaza, Camden, NJ 08103, USA. Email: sabia-michael@cooperhealth.edu
Received: November 20, 2024; Accepted: December 10, 2024; Published: December 17, 2024
Abstract
Background: Trigeminal trophic syndrome is a rare neuropathic disorder that affects the trigeminal dermatome with a severe litany array of symptoms, including unilateral facial paresthesia and dysesthesia. In most cases, Management is typically conservative, and non-operative and, utilizesing the same similar treatment modalities utilized to address in more common forms of neuropathic pain. Unfortunately, these analgesic options are not consistently effective.
Case Presentation: We present the case of a 34-year-old male patient who presented to a pain management practice after years of intractable facial pain and skin changes following a cerebrovascular accident. Pharmacotherapy had been ineffective in providing adequate analgesia. Interventions provided by multiple specialists failed to address his symptoms, leaving the patient unable to work or sleep. Due to the failure of numerous conservative measures, spinal cord stimulator use was hypothesized to provide relief from his uncontrollable pain and was subsequently implemented. Immediate pain relief was achieved thereafter, and the patient reported satisfactory pain control and improvement in his daily life.
Conclusion: Definitive analgesia options are limited in trigeminal trophic syndrome, and there is no standardized treatment pathway. We present a unique case in which a spinal cord stimulator successfully addresses the patient’s symptoms. This case demonstrates the potential utility of spinal cord stimulation in addressing intractable pain associated with TTS, underscoring the need for further research into SCS as a treatment modality.
Introduction
Trigeminal trophic syndrome is a rare disease process neuropathic condition caused by injury damage to the trigeminal ganglia and characterized by symptoms localized along the trigeminal dermatome trigeminal ganglia presenting along the trigeminal dermatome and can occur as part of secondary to a cerebrovascular event. This syndrome is increasingly challenging to address with no definitive treatment algorithm and requires input from multiple specialists for diagnosis and subsequent care. The general principle aligns with those of other neuropathic pain syndromes, involving pharmacologic therapies, nerve blocks, and surgical options if conservative measures fail for management is similar to the treatment of other neuropathic pain syndromes: analgesia via oral and injectable interventions with consideration of surgery if conservative measures fail. However, these treatment modalities vary in efficacy and subsequent symptom resolution. Due to this gap in care, spinal cord stimulator use was theorized to address this patient’s pain.
To our knowledge, this is the first report of successful pain management using SCS for Trigeminal Trophic Syndrome following a cerebrovascular accident. To the best of our knowledge, we present a novel case report of the successful use of spinal cord stimulation for the analgesic management of trigeminal trophic syndrome following a cerebrovascular accident. Generally used for other types of neuropathic pain, this specific use of spinal cord stimulation resulted in significant relief for the patient suffering from this relatively rare condition. In doing so, this opens the possibility that spinal cord stimulation may provide a definitive and alternative treatment optionmodality, especially when compared to alternative interventions, such as current therapies i.e. stellate ganglionectomy and radiotherapy, that carry their respective complications.
Case Presentation
A 34-year-old male suddenly noticed the onset of left-sided weakness, dysarthria, dizziness, drooling, and headache while exercising. Upon admittance to the nearest medical center, he was diagnosed with subacute infarcts in the left medulla and left cerebellum due to a left vertebral artery dissection seen on MRI of the brain and MRA & CTA of the head. He was treated non-operatively for this diagnosis via anticoagulation agents initially and then transitioned to antiplatelet agents. Upon resolution of his hospital course, he was discharged to inpatient rehabilitation to address nystagmus, left lateral gaze, dysphagia, left-sided pronator drift, and left-sided dysmetria, all of which eventually resolved.
However, since the cerebrovascular event was secondary to his left vertebral artery dissection, the patient complained of ongoing neuropathic pain along the left side of his face into his nose that had persisted for years thereafter and had workup completed by multiple specialists with varied yet ineffective results. Otolaryngology formally diagnosed the patient with trigeminal trophic syndrome, a relatively rare disease process with no definitive treatment modalities and felt an infraorbital nerve transection would be aggressive without guaranteed relief of his pain. Neurosurgery concluded that microvascular decompression, glycerol rhizotomy, or stereotactic radiosurgery would likely not be effective. Oro-maxillofacial surgery discussed the possibility of a left-sided trigeminal neurectomy, however, recommended against the procedure due to the possibility of anesthesia dolorosa and subsequent failure to relieve pain. He tried multiple medications prescribed by neurology as well as psychiatry to improve his presentation, including nortriptyline, amitriptyline, pregabalin, venlafaxine, lidocaine cream, and lidocaine & ketamine topical compound cream, all of which failed to address his concerns without meaningful relief. The patient pursued interventional treatment modalities as well; he had radiofrequency ablations of the left infraorbital nerve, which were ineffective, botulinum injections, which were inadequate, and lidocaine injections, that only provided relief for one day. He presented to interventional pain management practice six years after his cerebrovascular accident for alternative options to address his symptomatology.
When seen by pain management, the patient described his symptoms as an electric shock-like sensation in addition to burning pain on the left side of his face below his eye. He reported that the excruciating, intolerable facial pain would significantly affect his sleep on a nightly basis, resulting in insomnia. A physical exam revealed erythema of the left nasolabial fold inspection. There was tenderness to palpation of the area as well as allodynia, consistent with dysesthesia of the left trigeminal nerve within the infraorbital distribution of the maxillary division. Pain management initially attempted to address the pain via a targeted left V2 trigeminal nerve block under fluoroscopy, stellate ganglion blocks, and repeated radiofrequency ablation aimed at the nasociliary nerve, all of which failed to fully resolve the patient’s neuropathic facial pain. A Diagnostic Left Deep Cervical Plexus block under fluoroscopic guidance at C2 was performed, which provided about 24 hours of pain relief. This was repeated about a month later; the same analgesic results were noted. Therefore, a Left Deep C2 Cervical Plexus Thermal Radiofrequency ablation procedure was performed with the hopes of providing more sustained pain relief by denervating the cervical plexus. The patient only received a few days of analgesic relief from the aforementioned cervical plexus thermal radiofrequency ablation. Based on these outcomes, the decision was made to trial spinal cord stimulation (SCS) targeting the C2 dorsal sensory nerves to achieve long-term pain relief.
This sparked the idea of providing sustained stimulation to the C2 Dorsal Sensory Nerves through spinal cord stimulation as an idea of providing long-term pain control for the patient.
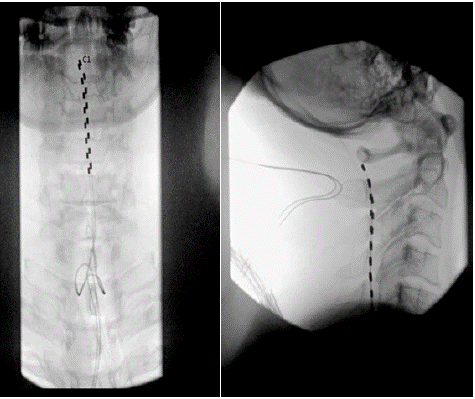
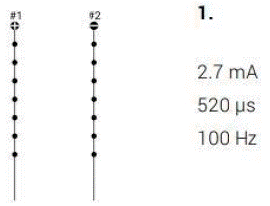
A high-level cervical spinal cord stimulation trial using the Medtronic spinal cord stimulator system via Medtronic was conducted to address the symptoms of allodynia and dysesthesia. Following appropriate treatment protocol, a 14-gauge Tuohy needle was placed under fluoroscopic guidance mid-way between the spinous process and the pedicle of the T1 vertebral body on the right and left side, and two a 45-centimeter octrode leads were advanced cephalad via the Tuohy needle into the epidural space, spanning the C1 to C5 spinal levels 45-centimeter octrode lead was threaded through the Tuohy needle in the cephalad direction maintaining midline position and the posterior position of the epidural space. The leads were threaded up the C1, C2, C3, C4, and C5 level.
The spinal cord stimulator leads were secured to the skin, and once all programming was complete, the patient was discharged home in hemodynamically and neurologically stable condition. Programming was optimized with assistance from a Medtronic representative. The Medtronic representative assisted in device programming andInitial trialing settings that included intensities of 3.0-3.2 mA, pulse widths of 350-370 μs, and frequencies of 100 Hz. The patient gained the most relief from settings consisting of an intensity set at 2.7 mA, a pulse width of 520 μs, and a frequency of 100 Hz.
Discussion
Spinal cord stimulation was first introduced in 1967 as spinal electroanalgesia, a new concept that used gate control theory to treat chronic pain symptoms [1]. The gate control theory, developed in 1965, proposes that multiple “gates” control the noxious level input to the spinal cord via small neuronal fibers that other large sensory neurons can modulate, higher inputs from the central nervous system, or both [2,3]. Postured gating mechanisms include Aβ fiber activation in the dorsal column of the spinal cord, which antidromically activates inhibitory interneurons, thus modulating peripheral nociceptive signals from Ad and C fibers [3]. Additionally, activation of Aβ fibers releases γ-Aminobutyric Acid (GABA), an inhibitory neurotransmitter that "closes the gate” [3]. This gate closure prevents pain signals from being transmitted to the brain, resulting in the suppression of pain sensations [3].
In addition to stimulating the spinal cord, there has been evidence that spinal cord stimulators can modulate pain cerebrally through a supraspinal-spinal feedback loop [4]. Studies have shown that spinal cord stimulation altered the activation of supraspinal areas associated with the lateral spinothalamic tract on fMRI, modulating incoming nociceptive signaling at the spinal levels through their descending projections [5]. Similarly, there is evidence of brainstem rostral ventromedial medulla descending modulation of pain via increased serotonergic input to the dorsal horn, as well as increased synthesis of norepinephrine in the locus coeruleus, wherein the effects of increased serotonin and norepinephrine resulted in improved McGill pain questionnaire outcomes [6,7].
Spinal cord stimulators achieve this mechanism via utilizing implanted leads connected to a remote-controlled pulse generator, and various stimulation programming is available which can be programmed with variable settings [8]. There are three main programming settings: amplitude of the signal signifies how intensely the stimulation will be felt, pulse width determines the length of time in which the amplitude will be delivered, and frequency is defined as the number of impulses in one second [8]. Conventional spinal cord programming, such as tonic stimulation, includes frequencies of 30-80 Hz, 100 to 500 μs of pulse width, and an amplitude above the sensory threshold [8]. Unfortunately, tonic spinal cord stimulation results in orthodromic activation of Aβ-fibers, causing paresthesia, which is often uncomfortable for patients, and tonic stimulation has been shown to decrease in effect over extended periods of time.
These findings have resulted in the development of alternate programming modalities. One example is high-frequency stimulation, firing at a frequency of 1-10 kHz (delivering more charge per second compared to tonic stimulation), a pulse width of 30 μs, and an amplitude of 1 to 5 mA. It has been hypothesized that the difference in frequency and energy delivery between the two paradigms seems to result in the activation of different neuronal mechanisms, whereas high-frequency stimulation does not activate Aβ axons in the dorsal column, resulting in the absence of paresthesia [8,9]. Burst stimulation fires at a frequency of 40 Hz with 5 closely spaced pulses at 500 Hz per burst. This differs from tonic and high-frequency stimulation in that burst stimulation pulses are delivered to the dorsal column in a cluster of high-frequency, low-charge pulses separated by a longer time duration (the inter-pulse interval) while the charge per second is higher. Similar to tonic stimulation, burst stimulation has been shown to utilize GABAergic interneurons in the spinal dorsal horn. [8,9]
Conventional stimulation of the dorsal column generates Evoked Compound Action Potentials, which can be used to measure Aβ fiber recruitment, where ECAP amplitude increases with increasing SCS current [10]. This information contributed to the development of a closed-loop SCS system where the intensity of conventional stimulation paradigms is continuously adapted by measuring ECAPs, comparing them to a set point of comfortable stimulation and optimal pain relief, and changing input current (i.e., amplitude) through a feedback algorithm that can allow alternate programming based on the patient's needs [10].
Pathologically, the current indications approved by the Food and Drug Administration for spinal cord stimulators include treatment of chronic, intractable trunk or extremity pain, bilateral or unilateral, associated with the following: failed back surgery syndrome, Complex Regional Pain Syndrome (CRPS) type I, & II, and persistent pain in the lower back and legs. Additional pathology includes radicular pain syndrome, radiculopathies causing pain secondary to failed back syndrome or herniated disc, epidural fibrosis, degenerative disc disease (pain due to herniated disc that does not respond to conservative and surgical interventions), arachnoiditis and multiple back operations [11].
Compared to the aforementioned conditions, trigeminal Trophic Syndrome (TTS) is a rare disorder believed to have resulted from injury to the trigeminal ganglia and is associated with pathology in the distribution of the trigeminal dermatome [12,13]. The disease often presents with the triad of trigeminal dermatome anesthesia, paresthesia, and facial ulceration, though presentations can vary [14,15]. Due to limited cases and the rarity of the disease, the exact incidence, etiology, and pathophysiology of TTS remains unclear. The most common etiology of TTS is iatrogenic, with the first reports of the disease following trigeminal rhizotomies, andrhizotomies and has been most associated with intervention for trigeminal neuralgia [13,16]. Various other causes have been identified to result in TTS, including trauma, herpes zoster, acoustic neuroma, and Cerebrovascular Accident (CVA) [17,18,19,20].
There is no definitive treatment algorithm for TTS, and there have been no randomized controlled trials to investigate the most appropriate management of the disease [1]. However, there are various case reports providing evidence for management. Most treatment options have included agents targeting dysesthesia and paresthesia via oral medication modalities, including amitriptyline, gabapentin, pimozide, diazepam, and carbamazepine [17,22]. There have also been reports that suggest improvement of TTS with Transcutaneous Electrical Nerve Stimulation (TENS) [23,24]. Overall, the treatment guidelines to approach TTS remain unclear, with the prognosis of the disease relying on a multidisciplinary approach [12].
The utilization of a spinal cord stimulator to address TTS is a novel concept, and the effectiveness in addressing this presentation is especially encouraging. The mechanism of action regarding spinal cord stimulators aligns closely with the current treatment paradigm for TTS, which consists of agents designed to address neuropathic pain orally or via transcutaneous electrical stimulation, which also utilizes the gate control theory to address pain symptoms.
The pathophysiology of trigeminal trophic syndrome is not well understood; however, the neuropathic nature of the symptomatology does mimic that of trigeminal neuralgia [12,13,14]. The treatment paradigm of both conditions is relatively similar, however, there have been cases of spinal cord stimulator use in addressing trigeminal neuralgia [25, 26, 27]. Spinal cord stimulator use has similarly been used to address intractable facial pain, a common symptom of trigeminal trophic syndrome, as well as generalized trigeminal neuropathy [27, 28]. Such examples could lead to further discoveries regarding the use of spinal cord stimulators as it pertains to intractable pain that is not amenable to conventional treatment strategies or surgery. Our case suggests that SCS may provide an effective alternative for patients with refractory TTS, offering sustained analgesia with minimal side effects.
Conclusions
Trigeminal trophic syndrome is an extremely painful condition. , and pProviding optimal analgesia is vital yet and can be challenging to achieve. This case report illustrates the successful use of a spinal cord stimulationor to provide effective analgesia in the setting of refractory pain with subsequent improvement in symptomatology. While spinal cord stimulator useneuromodulation is not the standard treatment for trigeminal trophic syndrome, this report case provides preliminary evidence case example of a treatment modality to aid other patients suffering from the same disease process and warrants further research.
References
- Sdrulla AD, Guan Y, Raja SN. Spinal Cord Stimulation: Clinical Efficacy and Potential Mechanisms. Pain Pract. 2018; 18: 1048-1067.
- Gildenberg PL. History of electrical neuromodulation for chronic pain. Pain Med. 2006; 7: S7-13.
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science 1965; 150: 971-9.
- Sivanesan E, Maher DP, Raja SN, Linderoth B, Guan Y. Supraspinal Mechanisms of Spinal Cord Stimulation for Modulation of Pain: Five Decades of Research and Prospects for the Future. Anesthesiology. 2019; 130: 651- 665.
- Kinany N, Pirondini E, Micera S, Van De Ville D. Spinal Cord fMRI: A New Window into the Central Nervous System. Neuroscientist. 2023; 29: 715-731.
- Song Z, Ansah OB, Meyerson BA, Pertovaara A, Linderoth B. The rostroventromedial medulla is engaged in the effects of spinal cord stimulation in a rodent model of neuropathic pain. Neuroscience. 2013; 247: 134-144.
- Bu Y, McClure T, Rosalynn Conic, Jamison Burks Lerman I, Simmons A, et al. ID: 222114 Effects of High Frequency Spinal Cord Stimulation as Measured by Autonomic Tone and fMRI. Neuromodulation Technology at the Neural Interface. 2023; 26: S43-S43.
- Sheldon B, Staudt MD, Williams L, Harland TA, Pilitsis JG. Spinal cord stimulation programming: a crash course. Neurosurgical Review. 2020; 44: 709-720.
- Dirk De Ridder, Vanneste S. Burst and Tonic Spinal Cord Stimulation: Different and Common Brain Mechanisms. 2016; 19: 47-59.
- Mekhail NA, Levy RM, Deer TR, Kapural L, Li S, Amirdelfan K, et al. ECAPcontrolled closed-loop versus open-loop SCS for the treatment of chronic pain: 36-month results of the EVOKE blinded randomized clinical trial. Regional Anesthesia & Pain Medicine. 2024; 49: 346-354.
- Approach to Spinal Cord Stimulation Programming: A Problem-Based Learning Discussion. ASRA Pain Medicine. Published 2024. 2024.
- Khan AU, Khachemoune A. Trigeminal trophic syndrome: an updated review. Int J Dermatol. 2019; 58: 530-537.
- Rashid RM, Khachemoune A. Trigeminal trophic syndrome. J Eur Acad Dermatol Venereol. 2007; 21: 725-31.
- Mishra SN, Nayak CS, Deshpande DJ, Pereira RR. Trigeminal trophic syndrome: a rare entity. Indian J Dermatol Venereol Leprol. 2011; 77: 729.
- Palanisamy A, Rajappavu SD, Kothandapany S. Trigeminal trophic syndrome. An Bras Dermatol. 2017; 92: 593-594.
- Loveman AB. An unusual dermatosis following section of the fifth cranial nerve. Arch Dermatol Syph. 1933; 28: 369–75.
- Carr P, Martin S, Young J, Chiota-McCollum N. Trigeminal trophic syndrome: A possible dermatologic manifestation of stroke deficits. Neurol Clin Pract. 2020; 10: e27-e29.
- Sadeghi P, Papay FA, Vidimos AT. Trigeminal trophic syndrome--report of four cases and review of the literature. Dermatol Surg. 2004; 30: 807-12.
- Pichard DC, Cowen EW. Trigeminal trophic syndrome after stroke. Mayo Clin Proc. 2014; 89: e87-8.
- Curtis AR, Oaklander AL, Johnson A, Yosipovitch G. Trigeminal trophic syndrome from stroke: an under-recognized central neuropathic itch syndrome. Am J Clin Dermatol. 2012; 13: 125-8.
- Mayer RD, Smith NP. Improvement of trigeminal neurotrophic ulceration with pimozide in a cognitively impaired elderly woman--a case report. Clin Exp Dermatol. 1993; 18: 171-3.
- Fruhauf J, Schaider H, Massone C, Kerl H, Mullegger RR. Carbamazepine as the only effective treatment in a 52-year-old man with trigeminal trophic syndrome. Mayo Clin Proc. 2008; 83: 502-4.
- Westerhof W, Bos JD. Trigeminal trophic syndrome: a successful treatment with transcutaneous electrical stimulation. Br J Dermatol. 1983; 108: 601-4.
- Fredeking AE, Silverman RA. Successful treatment of trigeminal trophic syndrome in a 6-year-old boy with negative pressure wound therapy. Arch Dermatol. 2008; 144: 984-6.
- Edelbach BM, Lopez-Gonzalez MA. Percutaneous high cervical spinal cord stimulation for refractory trigeminal neuralgia. Surg Neurol Int. 2023; 14: 198.
- Gupta M, Chitneni A, Ghorayeb J, Schnetzer B, Klusek M. Cervical Spinal Cord Stimulation for Trigeminal Neuralgia: a Narrative Review. Curr Pain Headache Rep. 2022; 26: 639-645.
- Velásquez C, Tambirajoo K, Franceschini P, Eldridge PR, Farah JO. Upper Cervical Spinal Cord Stimulation as an Alternative Treatment in Trigeminal Neuropathy. World Neurosurg. 2018; 114: e641-e646.
- Osenbach R. Neurostimulation for the Treatment of Intractable Facial Pain. Pain Medicine. 2006; 7: S126-S136.