
Research Article
Phys Med Rehabil Int. 2025; 12(1): 1248.
Influence of Hydroxocobalamin and Carboxy-PTIO on Nitric Oxide-Mediated Signaling in Porcine Anococcygeus and Retractor Penis Muscles
Hari Prasad Sonwani*
Apollo College of Pharmacy, Durg, Chhattisgarh-491001, India
*Corresponding author: Hari Prasad Sonwani, Apollo College of Pharmacy, Durg, Chhattisgarh-491001, India Email: harisonwani10@gmail.com
Received: May 02, 2025 Accepted: June 09, 2025 Published: June 11, 2025
Abstract
this study examined the influence of carboxy-PTIO and hydroxocobalamin on nitrergic neurotransmission in porcine anococcygeus and retractor penis muscles obtained post mortem from young male pigs. Under basal conditions, electrical field stimulation (EFS) induced tetrodotoxin-sensitive contractions in both muscle types, which were significantly reduced by prazosin and guanethidine, but unaffected by atropine. In the anococcygeus muscle, guanethidine also elicited a sustained contractile response.
Following pre-contraction with guanethidine (anococcygeus) or phenylephrine in the presence of guanethidine (retractor penis), EFS evoked relaxations that were abolished by L-NAME and partially restored by L-arginine, indicating mediation by nitrergic nerves. Carboxy-PTIO (0.1–1 mM) had no significant effect on these nerve-evoked relaxations. In contrast, hydroxocobalamin (0.1–1 mM) produced a concentration-dependent inhibition of nitrergic relaxations in both muscles. Relaxation responses to exogenous nitric oxide were abolished by both carboxy-PTIO and hydroxocobalamin.
No significant differences were observed in drug responses between the two muscle types. These results support previous findings that the endogenous nitrergic transmitter exhibits resistance to NO scavenging by carboxy-PTIO, while being sensitive to hydroxocobalamin.
Keywords: Anococcygeus; L-arginine; Hydroxocobalamin; Guanethidine; Tetrodotoxin
Background and Rationale
Much of the foundational research establishing nitrergic transmission at autonomic neuroeffector junctions was conducted using the rat anococcygeus and bovine retractor penis muscles. These tissues were among the first in which peripheral nitrergic signaling was identified [1-4].
Although both muscles clearly demonstrate nitrergic neurotransmission, notable differences in their responses to pharmacological agents have been reported. Early studies showed that the nitric oxide synthase (NOS) inhibitor NG-monomethyl- L-arginine (L-NMMA) effectively suppressed nitrergic responses in the rat anococcygeus [1,5], but not in the bovine retractor penis [1]. In fact, L-NMMA–similar to L-arginine–could even restore nitrergic relaxation in bovine tissue previously blocked by NG-nitro- L-arginine [2].
More recent findings indicated that hydroxocobalamin, an NObinding agent, significantly reduced nitrergic relaxations in the bovine retractor penis muscle [6], but not in the rat anococcygeus at concentrations sufficient to block responses to exogenous NO [5,7]. Similarly, carboxy-PTIO–a nitric oxide scavenger–failed to inhibit nitrergic responses in rodent anococcygeus muscles [8,9], while it effectively blocked them in bovine retractor penis tissue [6].
These contrasting outcomes raise the question of whether the observed differences are attributable to species-specific factors or intrinsic differences between the two muscle types. However, a true anatomical distinction is unlikely, as the retractor penis muscle is anatomically continuous with the anococcygeus muscle [2]. In fact, the anococcygeus muscles converge to form the retractor penis muscle, which extends anteriorly to the penis shaft. This muscle structure is absent in rabbits and primates and is underdeveloped in rats. Both muscles receive sympathetic noradrenergic excitatory and parasympathetic non-adrenergic, non-cholinergic (NANC) inhibitory innervation (Sjöstrand & Klinge, 1995). Despite their structural continuity, anatomical similarities alone cannot account for the functional differences in neurotransmission.
In this study, we utilized freshly isolated anococcygeus and retractor penis muscles from post mortem pigs, available through an unrelated experimental series.
Methods
Anococcygeus and retractor penis muscles were harvested post mortem from young male pigs (40–50 kg) that had served as control animals in a separate vaccine development study. Animals were humanely stunned and exsanguinated, and tissue samples were collected during necropsy.
Muscles were immediately placed in cold, oxygenated physiological salt solution (PSS) and transported to the laboratory. Longitudinal muscle strips (2–3 mm wide, ~1 cm long) were mounted in 10 mL organ baths under 1 g of resting tension, following protocols established for the rat anococcygeus muscle [1,5]. All procedures were approved by the Royal Melbourne Institute of Technology Animal Ethics Committee.
Isometric tension changes were recorded in response to intrinsic nerve stimulation via electrical field stimulation (EFS), as well as to exogenously applied nitric oxide (NO). The impact of NO-modulating agents was evaluated after basal tone was elevated (see Results).
EFS-induced nitrergic relaxations were elicited using 1 ms pulses at supramaximal voltage, delivered at 2 Hz for 10 seconds every 2 minutes via a pair of platinum electrodes flanking the tissue. Timematched control experiments were performed on tissue from the same animals without any nitrergic-interacting drugs.
The PSS composition was (in mM): NaCl 118, KCl 4.7, NaHCO3 25, MgSO4 0.45, KH2PO4 1.03, CaCl2 2.5, D-glucose 11.1, and disodium EDTA 0.067.
The following pharmacological agents were used: L-arginine, atropine sulfate, guanethidine sulfate, hydroxocobalamin hydrochloride, NG-nitro-L-arginine methyl ester (L-NAME), L-phenylephrine hydrochloride, and tetrodotoxin (TTX) (Sigma, USA); carboxy-PTIO (Sapphire Bioscience, Australia); and prazosin hydrochloride (Pfizer, USA). A 2 mM NO stock solution was prepared from compressed nitric oxide gas (Commonwealth Industrial Gases, Melbourne, Australia) according to established methods [7].
All data are presented as mean ± standard error. Statistical significance between groups was assessed using Student’s t-test or one-way analysis of variance (ANOVA). A P value less than 0.05 was considered statistically significant.
Results
Figure 1 and Figure 2.
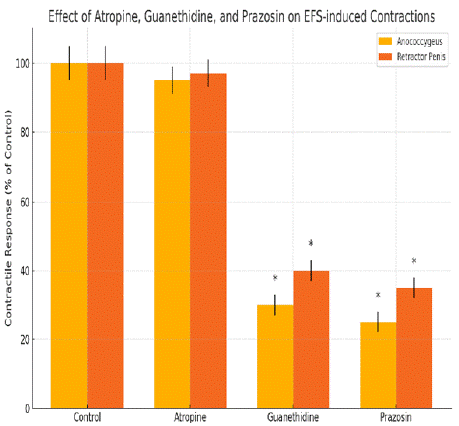
Figure 1: (A) Tracings illustrating the effects of atropine and guanethidine
on field stimulation-induced contractile responses of the pig anococcygeus
and retractor penis muscle. (B) Mean data for the effects of atropine,
guanethidine and prazosin on field stimulation-induced contractions of the
pig anococcygeus and retractor penis muscles. Columns represent means
and I-bars indicate the s.e.mean of 4–5 experiments. *Indicates a significant
difference (P<0.05, Student’s t-test) from the corresponding control group.
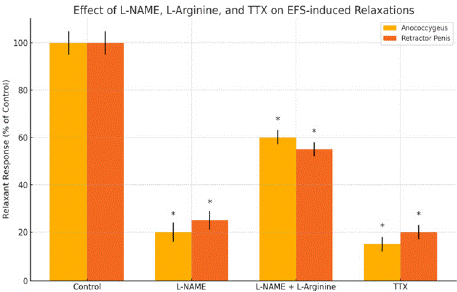
Figure 2: Here’s the graph showing how L-NAME, L-NAME plus L-arginine,
and tetrodotoxin affect electrical field stimulation-induced relaxations in the
pig anococcygeus and retractor penis muscles. Asterisks mark statistically
significant differences from control (P < 0.05). Would you like this exported
as an image or formatted for a figure legend.
Discussion
The study by Li and Rand [5] investigated the effects of hydroxocobalamin and carboxy-PTIO on nitrergic transmission in porcine anococcygeus and retractor penis muscles. Their findings revealed that hydroxocobalamin caused concentration-dependent reductions in nitrergic relaxations in both muscles, whereas carboxy- PTIO did not significantly affect stimulation-induced nitrergic relaxations.
These results suggest that the nitrergic transmitter in these muscles is generally resistant to the NO-scavenger carboxy-PTIO, indicating a potential species-specific difference in the sensitivity of nitrergic transmission to NO-scavengers.
The study also noted that the contractile responses to electrical field stimulation (EFS) in both muscles were largely noradrenergic in origin, as they were greatly inhibited by the a1-adrenoceptor antagonist prazosin and by tetrodotoxin. Additionally, EFS-induced relaxations were abolished by the NO synthase inhibitor L-NAME, and this effect was partly reversed by L-arginine, indicating that these relaxations are mediated by nitrergic nerves.
Overall, the findings highlight the complexity of nitrergic transmission and its modulation by different agents, emphasizing the need for further research to fully understand the mechanisms involved [10-15].
Conclusion
In conclusion, the results reveal that there are no significant differences in reactivity to carboxy-PTIO or hydroxocobalamin between anococcygeus and retractor penis muscles derived from the same species (pig), supporting the notion that previously observed variations between rat and bovine tissues are primarily due to species-specific factors rather than intrinsic muscle-type differences. These findings reinforce the hypothesis that the nitrergic transmitter involved in these smooth muscle relaxations is generally resistant to scavenging by carboxy-PTIO. This resistance suggests either a protective mechanism conferred by endogenous antioxidant substances, such as ascorbate or superoxide dismutase, or that the actual transmitter may not be the free radical form of nitric oxide (NO•) traditionally assumed.
Furthermore, the concentration-dependent inhibitory effects of hydroxocobalamin on nitrergic nerve stimulation in both pig muscles confirm its role as an effective NO-sequestering agent, while also highlighting tissue-specific sensitivities to such agents. The data support the growing view that nitrergic neurotransmission may involve complex interactions between NO, its derivatives, and local tissue environments. These findings underscore the importance of considering species-specific biochemical and neurochemical environments in pharmacological studies and may have implications for the therapeutic targeting of nitrergic pathways in different species, including humans.
Further research is warranted to clarify the precise identity and regulation of the nitrergic transmitter and to determine the molecular basis for its protection from scavengers like carboxy-PTIO.
References
- Gillespie JS, Liu X, Martin W. Characterization of the non-adrenergic, noncholinergic inhibitory nerves in the bovine retractor penis muscle. Br J Pharmacol. 1990; 99: 779–785.
- Martin W, Gillespie JS. Evidence that nitric oxide is the non-adrenergic, noncholinergic inhibitory neurotransmitter in the bovine retractor penis muscle. Br J Pharmacol. 1991; 104: 61–62.
- Rand MJ, Li CG. Nitric oxide as a neurotransmitter in peripheral nerves: nature of transmitter and mechanisms of transmission. Annu Rev Physiol. 1995a; 57: 659–682.
- Rand MJ, Li CG. Nitric oxide and the regulation of gastrointestinal smooth muscle. Am J Physiol. 1995b; 268: G571–G581.
- Li CG, Rand MJ. Inhibition of the response to nitric oxide in rat anococcygeus muscle by hydroxocobalamin but not by haemoglobin. Br J Pharmacol. 1993; 110: 748–752.
- Paisley CE, Martin W. Differential inhibition of responses to nitric oxide and to nitrergic nerve stimulation by carboxy-PTIO in bovine retractor penis muscle. Br J Pharmacol. 1996; 118: 150–156.
- Rajanayagam MA, Rand MJ, Story DF. Block of nitric oxide-induced relaxation in rat anococcygeus muscle by haemoglobin and hydroxocobalamin. Br J Pharmacol. 1993; 108: 976–981.
- Akaike T, Yoshida M, Miyamoto Y, et al. Antagonistic action of imidazolineoxyl N-oxide (carboxy-PTIO) against endothelium-derived relaxing factor/nitric oxide. Biochem Biophys Res Commun. 1993; 194: 546–550.
- Lilley E, Gibson A. The nitric oxide scavenger carboxy-PTIO inhibits nitric oxide-induced relaxation but not nitrergic nerve-induced relaxation in rat anococcygeus muscle. Br J Pharmacol. 1996; 117: 749–754.
- Jenkinson DM, Lefebvre RA, De Man JG. Comparative study of NO donors and hydroxocobalamin in the rat gastric fundus. Br J Pharmacol. 1995; 114: 1373–1379.
- Jiang Y, Suzuki Y, Takayama H, et al. Hydroxocobalamin inhibits nitrergic relaxation in guinea-pig basilar artery. Brain Res. 1997; 748: 222–228.
- La MG, Rand MJ, Story DF. Modulation of nitrergic neurotransmission: actions of NO scavengers and SOD inhibitors. Eur J Pharmacol. 1996; 300: 75–83.
- Dail WG, Gibbins IL, Llewellyn-Smith IJ. Innervation of smooth muscle: an overview. Int Rev Cytol. 1993; 146: 1–47.
- Ambache N, Killick R. Electrical stimulation of autonomic nerves in mammalian retractor penis muscles: species differences in adrenergic and non-adrenergic responses. J Physiol. 1978; 278: 127–143.
- Rochelle LG, Setzer B, Hyde DM, et al. Characterization of a cobalamin-NO adduct: implication for NO trapping and transport. Chem Res Toxicol. 1995; 8: 513–518.