
Research Article
Phys Med Rehabil Int. 2022; 9(2): 1201.
Less Medicines in Older Patients in the Netherlands, The Lemon Study: Study Protocol of a Cluster Randomised Controlled Trial
Abou J¹*, Elders PJM², Huijts D¹, Domic J¹, Marum RV3,4 and Hugtenburg J¹
1Department of Clinical Pharmacology & Pharmacy, Amsterdam University Medical Centers, The Netherlands
2Department of General Practice, Amsterdam UMC, location VU, Amsterdam Public Health research institute, The Netherlands
3Department of Elderly Care Medicine, Amsterdam Public Health Research Institute, The Netherlands
4Department of Clinical Pharmacology, Jeroen Bosch Hospital, s-Hertogenbosch, The Netherlands
*Corresponding author: Jamila Abou, Department of Clinical Pharmacology & Pharmacy, Amsterdam University Medical Centres, location VUMC, Amsterdam, The Netherlands
Received: June 14, 2022; Accepted: July 18, 2022; Published: July 25, 2022
Abstract
Background and Objective: Overtreatment with cardiometabolic medication is a common phenomenon in older patients. Up to 20% of these patients may be eligible for deprescribing. Deprescribing may decrease the risk of adverse drug events and is indicated when a drug may lead to more harm than benefits. The LeMON study aims to develop, implement and evaluate a standardized template for the performance of clinical medication reviews (CMR) using evidence based tools and training to support deprescribing of cardiometabolic medication.
Method: A clustered randomized controlled study involving twenty community pharmacists (CP). CP will be asked to conduct a CMR in ten patients. The intervention group will receive training on the background of deprescribing cardiometabolic medication and the use of tools and the control group will perform a CMR according to standard practice. Follow-up will take place within four weeks (T1) and after three months (T2) following the CMR. Patients 70 years or older; polypharmacy and chronic use of at least one blood pressure medicine and having a systolic blood pressure below 140 mmHg, or chronic use of glucose lowering medication and HbA1c level below 54 mmol/ mol were included.
Discussion: The LeMON study will assess whether a primary care-based intervention educating CPs about deprescribing cardiometabolic medication reduces the number of cardiometabolic medication used by older patients with a blood pressure or HbA1c lower than the treatment targets tment. The use of algorithms including information on blood pressure and/or HbA1c and cardiometabolic medication use has not been studied previously.
Keywords: Deprescribing; Polypharmacy; Cardiometabolic medication
Introduction
Patients with cardiometabolic diseases are treated with a variety of medication to decrease the risk of complications, such as cerebrovascular disease and myocardial infarction [1]. Treatment plans including the prescription of medication are generally based on national and international guidelines for the treatment of diseases. These guidelines include recommendations aimed at specific treatment targets, infrequently taking into account patient characteristics such as co-morbidities, co-medication and the patients’ age. In case treatment goals are not reached with first line medicine choices, second and third line medicines can be added to the treatment regimen. As a result, and particular in case of comorbidities, patients may use more than 5 medicines, which is recognized as polypharmacy [2].
Polypharmacy is associated with an elevated risk of drug related problems, such as adverse drug reactions, drug-drug interactions, contra-indications and non-adherence, which may result in increased morbidity and hospitalization, and unnecessary health care costs [3-7]. This risk is more profound in older adults due to age related changes in pharmacodynamics and -kinetics but also due to increasing frailty which increases susceptibility to negative effects of medication [8].
In order to provide guidance on the prescribing of adequate medication in older patients in 2012 the Dutch Multidisplinary Guideline ‘Polypharmacy in Older Patients’ (MDR Polypharmacy [MDRP]) was published. An important tool to optimize the medication of older patients with polypharmacy is the periodicallyconducted clinical medication review (CMR). Applying the MDRP in primary care was stimulated by obliging CPs and GPs to annually perform a minimum number of CMRs for patients at risk and reimbursing these HCPs.
Several studies show that (frail) older adults may benefit from less strict target values of blood pressure and blood glucose than recommended in younger individuals [9]. The Dutch guidelines for cardiovascular risk management and diabetes recommend =150 mmHg and 53-69 mmol/mol as target values for systolic blood pressure and HbA1c in (frail) older adults [10,11]. With these less strict treatment targets, medication often can be tapered or discontinued. Deprescribing has recently been introduced as ‘the process of withdrawal of an inappropriate medication, supervised by a healthcare provider (HCP) with the goal of managing polypharmacy and improving outcomes’ [3]. Deprescribing may improve quality of life and decrease the risk of adverse drug events [4] and is indicated when a medication may lead to more harm than benefits [12]. Several tools with regard to deprescribing have been developed [13-15]. Deprescribing of glucose-lowering and antihypertensive medication in patients with multiple comorbidities, frailty, hypoglycemic risk, or a limited life expectancy seems feasible and safe [16,19]. Although deprescribing of cardiometabolic medication seems feasible, its implementation needs further support [9].
However, in daily practice the MDRP turned out to be difficult to apply and results were somewhat disappointing, particularly because CP recommendations to deprescribe medication often did not result in dose reductions and discontinuation [20,23]. The Dutch MDRP has therefore recently been supplemented by a guideline on deprescribing, partially filling the evidence and information gap by adding fact sheets on the most common disorders in older patients [24]. In these documents considerations and criteria have been summarized that support the decision-making to discontinue or continue the use of certain medications.
To implement the MDRP in daily practice, CP needs training and support. We therefore developed a practical model for the performance of CMR for CPs with an integrated module for deprescribing cardiometabolic medication with a training including information from existing guidelines, which will be implemented and evaluated in a cluster randomized trial. The protocol for this study is described in the present article.
Methods
Study Design and Setting
A clustered randomized controlled trial will be performed. In total, 20 community pharmacies will be recruited. Each CP will be randomly assigned to either the intervention or control group. CPs in the intervention group will receive training on deprescribing cardiometabolic medication. Subsequently, intervention CPs will perform a CMR in 10 patients. Each control CP will perform CMRs in 10 patients according to usual care. Follow-ups will take place within four weeks (T1) and after three months (T2) following the CMR. A process evaluation will be performed at the end of the study. A flowchart of the study is presented in (Figure 1).
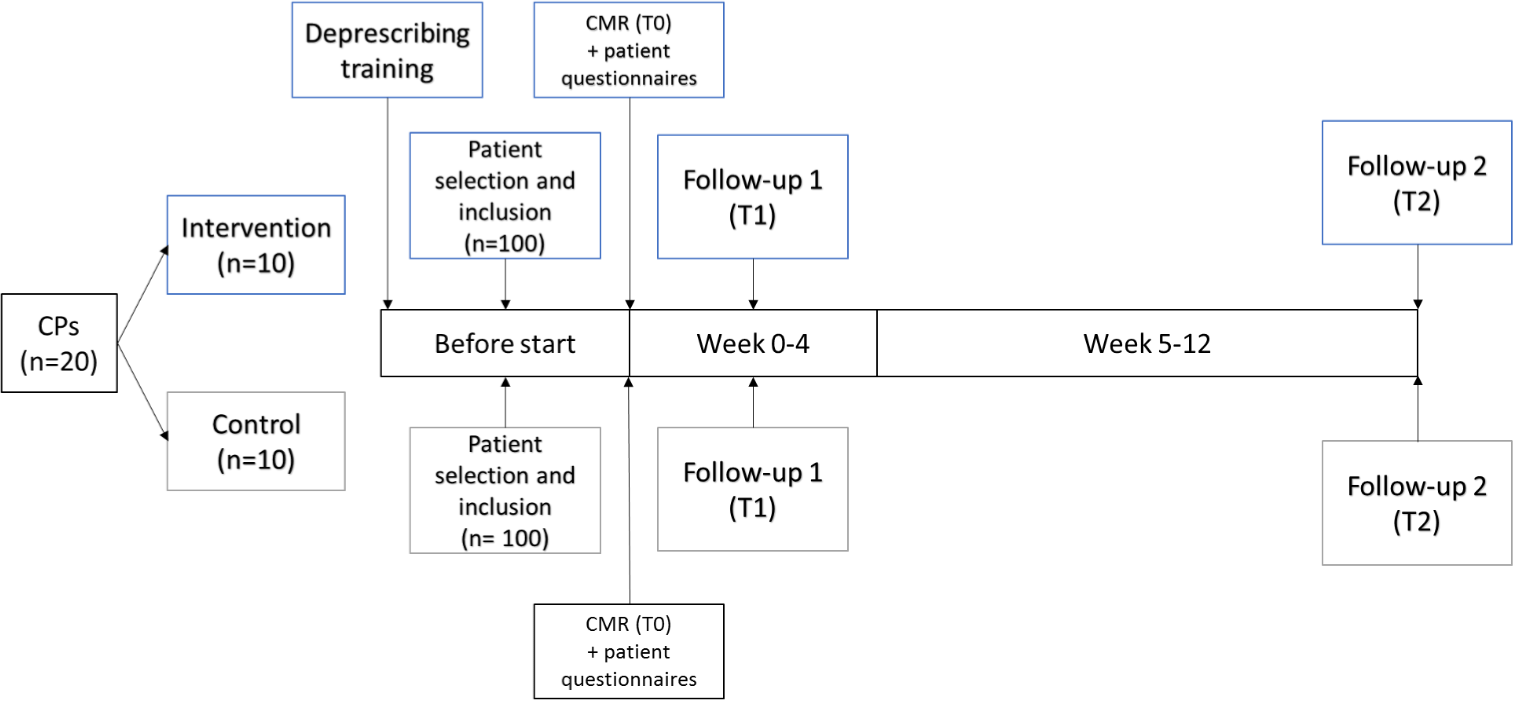
Figure 1: Flowchart of the study.
Abbreviations: CP = community pharmacist, CMR = clinical medication review.
Community pharmacies, part of the international BENU pharmacy chain, will participate in this trial. A total of 35 BENU community pharmacies, provided by a BENU pharmacy manager, will be approached by the researchers. The intervention consists of a CMR initiated by CP in collaboration with GPs. For the description of the design of the LeMON study, the Consolidated Standards of Reporting Trials (CONSORT) statement with extension to cluster randomized trials is followed.
Community Pharmacists and General Practitioners
CP cooperates with several GP and during the training the CP will be instructed to inform the GP about the study. CP also receives instructions on how to communicate about deprescribing with other GP and other HCP.
Study Population
Patients will be selected by the CPs using the Pharmacy Administration and Information System and data requested from GPs from their General Practice Information Systems. Inclusion criteria for the patients are: 70 years or older; the use of five or more chronic medications; chronic use of at least one blood pressure medicine and having a systolic blood pressure below 140 mmHg, and/or chronic use of glucose lowering medication and HbA1c level below 54 mmol/ mol. Exclusion criteria: a life expectancy of less than three months will be excluded. Patients will be invited by the CP, informed about the study by means of an information leaflet and will be asked for their informed consent. The selection of the specific patient group, older patient using cardiometabolic medication having a systolic blood pressure below 140 mmHg, or/and a HbA1c level below 54 mmol/mol, seems feasible by collecting information from GPs by CPs. In this study we use this algorithm to select cardiometabolic patients whom could benefit most from deprescribing.
Recruitment
Participants will be recruited by their CP according to the selection algoritme. Usually CP discusses the selected list with GPs. The participants are then invited by letter or telephone consultation by their pharmacist.
Clinical Medication Review
Participants from intervention and control group will have a CMR. A CMR is an evaluation of the pharmacological treatment of a patient, usually including a patient interview. Performing regular CMRs is included in the national guidelines for Dutch pharmacists and GPs and may positively influence the quality of drug therapy and health outcomes [20]. A method in these guidelines is the ‘Systematic Tool to Reduce Inappropriate Prescribing’ (STRIP) method and consists of different five steps [25]. In the first step the pharmacist explores in a patient interview the health complaints, adherence and possible side effects of their medication of the patients. In the second step the CP will conduct a medication review combining clinical data (laboratory values), medication data and patient information from the interviews. Recommendations for deprescribing medication will be addressed. In the third step the CP and GP discuss a pharmaceutical care plan with prioritized treatment goals. In the next step the pharmaceutical care plan will be discussed with the patient by CP or GP and the actions will be implemented. In the fifth step the follow up moments are scheduled by CP.
Thus, deprescribing within the context of CMR fit very well in the usual working procedures of CPs and GPs, will enable the full implementation and integration of deprescribing in the current workflow of these HCPs. The decision to discuss deprescribing with a patient may be prompted by several factors, such as the total quantity of medication taken, use of potentially inappropriate medications, new symptoms, or changed treatment goals [1,26].
Intervention
Deprescribing training: Our previous research showed that lack of knowledge, lack of self-efficacy, and fear for the consequences of deprescribing are important barriers for CP and GP regarding deprescribing cardiometabolic medication [27]. Overcoming these barriers, requires tailored training. HCP’s emphasized that additional information regarding polypharmacy, the risks of continuing chronic medication, and the possible benefits of deprescribing would enable deprescribing. Anderson et al. [28] also emphasized the importance of educating prescribers on deprescribing to shift the focus from treatment intensification towards a more balanced approach where deprescribing is considered an essential approach of good prescribing practice as well. Hence, for our study we will develop an interactive training to provide the CPs with guidance on evidence based treatment guidelines and tools on deprescribing in cardiometabolic diseases. The training will provide an extensive explanation on existing evidence and guidelines regarding deprescribing cardiometabolic medication. By interactively discussing cases, CPs can directly practice the application of information provided during the training while receiving feedback from the researchers. Furthermore, studies indicate that clear communication skills and a trusting relationship with the patient are important enablers in the deprescribing process [29]. Therefore, the training will include information on tools and discussion of examples of patient counselling on deprescribing to address these aspects as well. Eventually, the goal of the training will be to draw attention to deprescribing cardiometabolic medication, stir up discussion on the topic and provide tools and information that CPs can adaptively use in their CMR routines.
The training will last approximately 1.5 hours. The training will provide background information regarding polypharmacy, overuse and deprescribing in older diabetes and cardiovascular patients. Multimorbidity, polypharmacy and overtreatment with antihypertensive medication and may be harmful and was associated with increased risk of fall injuries. In the training background on minimizing treatment related harms will be addressed. Subsequently, five topics of interest concerning deprescribing will be addressed: [1] strategies and tools, [2] collaborating with other HCPs (multidisciplinary approach), [3] involvement of the patient and patient counselling, [4] composing a treatment plan, and [5] monitoring.
Specific strategies and tools that will be presented are based on existing evidence [4,13,29,30-36]. Guidelines that will be addressed include the STOPP-frail [13], the Dutch national guidelines for the treatment of cardiometabolic diseases and diabetes [10,11], the BEERs list [14], and the Dutch multidisciplinary guideline polypharmacy in elderly [37]. Concerning the topics [2] multidisciplinary approach and [3] involvement of the patient, research has been conducted to gain and the patient centered process proposed by Reeve et al. [29] will be discussed. Also, these topics will cover several aspects of conversational strategies regarding deprescribing in patients. The fourth topic, composing a treatment plan, will address several tools that could help compiling such a plan, for example the Outcome Prioritization Tool [36]. Furthermore, there will be interactive discussions with the CPs about two patient cases in which deprescribing could be applied. At the end of the training, several study procedures will be explained. The CPs will receive handouts of the training.
The CPs in the intervention group will be trained one by one digitally via Zoom Video Communications (San Jose, USA). Each CP will attend the deprescribing training once. The CMRs performed by the CP will take place at the pharmacy, digitally, or via telephone, depending on the CPs’ working procedures.
Control Group
Regarding the control group, patient selection will be carried out similar as for the intervention group. The control group will not receive the deprescribing training, but a handbook with study procedures on how to conduct CMR based on the DMDG [24]. Evaluations of the proposed and implemented interventions will be performed within four weeks (T1) and after three months (T2) as well. After the study the deprescribing training will be offered to all control CPs.
Implementation and Follow-up
Once the CMR has been performed, the CP will discuss proposed changes in medication with the GP or specialist to compile a treatment plan. Depending on their preferences, either the GP or CP will discuss the treatment plan with the patient within four weeks after the CMR (T1). Changes in medication use will only be implemented after discussion and agreement with the patient. After three months (T2), the CP will evaluate which changes are still implemented and collect the latest HbA1c, blood pressure, and lipid concentrations.
Materials
All CPs will receive an information letter, a protocol for the performance of the patient selection and CMR, a manual for the performance of the data collection, information letters and informed consent forms for the patients. The intervention group will also receive an overview containing guidelines about deprescribing and a handout of the deprescribing training. The overview and handouts also contains a summary of targets of glucose levels and blood pressure thresholds.
Randomisation
Randomisation and data collection: The community pharmacies will be randomized using Castor EDC [38] to the intervention or control group. Data regarding demographics, medication use and medical history will be collected at baseline. During the followup moments, information regarding proposed and implemented changes will be collected. All CPs will report back to the researchers following T0, T1, and T2 using Castor EDC [38]. CPs will be asked to create an account which will allow them to fill in the anonymized data regarding their patients. The researchers will monitor when a follow-up needs to take place and informs the CPs by sending them a reminder. In the case of an adverse event, additional data concerning this event will be collected. Monitoring the medication use will be carried out in Castor by the CP to identify medication changes.
Outcome measures
Primary outcome measures: The primary outcome measure of the study will be the proportion of patients with 1 or more cardiometabolic medication deprescribed at T2.
Secondary outcome measures: Secondary outcome measures will be the type and number of proposed deprescribing interventions by the CP at T1 and whether these interventions have been successfully implemented at T2. The type of intervention will be categorized by blood glucose medication, cardiovascular medication, or other. In addition, the results of the process evaluations using NPT and the PREM and TSQM questionnaires will also be assessed as secondary outcome measures.
Sample Size
It is hypothesized that medication will be reduced in 40% of the patients from the CPs assigned to the intervention group and in 10% of the patients within the control group [39]. Considering a power of 80%, type 1 error of 0.05 and a correction of 15% for the cluster design, 76 patients will have to be included in each group. Taking into account a drop-out of approximately 30%, a total of 200 patients will be recruited.
Analyses
Descriptive statistics will be used to describe the demographics, total amount of drug use, number of antihypertensive and diabetes medication, blood pressure and HbA1c value at baseline.
Multilevel logistic regression will be conducted to study the difference between patients in the interventions group and the control group. Using multilevel analyses enables taking into account clustering of observations of participants receiving care from the same CP. We will adjust results for possible confounders and effect modifiers (age, sex, number of medication). Explorative subgroup analyses will be performed in order to gain a better understanding which subgroups benefit most from the intervention.
Process Evaluation
Normalization process theory: The Normalization Process Theory (NPT) will be used to evaluate the intervention. It has been observed that interventions that have been tested in study settings, are scarcely implemented in practice [43]. This seems contradicting, since testing an intervention in a study setting often includes its fit into daily working procedures. Hence, proper evaluation of the intervention is needed to assess the likelihood of the intervention being implemented in work flow. NPT provides guidance in the evaluation of complex interventions using four core constructs: coherence, cognitive participation, collective action, and reflexive monitoring [43]. Based on these constructs a topic list will be created that will be used during semi-structured interviews with the pharmacists that received the intervention.
To evaluate the process of the intervention, semi-structured interviews will be conducted with the CPs allocated to the intervention group. The interviews are held by a member of the research team and will last approximately 30 minutes each. A topic-list based on NPT [43] will be used to guide the interview. The topic list can be found in appendix 1. After consent from the interviewee, the interviews will be recorded and transcribed verbatim. The transcript will be coded using direct content analysis and NPT.
To assess the satisfaction of the patients regarding the therapy provided by the CPs, the Treatment Satisfaction Questionnaire for Medication (TSQM) will be used. Also, the Patient Reported Experience Measure (PREM) questionnaire will be used to evaluate the patients’ satisfaction about the CMR. Members of the research team will contact the patients by telephone and the questionnaires are filled out together with the patients. This will be done after the CMR. In case patients are not able to answer the questionnaires themselves the partner or a close family member will be asked to help or fill out the questionnaire on behalf of the patient. It will take approximately 30 minutes to complete one questionnaire.
Discussion
This paper describes the background and design of a cluster RCT aiming to implement and evaluate deprescribing of cardiometabolic medication within CMRs in older cardiometabolic patients using a training of CPs as compared to the performance of regular CMRs without training on deprescribing. The training is supposed to increase knowledge of CPs on deprescribing cardiometabolic medication, to increase their communication skills regarding deprescribing, to deal with barriers for deprescribing and to get acquainted with the use of various tools to support deprescribing. We have previously investigated the barriers and facilitators of HCP to deprescribe cardiometabolic medication in a qualitative study [40]. The results of this study were input for the current training. HCP needed synopsis of the knowledge on deprescribing, more communication skills on how to implement deprescribing in their daily practice in collaboration with other HCP, patients and caregivers. The training is based on actual guidelines on how to perform CMR, and in particular deprescribing unnecessary cardiometabolic medication. Although the importance of deprescribing is recognized and considered safe, the implementation in daily clinical practice remains challenging [9]. This is largely explained by lack of knowledge, skills, and selfefficacy of HCPs regarding the deprescribing process in preventive medication and its consequences for HCPs and patients [28,41]. Organizational factors, poor communication, and level of trust from the patient are other important aspects that influence the process of deprescribing. Anderson et al. mention that deprescribing of inappropriate medication must be considered as evenly important as the prescription of new medications [28]. Their study showed that increased information regarding balancing benefits and harms, creating confidence for HCPs to deviate from existing guidelines, experience, improved communication with patients and other HCPs, and targeted training may contribute to appropriate deprescribing [28]. Previous study has shown that CMR on large scale seems not efficient [42]. Therefore it might be more beneficial to select a specific group of patients. In Abou et al. HCP expressed the need to use blood pressures and HbA1c in the process of deprescribing cardiometabolic medication. The LeMON study combines the information of the values of blood pressure and HbA1c with polypharmacy data from the pharmacies. After selection based on polypharmacy of cardiometabolic medication, the CPs ask the eligible patient for inclusion based on low blood pressure or low HbA1c values. These values are obtained from GPs. With these selection criteria, patients with potential overtreatment are selected. We believe that these patients are potentially more likely to benefit from a CMR with a specific focus on deprescribing. Several studies suggest that low blood pressure and overtreatment may be harmful in frail patients with polypharmacy. The training of CPs is hypothesized to result in more patients who may be eligible for deprescribing. Although this study does not investigated the effects of deprescribing cardiometabolic outcomes, it is hypothesized that in patients with overtreatment of cardiometabolic medication deprescribing of these medication will lead to a lower risk of adverse events such as falls, fractures and hypoglucosis. Monitoring the patient is an important part of the process of CMR, and deprescribing.
A major strength of this study is that we designed a cluster randomised control trial after conducting preliminary research. The findings of that study were applied in the development of the methodology of this study and the deprescribing training. In this way, we aim to tackle the barriers experienced by CPs in the deprescribing process. It has been observed that there is ambiguity about who is responsible for the initiation of the deprescribing process. This may be an obstacle during the study, since we will not include all HCPs related to the prescribing process. On the other hand, it may provide clarity as well, since the CP will be asked to take lead in the initiation of the deprescribing process. Also strength of this study is the special attention for follow up moments. Although the CPs will only be asked to attend the training once, reporting data to the researchers and evaluations of possible changes in medication might be considered time consuming by the CPs. However, the process of CMR of this protocol is almost similar to the daily practice of CPs. The design allows it to be adapted to the usual practice of each CP. In this way, the implementation of the intervention fits very well in routine clinical practice, which increases the probability of the intervention to be executed by CPs.
Finally, a process-evaluation will provide insight into the facilitators and barriers for implementation. In addition, NPT will be used to assess whether this it is feasible to implement the intervention in practice and if adaptions are necessary for implementation. Lastly, the CPs allocated to the control group will receive the training after the study so that all CPs included in the study will receive the deprescribing training. The inclusion of the patients has been performed between March 2020 and December 2021. If implementation of deprescribing cardiometabolic medication within CMR leads to appropriate and feasible deprescribing cardiometabolic medication in older patients, this intervention based on training of CPs in deprescribing can be implemented in other community pharmacies.
Ethics and Dissemination
The study design, study protocol, procedure and informed consent are approved by the Medical Ethics Committee of the University Medical Centre of Amsterdam, location VUmc (FWA00017598). Participation is voluntary and all participants will sign informed consent. The trial was registered in the Netherlands Trial Register number: NL8082
Acknowledgements
We would like to thank BENU Apotheken for their support in recruiting the pharmacists and for the project management support during this study. Also we would like to thank all participating CPs.
Funding
This study was supported by a research grant by the Dutch Organization for Pharmacists (KNMP).
Conflict of Interest Statement
All authors declare that they have no conflict of interest.
References
- Krishnaswami A, Steinman MA, Goyal P, Zullo AR, Anderson TS, Birtcher KK, et al. Deprescribing in Older Adults With Cardiovascular Disease. Journal of the American College of Cardiology. 2019; 73: 2584-2595.
- Payne RA, Avery AJ, Duerden M, Saunders CL, Simpson CR, Abel GA. Prevalence of polypharmacy in a Scottish primary care population. European Journal of Clinical Pharmacology. 2013; 70: 575-581.
- Reeve E, Moriarty F, Nahas R, Turner JP, O’Donnell LK, Hilmer SN. A narrative review of the safety concerns of deprescribing in older adults and strategies to mitigate potential harms. Expert Opinion on Drug Safety. 2018; 17: 39-49.
- Reeve E, Gnjidic D, Long J, Hilmer S. A systematic review of the emerging definition of ‘deprescribing’ with network analysis: implications for future research and clinical practice. British journal of clinical pharmacology. 2015; 80: 1254-1268.
- Scott IA, Couteur DGL. Physicians need to take the lead in deprescribing. Internal Medicine Journal. 2015; 45: 352-356.
- Leendertse AJ, Koning GHP, Goudswaard AN, Belitser SV, Verhoef M, Gier HJ, et al. Preventing hospital admissions by reviewing medication (PHARM) in primary care: an open controlled study in an elderly population. Journal of Clinical Pharmacy and Therapeutics. 2013; 38: 379-387.
- Barnett AH, Cradock S, Fisher M, Hall G, Hughes E, Middleton A. Key considerations around the risks and consequences of hypoglycaemia in people with type 2 diabetes. International Journal of Clinical Practice. 2010; 64: 1121-1129.
- Reeve E, Wiese MD, Mangoni AA. Alterations in drug disposition in older adults. Expert Opinion on Drug Metabolism & Toxicology. 2015; 11: 491-508.
- Sussman JB, Kerr EA, Saini SD, Holleman RG, Klamerus ML, Min LC, et al. Rates of Deintensification of Blood Pressure and Glycemic Medication Treatment Based on Levels of Control and Life Expectancy in Older Patients With Diabetes Mellitus. JAMA internal medicine. 2015; 175: 1942.
- Guideline Cardiovascular Risk Management (CVRM). Dutch General Practitioners Association (NHG), Utrecht. 2019.
- Guideline Diabetes Mellitus Type 2. Dutch General Practitioners Association (NHG), Utrecht. 2018.
- Gillespie RJ, Harrison L, Mullan J. Deprescribing medications for older adults in the primary care context: A mixed studies review. Health Science Reports. 2018; 1: e45.
- Lavan AH, Gallagher P, Parsons C, O’Mahony D. STOPPFrail (Screening Tool of Older Persons Prescriptions in Frail adults with limited life expectancy): consensus validation. Age and Ageing. 2017; 46: 600–607.
- Greenberg SA. American Geriatrics Society Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. Journal of the American Geriatrics Society. 2012; 60: 616-631.
- O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age and Ageing. 2015; 44: 213-218.
- Farrell B, Black C, Thompson W, McCarthy L, Rojas-Fernandez C, Lochnan H, et al. Deprescribing antihyperglycemic agents in older persons: Evidencebased clinical practice guideline. Canadian family physician Medecin de famille canadien. 2017; 63: 832-843.
- Oktora MP, Kerr KP, Hak E, Denig P. Rates, determinants and success of implementing deprescribing in people with type 2 diabetes: A scoping review. Diabetic Medicine. 2020; 38(2).
- Silverii GA, Caldini E, Dicembrini I, Pieri M, Monami M, Mannucci E. Deprescription in Elderly Patients with Type 2 Diabetes Mellitus. Diabetes research and clinical practice. 2020; 170: 108498.
- Reeve E, Jordan V, Thompson W, et al. Withdrawal of antihypertensive drugs in older people. Cochrane Database Syst Rev. 2020; 10: CD012572.
- Chau SH, Jansen APD, Ven PMVD, Hoogland P, Elders PJM, Hugtenburg JG. Clinical medication reviews in elderly patients with polypharmacy: a crosssectional study on drug-related problems in the Netherlands. International Journal of Clinical Pharmacy. 2015; 38: 46-53.
- Geurts MME, Stewart RE, Brouwers JRBJ, Graeff PAD, Gier JJD. Implications of a clinical medication review and a pharmaceutical care plan of polypharmacy patients with a cardiovascular disorder. International Journal of Clinical Pharmacy. 2016; 38: 808-815.
- Hurmuz MZM, Janus SIM, Manen JGV. Changes in medicine prescription following a medication review in older high-risk patients with polypharmacy. International Journal of Clinical Pharmacy. 2018; 40: 480-487.
- Bosch-Lenders D, Jansen J, Stoffers HEJHJ, Winkens B, Aretz K, Twellaar M, et al. The Effect of a Comprehensive, Interdisciplinary Medication Review on Quality of Life and Medication Use in Community Dwelling Older People with Polypharmacy. Journal of Clinical Medicine. 2021; 10.
- MDRP guideline, december 2021.
- Dutch STRIP method, https://richtlijnen.nhg.org/files/2020-05/uitwerking_ stappenplan.pdf
- Jansen J, Naganathan V, Carter SM, McLachlan AJ, Nickel B, Irwig L, et al. Too much medicine in older people? Deprescribing through shared decision making. British Medical Journal. 2016; 353: i2893.
- Abou J, Crutzen S, Tromp V, Heringa M, Marum RV, Elders P, et al. Barriers and Enablers of Healthcare Providers to Deprescribe Cardiometabolic Medication in Older Patients: A Focus Group Study. Drugs & Aging. 2022; 39: 209-221.
- Anderson K, Stowasser D, Freeman C, Scott I. Prescriber barriers and enablers to minimising potentially inappropriate medications in adults: a systematic review and thematic synthesis. BMJ Open. 2014; 4: e006544.
- Reeve E, Shakib S, Hendrix I, Roberts MS, Wiese MD. Review of deprescribing processes and development of an evidence-based, patientcentred deprescribing process. British Journal of Clinical Pharmacology. 2014; 78: 738-747.
- Scott IA, Hilmer SN, Reeve E, Potter K, Couteur DL, Rigby D, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA internal medicine. 2015; 175: 827.
- Currie CJ, Peters JR, Tynan A, Evans M, Heine RJ, Bracco OL, et al. Survival as a function of HbA1c in people with type 2 diabetes: a retrospective cohort study. The Lancet. 2010; 375: 481-489.
- Cushman WC, Evans GW, Byington RP, Goff DC, Grimm RH, Cutler JA, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. The New England journal of medicine. 2010; 362: 1575-1585.
- Herwaarden MFW, Kramers C, Sturkenboom MC, Bemt PM, Smet PA. Targeting Outpatient Drug Safety. Drug Safety. 2012; 35: 245-259.
- Reeve E, To J, Hendrix I, Shakib S, Roberts MS, Wiese MD. Patient Barriers to and Enablers of Deprescribing: a Systematic Review. Drugs & Aging. 2013; 30: 793-807.
- Verdoorn S, Blom J, Vogelzang T, Kwint H, Gussekloo J, Bouvy ML. The use of goal attainment scaling during clinical medication review in older persons with polypharmacy. Research in social & administrative pharmacy: RSAP. 2018; 15: 1259-1265.
- Stegmann ME, Festen S, Brandenbarg D, Schuling J, Leeuwen BV, Graeff PD, et al. Using the Outcome Prioritization Tool (OPT) to assess the preferences of older patients in clinical decision-making: A review. Maturitas. 2019; 128: 49-52.
- Multidisciplinary Guideline Polypharmacy in The Elderly (MDGP). Dutch General Practitioners Association (NHG), Utrecht. 2012.
- Castor EDC. Castor Electronic Data Capture 2019 [27 Aug. 2019]. Available from: https://castoredc.com.
- Ibrahim K, Cox NJ, Stevenson JM, Lim S, Fraser SDS, Roberts HC. A systematic review of the evidence for deprescribing interventions among older people living with frailty. BMC Geriatrics. 2021; 21.
- Abou J, Crutzen S, Tromp V, Heringa M, Marum RV, Elders P, et al. Barriers and Enablers of Healthcare Providers to Deprescribe Cardiometabolic Medication in Older Patients: A Focus Group Study. Drugs & Aging. 2022; 39: 209-221.
- Schuling J, Gebben H, Veehof LJG, Haaijer-Ruskamp FM. Deprescribing medication in very elderly patients with multimorbidity: the view of Dutch GPs. A qualitative study. BMC Family Practice. 2012; 13: 56-56.
- Willeboordse F, Schellevis FG, Chau SH, Hugtenburg JG, Elders PJM. The effectiveness of optimised clinical medication reviews for geriatric patients: Opti-Med a cluster randomised controlled trial. Family Practice. 2017; 34: 437-445.
- Murray E, Treweek S, Pope C, MacFarlane A, Ballini L, Dowrick C, et al. Normalisation process theory: a framework for developing, evaluating and implementing complex interventions. BMC Medicine. 2010; 8: 63-63.